Abstract
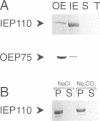
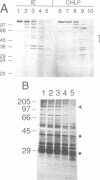
Proteins from both the inner and outer envelope membranes are engaged in the recognition and translocation of precursor proteins into chloroplasts. A 110 kDa protein of the chloroplastic inner envelope membrane was identified as a component of the protein import apparatus by two methods. First, this protein was part of a 600 kDa complex generated by cross-linking of precursors trapped in the translocation process. Second, solubilization with detergents of chloroplasts containing trapped precursors resulted in the identification of a complex containing both radiolabeled precursor and IEP110. Trypsin treatment of intact purified chloroplasts was used to study the topology of IEP110. The protease treatment left the inner membrane intact while simultaneously degrading domains of inner envelope proteins exposed to the intermembrane space. About 90 kDa of IEP110 was proteolitically removed, indicating that large portions protrude into the intermembrane space. Hydropathy analysis of the protein sequence deduced from the isolated cDNA clone in addition to Western blot analysis using an antiserum of IEP110 specific to the N-terminal 20 kDa, suggests that the N-terminus serves to anchor the protein in the membrane. We speculate that IEP110 could be involved in the formation of translocation contact sites due to its specific topology.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M. A., Dorne A. J., Joyard J., Douce R. Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts. II. Biochemical characterization. J Biol Chem. 1983 Nov 10;258(21):13281–13286. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Transport of proteins into mitochondria and chloroplasts. J Cell Biol. 1979 Jun;81(3):461–483. doi: 10.1083/jcb.81.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Andrews J., Mersey B., Newcomb E. H., Keegstra K. Separation and characterization of inner and outer envelope membranes of pea chloroplasts. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3595–3599. doi: 10.1073/pnas.78.6.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B., Wachter C., Schatz G. Protein import into mitochondria: two systems acting in tandem? Trends Cell Biol. 1991 Oct;1(4):99–103. doi: 10.1016/0962-8924(91)90037-a. [DOI] [PubMed] [Google Scholar]
- Hachiya N., Mihara K., Suda K., Horst M., Schatz G., Lithgow T. Reconstitution of the initial steps of mitochondrial protein import. Nature. 1995 Aug 24;376(6542):705–709. doi: 10.1038/376705a0. [DOI] [PubMed] [Google Scholar]
- Hirsch S., Muckel E., Heemeyer F., von Heijne G., Soll J. A receptor component of the chloroplast protein translocation machinery. Science. 1994 Dec 23;266(5193):1989–1992. doi: 10.1126/science.7801125. [DOI] [PubMed] [Google Scholar]
- Horst M., Hilfiker-Rothenfluh S., Oppliger W., Schatz G. Dynamic interaction of the protein translocation systems in the inner and outer membranes of yeast mitochondria. EMBO J. 1995 May 15;14(10):2293–2297. doi: 10.1002/j.1460-2075.1995.tb07223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard J., Block M. A., Douce R. Molecular aspects of plastid envelope biochemistry. Eur J Biochem. 1991 Aug 1;199(3):489–509. doi: 10.1111/j.1432-1033.1991.tb16148.x. [DOI] [PubMed] [Google Scholar]
- Kessler F., Blobel G., Patel H. A., Schnell D. J. Identification of two GTP-binding proteins in the chloroplast protein import machinery. Science. 1994 Nov 11;266(5187):1035–1039. doi: 10.1126/science.7973656. [DOI] [PubMed] [Google Scholar]
- Kiebler M., Pfaller R., Söllner T., Griffiths G., Horstmann H., Pfanner N., Neupert W. Identification of a mitochondrial receptor complex required for recognition and membrane insertion of precursor proteins. Nature. 1990 Dec 13;348(6302):610–616. doi: 10.1038/348610a0. [DOI] [PubMed] [Google Scholar]
- Ko K., Budd D., Wu C., Seibert F., Kourtz L., Ko Z. W. Isolation and characterization of a cDNA clone encoding a member of the Com44/Cim44 envelope components of the chloroplast protein import apparatus. J Biol Chem. 1995 Dec 1;270(48):28601–28608. doi: 10.1074/jbc.270.48.28601. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marshall J. S., DeRocher A. E., Keegstra K., Vierling E. Identification of heat shock protein hsp70 homologues in chloroplasts. Proc Natl Acad Sci U S A. 1990 Jan;87(1):374–378. doi: 10.1073/pnas.87.1.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen L. J., Keegstra K. The binding of precursor proteins to chloroplasts requires nucleoside triphosphates in the intermembrane space. J Biol Chem. 1992 Jan 5;267(1):433–439. [PubMed] [Google Scholar]
- Olsen L. J., Theg S. M., Selman B. R., Keegstra K. ATP is required for the binding of precursor proteins to chloroplasts. J Biol Chem. 1989 Apr 25;264(12):6724–6729. [PubMed] [Google Scholar]
- Perry S. E., Keegstra K. Envelope membrane proteins that interact with chloroplastic precursor proteins. Plant Cell. 1994 Jan;6(1):93–105. doi: 10.1105/tpc.6.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon M., Fischer K., Flügge U. I., Soll J. Sequence analysis and protein import studies of an outer chloroplast envelope polypeptide. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5778–5782. doi: 10.1073/pnas.87.15.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell D. J., Blobel G. Identification of intermediates in the pathway of protein import into chloroplasts and their localization to envelope contact sites. J Cell Biol. 1993 Jan;120(1):103–115. doi: 10.1083/jcb.120.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell D. J., Kessler F., Blobel G. Isolation of components of the chloroplast protein import machinery. Science. 1994 Nov 11;266(5187):1007–1012. doi: 10.1126/science.7973649. [DOI] [PubMed] [Google Scholar]
- Seedorf M., Soll J. Copper chloride, an inhibitor of protein import into chloroplasts. FEBS Lett. 1995 Jun 19;367(1):19–22. doi: 10.1016/0014-5793(95)00529-i. [DOI] [PubMed] [Google Scholar]
- Seedorf M., Waegemann K., Soll J. A constituent of the chloroplast import complex represents a new type of GTP-binding protein. Plant J. 1995 Mar;7(3):401–411. doi: 10.1046/j.1365-313x.1995.7030401.x. [DOI] [PubMed] [Google Scholar]
- Stuart R. A., Cyr D. M., Craig E. A., Neupert W. Mitochondrial molecular chaperones: their role in protein translocation. Trends Biochem Sci. 1994 Feb;19(2):87–92. doi: 10.1016/0968-0004(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Theg S. M., Bauerle C., Olsen L. J., Selman B. R., Keegstra K. Internal ATP is the only energy requirement for the translocation of precursor proteins across chloroplastic membranes. J Biol Chem. 1989 Apr 25;264(12):6730–6736. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel P. J., Froehlich J., Goyal A., Keegstra K. A component of the chloroplastic protein import apparatus is targeted to the outer envelope membrane via a novel pathway. EMBO J. 1995 Jun 1;14(11):2436–2446. doi: 10.1002/j.1460-2075.1995.tb07241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebst A. Measurement of Hill reactions and photoreduction. Methods Enzymol. 1972;24:146–165. doi: 10.1016/0076-6879(72)24065-4. [DOI] [PubMed] [Google Scholar]
- Waegemann K., Soll J. Characterization and isolation of the chloroplast protein import machinery. Methods Cell Biol. 1995;50:255–267. doi: 10.1016/s0091-679x(08)61035-3. [DOI] [PubMed] [Google Scholar]
- Werner-Washburne M., Cline K., Keegstra K. Analysis of pea chloroplast inner and outer envelope membrane proteins by two-dimensional gel electrophoresis and their comparison with stromal proteins. Plant Physiol. 1983 Nov;73(3):569–575. doi: 10.1104/pp.73.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Seibert F. S., Ko K. Identification of chloroplast envelope proteins in close physical proximity to a partially translocated chimeric precursor protein. J Biol Chem. 1994 Dec 23;269(51):32264–32271. [PubMed] [Google Scholar]
- von Heijne G., Blomberg C. Trans-membrane translocation of proteins. The direct transfer model. Eur J Biochem. 1979 Jun;97(1):175–181. doi: 10.1111/j.1432-1033.1979.tb13100.x. [DOI] [PubMed] [Google Scholar]