Abstract
Lactococcus lactis possesses an ATP-dependent drug extrusion system which shares functional properties with the mammalian multidrug resistance (MDR) transporter P-glycoprotein. One of the intriguing aspects of both transporters is their ability to interact with a broad range of structurally unrelated amphiphilic compounds. It has been suggested that P-glycoprotein removes drugs directly from the membrane. Evidence is presented that this model is correct for the lactococcal multidrug transporter through studies of the extrusion mechanism of BCECF-AM and cationic diphenylhexatriene (DPH) derivatives from the membrane. The non-fluorescent probe BCECF-AM can be converted intracellularly into its fluorescent derivative, BCECF, by non-specific esterase activities. The development of fluorescence was decreased upon energization of the cells. These and kinetic studies showed that BCECF-AM is actively extruded from the membrane before it can be hydrolysed intracellularly. The increase in fluorescence intensity due to the distribution of TMA-DPH into the phospholipid bilayer is a biphasic process. This behaviour reflects the fast entry of TMA-DPH into the outer leaflet followed by a slower transbilayer movement to the inner leaflet of the membrane. The initial rate of TMA-DPH extrusion correlates with the amount of probe associated with the inner leaflet. Taken together, these results demonstrate that the lactococcal MDR transporter functions as a 'hydrophobic vacuum cleaner', expelling drugs from the inner leaflet of the lipid bilayer. Thus, the ability of amphiphilic substrates to partition in the inner leaflet of the membrane is a prerequisite for recognition by multidrug transporters.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenberg G. A., Vanoye C. G., Horton J. K., Reuss L. Unidirectional fluxes of rhodamine 123 in multidrug-resistant cells: evidence against direct drug extrusion from the plasma membrane. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4654–4657. doi: 10.1073/pnas.91.11.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
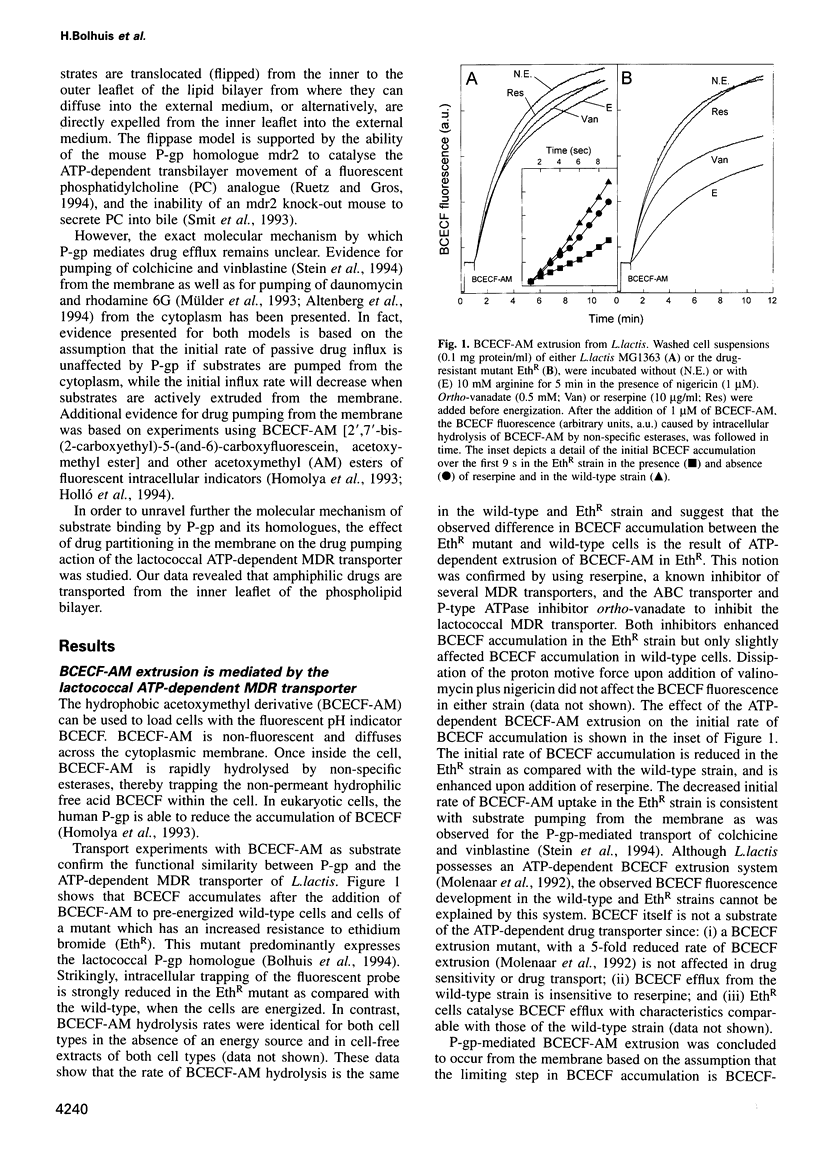
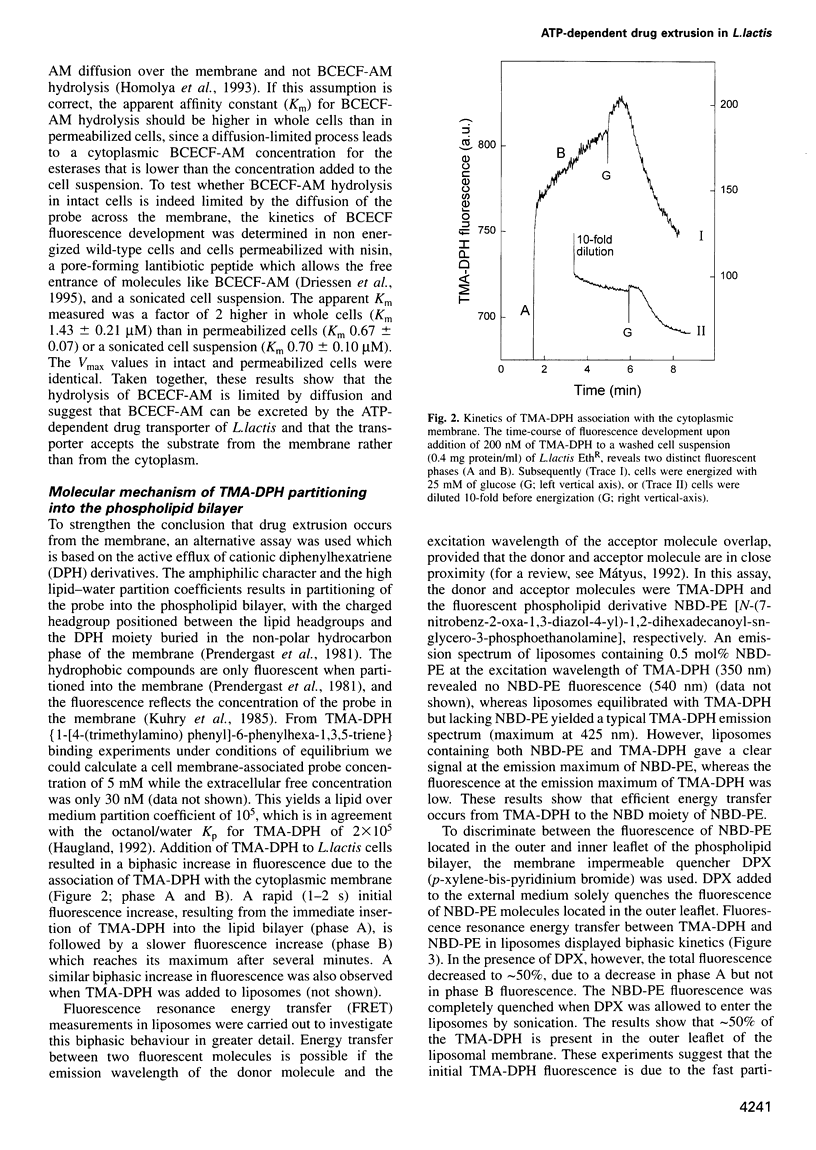
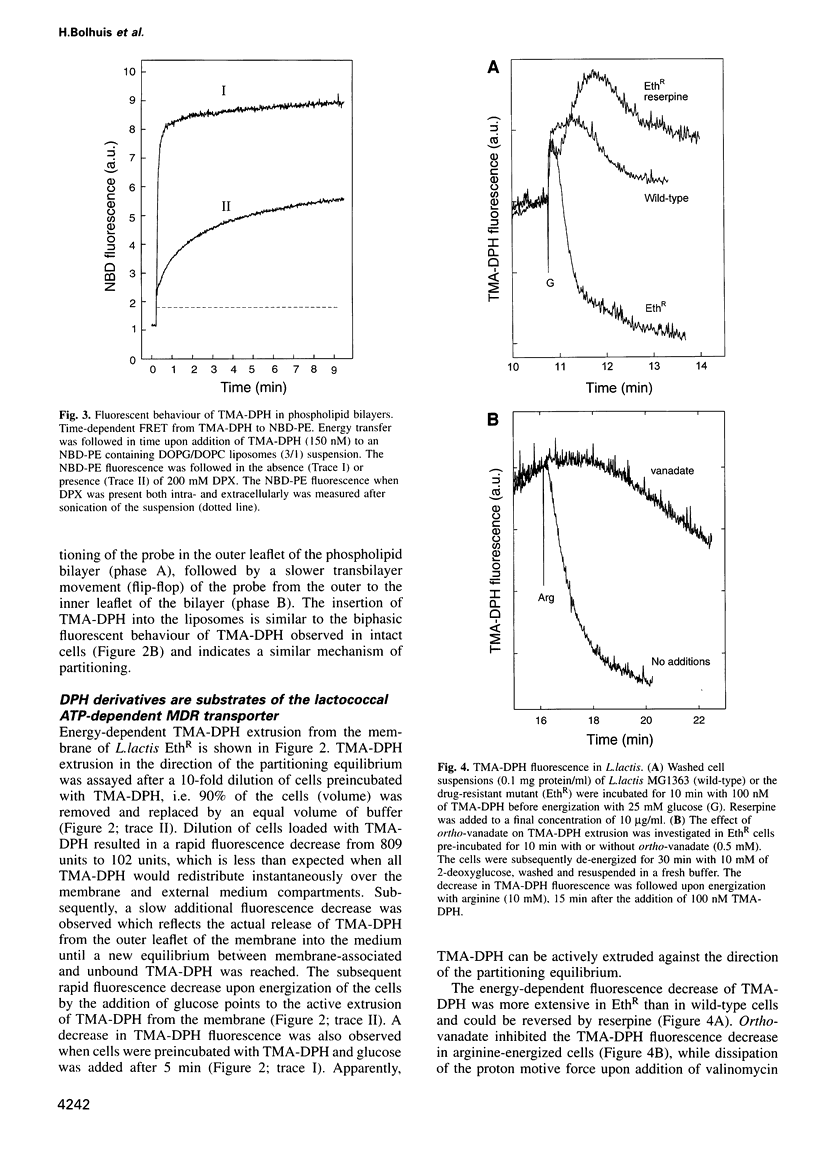
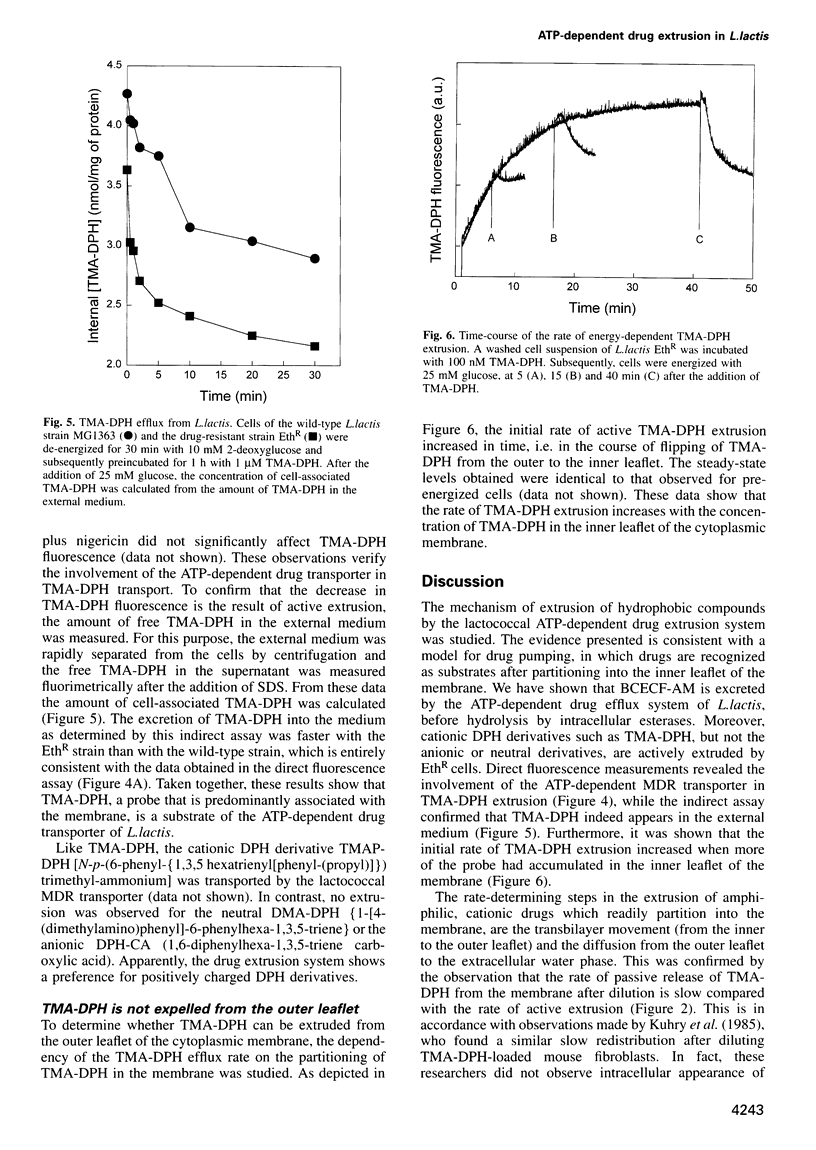
- Bolhuis H., Molenaar D., Poelarends G., van Veen H. W., Poolman B., Driessen A. J., Konings W. N. Proton motive force-driven and ATP-dependent drug extrusion systems in multidrug-resistant Lactococcus lactis. J Bacteriol. 1994 Nov;176(22):6957–6964. doi: 10.1128/jb.176.22.6957-6964.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis H., Poelarends G., van Veen H. W., Poolman B., Driessen A. J., Konings W. N. The Lactococcal lmrP gene encodes a proton motive force-dependent drug transporter. J Biol Chem. 1995 Nov 3;270(44):26092–26098. doi: 10.1074/jbc.270.44.26092. [DOI] [PubMed] [Google Scholar]
- Driessen A. J., van den Hooven H. W., Kuiper W., van de Kamp M., Sahl H. G., Konings R. N., Konings W. N. Mechanistic studies of lantibiotic-induced permeabilization of phospholipid vesicles. Biochemistry. 1995 Feb 7;34(5):1606–1614. doi: 10.1021/bi00005a017. [DOI] [PubMed] [Google Scholar]
- Foote S. J., Thompson J. K., Cowman A. F., Kemp D. J. Amplification of the multidrug resistance gene in some chloroquine-resistant isolates of P. falciparum. Cell. 1989 Jun 16;57(6):921–930. doi: 10.1016/0092-8674(89)90330-9. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Higgins C. F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Higgins C. F. Flip-flop: the transmembrane translocation of lipids. Cell. 1994 Nov 4;79(3):393–395. doi: 10.1016/0092-8674(94)90248-8. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Gottesman M. M. Is the multidrug transporter a flippase? Trends Biochem Sci. 1992 Jan;17(1):18–21. doi: 10.1016/0968-0004(92)90419-a. [DOI] [PubMed] [Google Scholar]
- Holló Z., Homolya L., Davis C. W., Sarkadi B. Calcein accumulation as a fluorometric functional assay of the multidrug transporter. Biochim Biophys Acta. 1994 May 11;1191(2):384–388. doi: 10.1016/0005-2736(94)90190-2. [DOI] [PubMed] [Google Scholar]
- Homolya L., Holló Z., Germann U. A., Pastan I., Gottesman M. M., Sarkadi B. Fluorescent cellular indicators are extruded by the multidrug resistance protein. J Biol Chem. 1993 Oct 15;268(29):21493–21496. [PubMed] [Google Scholar]
- Horio M., Gottesman M. M., Pastan I. ATP-dependent transport of vinblastine in vesicles from human multidrug-resistant cells. Proc Natl Acad Sci U S A. 1988 May;85(10):3580–3584. doi: 10.1073/pnas.85.10.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa S., Matsubayashi M., Kotani K., Usui K., Kametani F. Asymmetry of membrane fluidity in the lipid bilayer of blood platelets: fluorescence study with diphenylhexatriene and analogs. J Membr Biol. 1991 Feb;119(3):221–227. doi: 10.1007/BF01868727. [DOI] [PubMed] [Google Scholar]
- Kuhry J. G., Duportail G., Bronner C., Laustriat G. Plasma membrane fluidity measurements on whole living cells by fluorescence anisotropy of trimethylammoniumdiphenylhexatriene. Biochim Biophys Acta. 1985 Apr 22;845(1):60–67. doi: 10.1016/0167-4889(85)90055-2. [DOI] [PubMed] [Google Scholar]
- Läuger P., Neumcke B. Theoretical analysis of ion conductance in lipid bilayer membranes. Membranes. 1973;2:1–59. [PubMed] [Google Scholar]
- Mülder H. S., van Grondelle R., Westerhoff H. V., Lankelma J. A plasma membrane 'vacuum cleaner' for daunorubicin in non-P-glycoprotein multidrug-resistant SW-1573 human non-small cell lung carcinoma cells. A study using fluorescence resonance energy transfer. Eur J Biochem. 1993 Dec 15;218(3):871–882. doi: 10.1111/j.1432-1033.1993.tb18443.x. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science. 1994 Apr 15;264(5157):382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- Poolman B., Smid E. J., Veldkamp H., Konings W. N. Bioenergetic consequences of lactose starvation for continuously cultured Streptococcus cremoris. J Bacteriol. 1987 Apr;169(4):1460–1468. doi: 10.1128/jb.169.4.1460-1468.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast F. G., Haugland R. P., Callahan P. J. 1-[4-(Trimethylamino)phenyl]-6-phenylhexa-1,3,5-triene: synthesis, fluorescence properties, and use as a fluorescence probe of lipid bilayers. Biochemistry. 1981 Dec 22;20(26):7333–7338. doi: 10.1021/bi00529a002. [DOI] [PubMed] [Google Scholar]
- Raviv Y., Pollard H. B., Bruggemann E. P., Pastan I., Gottesman M. M. Photosensitized labeling of a functional multidrug transporter in living drug-resistant tumor cells. J Biol Chem. 1990 Mar 5;265(7):3975–3980. [PubMed] [Google Scholar]
- Ruetz S., Gros P. Phosphatidylcholine translocase: a physiological role for the mdr2 gene. Cell. 1994 Jul 1;77(7):1071–1081. doi: 10.1016/0092-8674(94)90446-4. [DOI] [PubMed] [Google Scholar]
- Shapiro A. B., Ling V. Reconstitution of drug transport by purified P-glycoprotein. J Biol Chem. 1995 Jul 7;270(27):16167–16175. doi: 10.1074/jbc.270.27.16167. [DOI] [PubMed] [Google Scholar]
- Smit J. J., Schinkel A. H., Oude Elferink R. P., Groen A. K., Wagenaar E., van Deemter L., Mol C. A., Ottenhoff R., van der Lugt N. M., van Roon M. A. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993 Nov 5;75(3):451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- Speelmans G., Staffhorst R. W., de Kruijff B., de Wolf F. A. Transport studies of doxorubicin in model membranes indicate a difference in passive diffusion across and binding at the outer and inner leaflets of the plasma membrane. Biochemistry. 1994 Nov 22;33(46):13761–13768. doi: 10.1021/bi00250a029. [DOI] [PubMed] [Google Scholar]
- Stein W. D., Cardarelli C., Pastan I., Gottesman M. M. Kinetic evidence suggesting that the multidrug transporter differentially handles influx and efflux of its substrates. Mol Pharmacol. 1994 Apr;45(4):763–772. [PubMed] [Google Scholar]
- de Wolf F. A., Maliepaard M., van Dorsten F., Berghuis I., Nicolay K., de Kruijff B. Comparable interaction of doxorubicin with various acidic phospholipids results in changes of lipid order and dynamics. Biochim Biophys Acta. 1990 Nov 14;1096(1):67–80. doi: 10.1016/0925-4439(90)90014-g. [DOI] [PubMed] [Google Scholar]



