Abstract
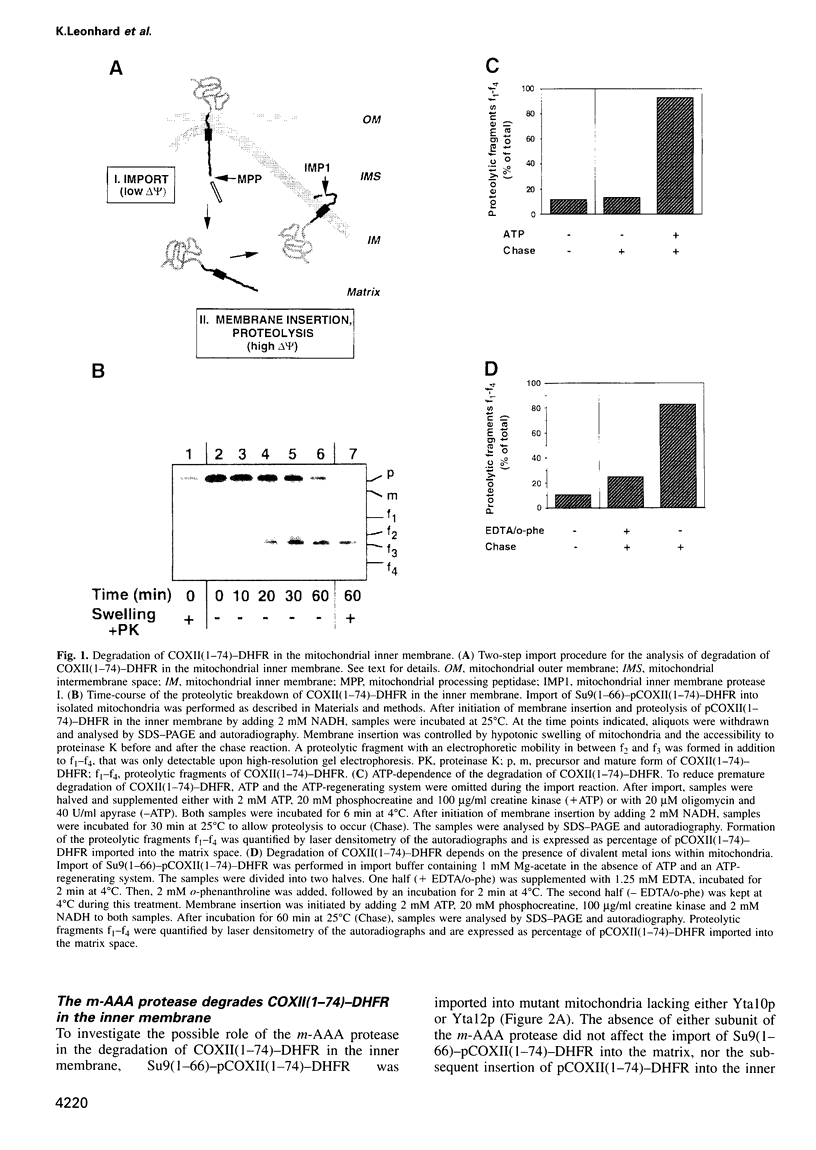
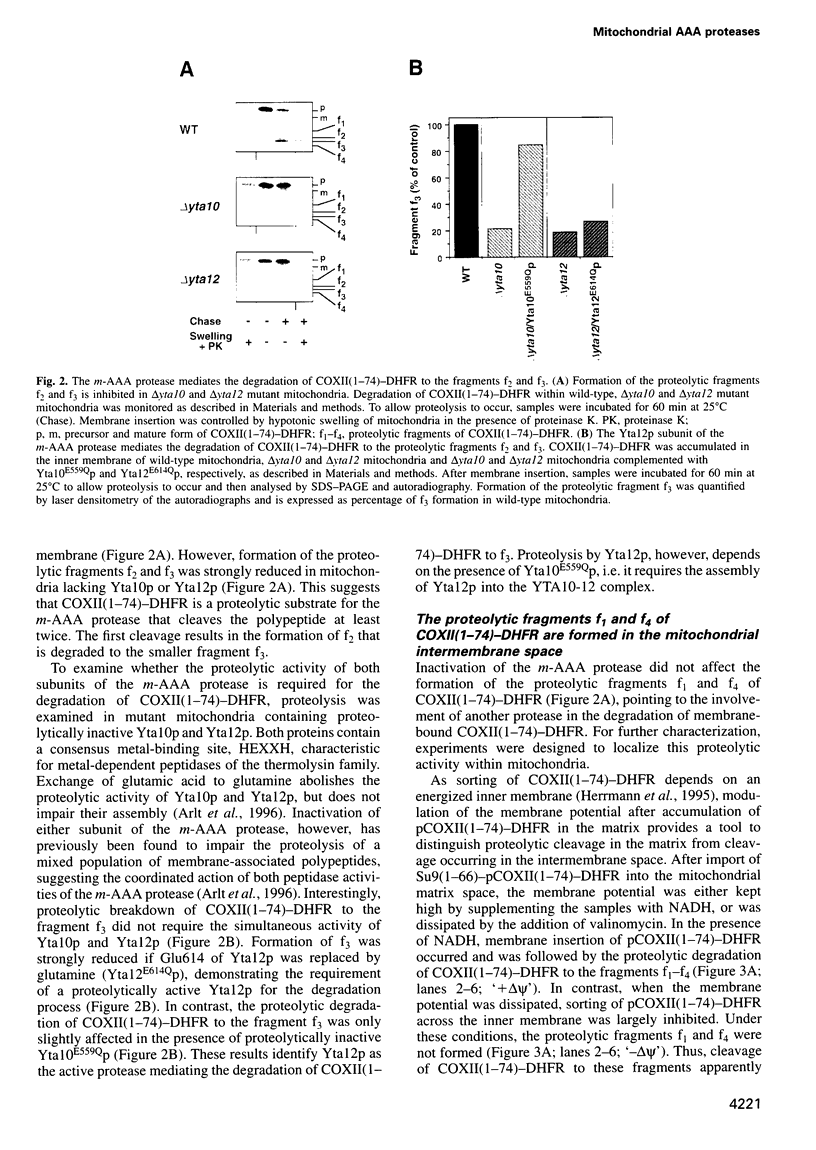
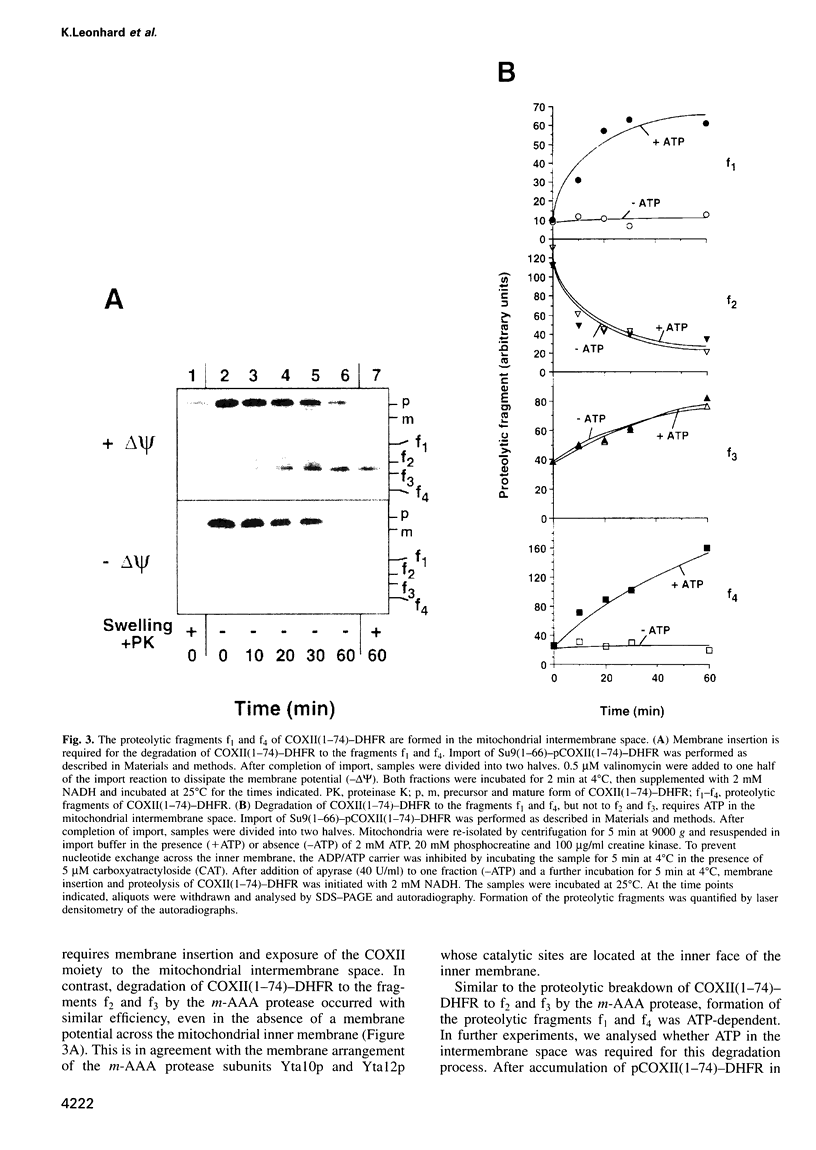
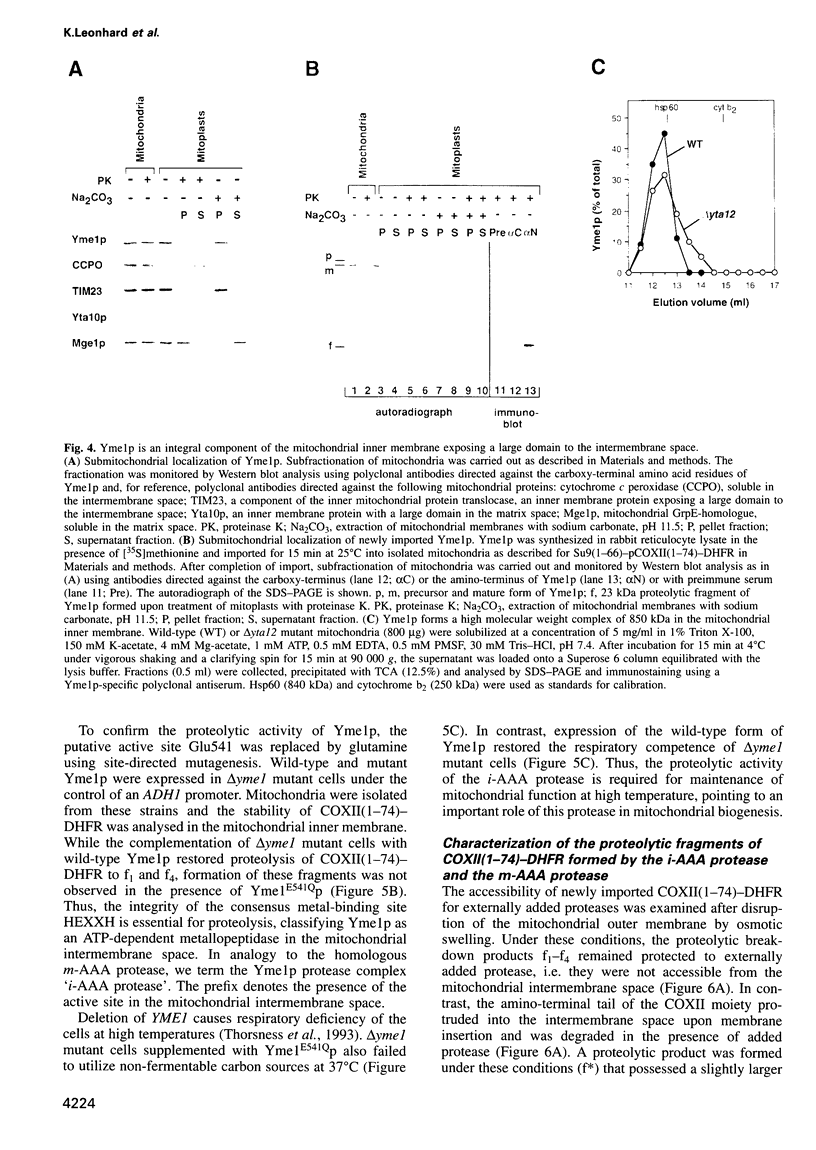
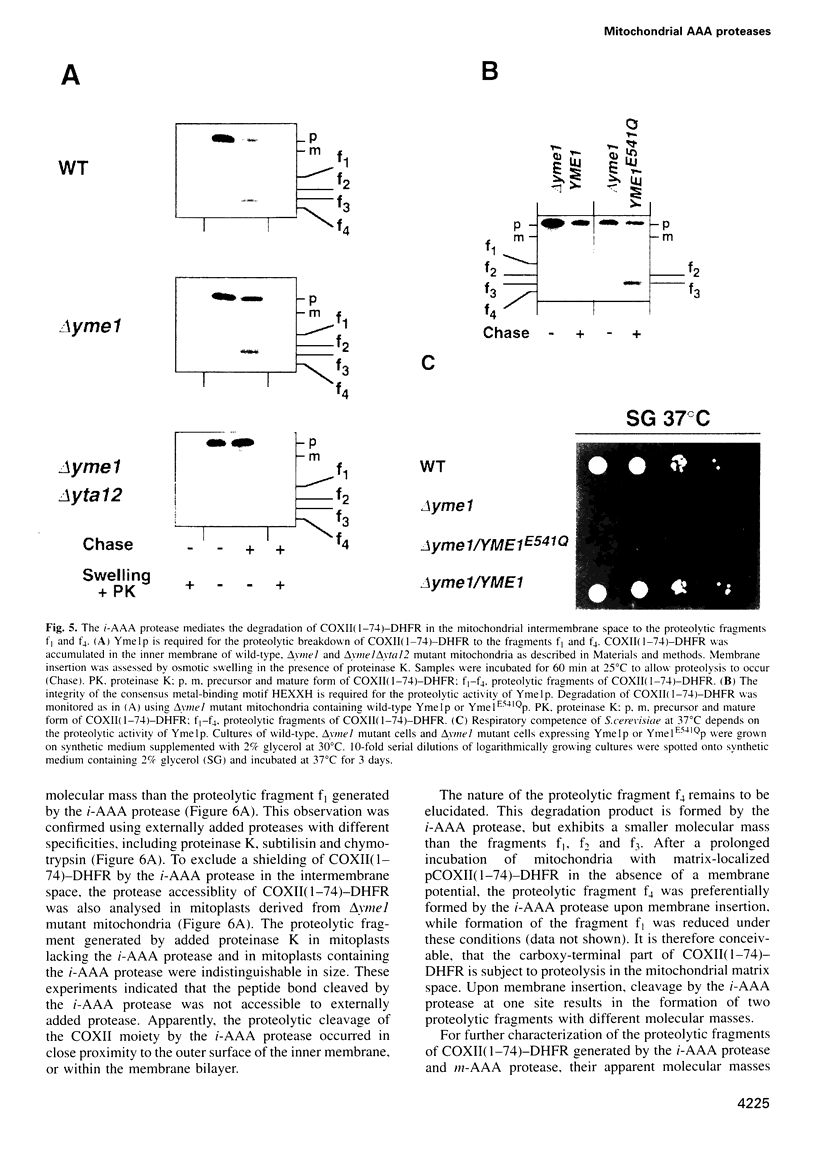
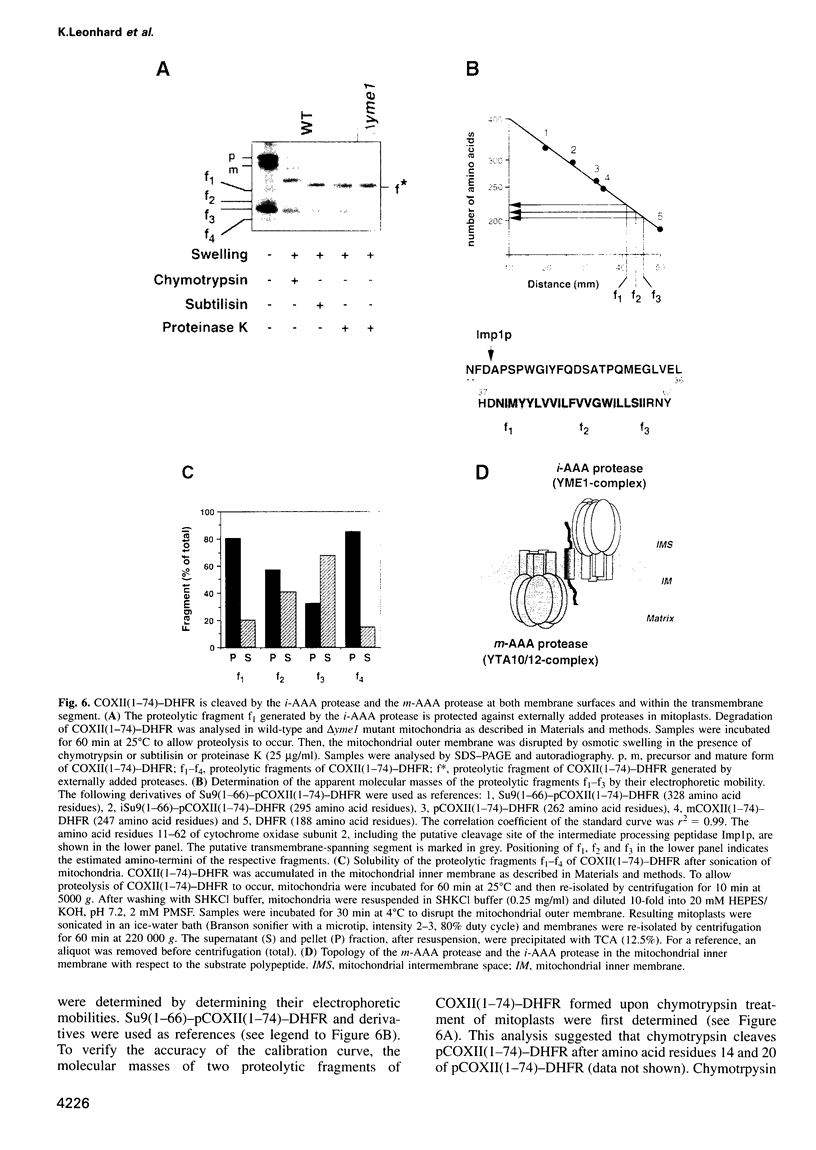
The mechanism of selective protein degradation of membrane proteins in mitochondria has been studied employing a model protein that is subject to rapid proteolysis within the inner membrane. Protein degradation was mediated by two different proteases: (i) the m-AAA protease, a protease complex consisting of multiple copies of the ATP-dependent metallopeptidases Yta1Op (Afg3p) and Yta12p (Rcalp); and (ii) by Ymelp (Ytallp) that also is embedded in the inner membrane. Ymelp, highly homologous to Yta1Op and Yta12p, forms a complex of approximately 850 kDa in the inner membrane and exerts ATP-dependent metallopeptidase activity. While the m-AAA protease exposes catalytic sites to the mitochondrial matrix, Ymelp is active in the intermembrane space. The Ymelp complex was therefore termed 'i-AAA protease'. Analysis of the proteolytic fragments indicated cleavage of the model polypeptide at the inner and outer membrane surface and within the membrane-spanning domain. Thus, two AAA proteases with their catalytic sites on opposite membrane surfaces constitute a novel proteolytic system for the degradation of membrane proteins in mitochondria.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Ogura T., Ito K. Involvement of FtsH in protein assembly into and through the membrane. I. Mutations that reduce retention efficiency of a cytoplasmic reporter. J Biol Chem. 1994 Feb 18;269(7):5218–5224. [PubMed] [Google Scholar]
- Akiyama Y., Yoshihisa T., Ito K. FtsH, a membrane-bound ATPase, forms a complex in the cytoplasmic membrane of Escherichia coli. J Biol Chem. 1995 Oct 6;270(40):23485–23490. doi: 10.1074/jbc.270.40.23485. [DOI] [PubMed] [Google Scholar]
- Arlt H., Tauer R., Feldmann H., Neupert W., Langer T. The YTA10-12 complex, an AAA protease with chaperone-like activity in the inner membrane of mitochondria. Cell. 1996 Jun 14;85(6):875–885. doi: 10.1016/s0092-8674(00)81271-4. [DOI] [PubMed] [Google Scholar]
- Campbell C. L., Tanaka N., White K. H., Thorsness P. E. Mitochondrial morphological and functional defects in yeast caused by yme1 are suppressed by mutation of a 26S protease subunit homologue. Mol Biol Cell. 1994 Aug;5(8):899–905. doi: 10.1091/mbc.5.8.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confalonieri F., Duguet M. A 200-amino acid ATPase module in search of a basic function. Bioessays. 1995 Jul;17(7):639–650. doi: 10.1002/bies.950170710. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- Guelin E., Rep M., Grivell L. A. Sequence of the AFG3 gene encoding a new member of the FtsH/Yme1/Tma subfamily of the AAA-protein family. Yeast. 1994 Oct;10(10):1389–1394. doi: 10.1002/yea.320101016. [DOI] [PubMed] [Google Scholar]
- Guzélin E., Rep M., Grivell L. A. Afg3p, a mitochondrial ATP-dependent metalloprotease, is involved in degradation of mitochondrially-encoded Cox1, Cox3, Cob, Su6, Su8 and Su9 subunits of the inner membrane complexes III, IV and V. FEBS Lett. 1996 Feb 26;381(1-2):42–46. doi: 10.1016/0014-5793(96)00074-9. [DOI] [PubMed] [Google Scholar]
- Herman C., Thévenet D., D'Ari R., Bouloc P. Degradation of sigma 32, the heat shock regulator in Escherichia coli, is governed by HflB. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3516–3520. doi: 10.1073/pnas.92.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. M., Koll H., Cook R. A., Neupert W., Stuart R. A. Topogenesis of cytochrome oxidase subunit II. Mechanisms of protein export from the mitochondrial matrix. J Biol Chem. 1995 Nov 10;270(45):27079–27086. doi: 10.1074/jbc.270.45.27079. [DOI] [PubMed] [Google Scholar]
- Kihara A., Akiyama Y., Ito K. FtsH is required for proteolytic elimination of uncomplexed forms of SecY, an essential protein translocase subunit. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4532–4536. doi: 10.1073/pnas.92.10.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunau W. H., Beyer A., Franken T., Götte K., Marzioch M., Saidowsky J., Skaletz-Rorowski A., Wiebel F. F. Two complementary approaches to study peroxisome biogenesis in Saccharomyces cerevisiae: forward and reversed genetics. Biochimie. 1993;75(3-4):209–224. doi: 10.1016/0300-9084(93)90079-8. [DOI] [PubMed] [Google Scholar]
- Landt O., Grunert H. P., Hahn U. A general method for rapid site-directed mutagenesis using the polymerase chain reaction. Gene. 1990 Nov 30;96(1):125–128. doi: 10.1016/0378-1119(90)90351-q. [DOI] [PubMed] [Google Scholar]
- Nakai T., Yasuhara T., Fujiki Y., Ohashi A. Multiple genes, including a member of the AAA family, are essential for degradation of unassembled subunit 2 of cytochrome c oxidase in yeast mitochondria. Mol Cell Biol. 1995 Aug;15(8):4441–4452. doi: 10.1128/mcb.15.8.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajic A., Tauer R., Feldmann H., Neupert W., Langer T. Yta10p is required for the ATP-dependent degradation of polypeptides in the inner membrane of mitochondria. FEBS Lett. 1994 Oct 17;353(2):201–206. doi: 10.1016/0014-5793(94)01046-3. [DOI] [PubMed] [Google Scholar]
- Paul M. F., Tzagoloff A. Mutations in RCA1 and AFG3 inhibit F1-ATPase assembly in Saccharomyces cerevisiae. FEBS Lett. 1995 Oct 2;373(1):66–70. doi: 10.1016/0014-5793(95)00979-j. [DOI] [PubMed] [Google Scholar]
- Santos D., De Almeida D. F. Isolation and characterization of a new temperature-sensitive cell division mutant of Escherichia coli K-12. J Bacteriol. 1975 Dec;124(3):1502–1507. doi: 10.1128/jb.124.3.1502-1507.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnall R., Mannhaupt G., Stucka R., Tauer R., Ehnle S., Schwarzlose C., Vetter I., Feldmann H. Identification of a set of yeast genes coding for a novel family of putative ATPases with high similarity to constituents of the 26S protease complex. Yeast. 1994 Sep;10(9):1141–1155. doi: 10.1002/yea.320100903. [DOI] [PubMed] [Google Scholar]
- Söllner T., Rassow J., Pfanner N. Analysis of mitochondrial protein import using translocation intermediates and specific antibodies. Methods Cell Biol. 1991;34:345–358. doi: 10.1016/s0091-679x(08)61689-1. [DOI] [PubMed] [Google Scholar]
- Tauer R., Mannhaupt G., Schnall R., Pajic A., Langer T., Feldmann H. Yta10p, a member of a novel ATPase family in yeast, is essential for mitochondrial function. FEBS Lett. 1994 Oct 17;353(2):197–200. doi: 10.1016/0014-5793(94)01045-5. [DOI] [PubMed] [Google Scholar]
- Taussig R., Gilman A. G. Mammalian membrane-bound adenylyl cyclases. J Biol Chem. 1995 Jan 6;270(1):1–4. doi: 10.1074/jbc.270.1.1. [DOI] [PubMed] [Google Scholar]
- Thorsness P. E., White K. H., Fox T. D. Inactivation of YME1, a member of the ftsH-SEC18-PAS1-CDC48 family of putative ATPase-encoding genes, causes increased escape of DNA from mitochondria in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Sep;13(9):5418–5426. doi: 10.1128/mcb.13.9.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoyasu T., Gamer J., Bukau B., Kanemori M., Mori H., Rutman A. J., Oppenheim A. B., Yura T., Yamanaka K., Niki H. Escherichia coli FtsH is a membrane-bound, ATP-dependent protease which degrades the heat-shock transcription factor sigma 32. EMBO J. 1995 Jun 1;14(11):2551–2560. doi: 10.1002/j.1460-2075.1995.tb07253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagoloff A., Yue J., Jang J., Paul M. F. A new member of a family of ATPases is essential for assembly of mitochondrial respiratory chain and ATP synthetase complexes in Saccharomyces cerevisiae. J Biol Chem. 1994 Oct 21;269(42):26144–26151. [PubMed] [Google Scholar]
- Weber E. R., Hanekamp T., Thorsness P. E. Biochemical and functional analysis of the YME1 gene product, an ATP and zinc-dependent mitochondrial protease from S. cerevisiae. Mol Biol Cell. 1996 Feb;7(2):307–317. doi: 10.1091/mbc.7.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]









