Abstract
Background
The presence of lymph nodes (LN) within the prostatic anterior fat pad (PAFP) has been reported in several recent reports. These PAFP LNs rarely harbor metastatic disease, and the characteristics of patients with PAFP LN metastasis are not well-described in the literature. Our previous study suggested that metastatic disease to the PAFP LN was associated with less severe oncologic outcomes than those that involve the pelvic lymph node (PLN). Therefore, the objective of this study is to assess the oncologic outcome of prostate cancer (PCa) patients with PAFP LN metastasis in a larger patient population.
Methods
Data were analyzed on 8800 patients from eleven international centers in three countries. Eighty-eight patients were found to have metastatic disease to the PAFP LNs (PAFP+) and 206 men had isolated metastasis to the pelvic LNs (PLN+). Clinicopathologic features were compared using ANOVA and Chi square tests. The Kaplan-Meier method was used to calculate the time to biochemical recurrence (BCR).
Results
Of the eighty-eight patients with PAFP LN metastasis, sixty-three (71.6 %) were up-staged based on the pathologic analysis of PAFP and eight (9.1 %) had a low-risk disease. Patients with LNs present in the PAFP had a higher incidence of biopsy Gleason score (GS) 8–10, pathologic N1 disease, and positive surgical margin in prostatectomy specimens than those with no LNs detected in the PAFP. Men who were PAFP+ with or without PLN involvement had more aggressive pathologic features than those with PLN disease only. However, there was no significant difference in BCR-free survival regardless of adjuvant therapy. In 300 patients who underwent PAFP LN mapping, 65 LNs were detected. It was also found that 44 out of 65 (67.7 %) nodes were located in the middle portion of the PAFP.
Conclusions
There was no significant difference in the rate of BCR between the PAFP LN+ and PLN+ groups. The PAFP likely represents a landing zone that is different from the PLNs for PCa metastasis. Therefore, the removal and pathologic analysis of PAFP should be adopted as a standard procedure in all patients undergoing radical prostatectomy.
Electronic supplementary material
The online version of this article (doi:10.1186/s12894-015-0070-1) contains supplementary material, which is available to authorized users.
Keywords: Lymph node metastases, Prostate anterior fat pad, Prostate cancer
Background
In men undergoing radical prostatectomy (RP), pelvic lymph node dissection (PLND) is the most accurate and reliable staging procedure for detecting lymph node (LN) metastasis in prostate cancer (PCa) [1–4]. Aside from providing clinicians with the most accurate LN staging, the therapeutic role of PLND in PCa has emerged as some have suggested that RP and removal of involved regional LNs has survival benefit [5–7]. Currently, an extended template for PLND has been accepted by many surgeons as the standard due to higher LN yield, increased removal of positive nodes, and fewer missed positive nodes [8, 9]. Nevertheless, the optimal extent of PLND that balances potential morbidity with therapeutic benefit remains controversial.
During RP, Ahlering et al. have proposed that the prostatic anterior fat pad (PAFP) should be dissected to aid in the identification of the puboprostatic ligaments and the anterior surface of the dorsal vein complex [10]. In addition, the potential oncologic rationale for the PAFP removal has been suggested initially by Kothari et al. in 2001 [11]. Since then, several groups have reported the presence of LNs in the PAFP and incidence of PAFP LN metastasis occurring in the range of 5.5 % to 17.0 % and 1.2 % to 2.5 %, respectively [12–16].
Most recently, we have reported the largest series on the pathologic analysis of LNs and LN metastasis in the PAFP after reviewing 4,261 patients from 8 institutions [17]. In this study, PAFP LNs were found in 11.9 % and 0.94 % harbored metastatic disease. More importantly, our initial study suggested that metastatic disease to the PAFP LN was associated with more favorable oncologic outcome than those that involve the PLNs. To further define the oncologic implications of PAFP LN metastasis, we have expanded the scope of the study to 8800 men from 11 international institutions.
Methods
Ethics statement
This study was approved by the institutional review board of all 13 participating institutions (see Additional file 1). Furthermore, the principles of the Helisinki Declaration were followed. Each board exempted informed consent because this was a retrospective study.
Study population
Prospectively maintained database approved by the institutional review board (IRB) at each institution was analyzed. Written informed consent was obtained from all study subjects during the study period between January of 2006 and February of 2014. In this study, only men who underwent PAFP excision and pathologic analysis during open retropubic radical prostatectomy (RRP) or robot-assisted radical prostatectomy (RARP) were included. All patients routinely undergo PAFP excision and pathologic analysis from the thirteen participating institutions. Initially, the outcomes of 9510 PCa patients were reviewed [RARP, N = 8747 and RRP, N = 763]. Of these, 8800 PCa patients from eleven institutions with complete data were selected for analysis.
The participating thirteen institutions are as follows: Rutgers Cancer Institute of New Jersey (New Brunswick, NJ, USA), University of Pennsylvania (Philadelphia, PA, USA), Yonsei University (Seoul, Korea), University of California Irvine (Orange, CA, USA), Asan Medical Center (Seoul, Korea), Samsung Medical Center (Seoul, Korea), Taichung Veterans General Hospital (Taichung, Taiwan), Temple University (Philadelphia, PA, USA), Associated Medical Professionals (Syracuse, NY, USA), Icahn school of Medicine at Mount Sinai Hospital (New York, NY, USA), City of Hope National Medical Center (Duarte, CA, USA), Kyungpook National University Medical Center (Daegu, Korea), and Georgetown University (Washington, D.C., USA).
PAFP removal and pathologic evaluation
PAFP removal and pathological analysis were performed as described previously [17].
Statistical analysis
For comparison of variables, a student t test or analysis of variance (ANOVA) test and Pearson χ2 test were used for analysis of each set of continuous and categorical data. Biochemical recurrence (BCR) was defined as 2 consecutive PSA increases with the last PSA 0.2 ng/ml or greater. Multivariate Cox regression analyses were performed to identify factors predictive of BCR. The time to BCR was used as the end point for the Kaplan-Meier model. The log-rank test was used for comparison with p ≤ 0.05 considered statistically significant. All statistical analyses were performed using the SPSS v.18.0 (IBM Corp., Armonk, NY).
Results
From the eleven international institutions, 8800 patients underwent pathologic analysis of the PAFP (data not shown; see Additional file 2) because the number of patients with LNs present in the PAFP was not available due to an institutional procedure on not reporting negative LNs at two sites. Metastatic disease in the PAFP was detected in eighty-eight patients out of 8800 (0.93 %). The overall incidence of LNs present in the PAFP was 10.3 % (909/8800).
2835 out of 5260 (53.9 %) patients with available data on pelvic LNs underwent pelvic LN dissection, with varying institutional range from 23.2 % to 100.0 %. For these patients, the mean (median) number of dissected total pelvic LN was 6.1 (7) with values in the range of 1–45. Of the ones who underwent pelvic LN dissection, 4.4 % of patients had metastasis to pelvic LN.
Pre- and post-operative patient characteristics of 8800 men with known LN status in the PAFP is known are summarized in Table 1 with a median follow-up of 18.0 months (range 3.0-84.0 months). In this cohort, 7891 patients were found to have no LNs in the PAFP. Preoperatively, biopsy Gleason score (GS) was the only variable significantly different between the two groups. Specifically, patients with LNs present in the PAFP had more frequent biopsy GS of 8–10 than those with LNs absent in the PAFP. Regarding pathologic characteristics of the RP specimens, statistically significant differences were found for pathologic LN (N) stage (P = 0.001) and surgical margin status (P < 0.001).
Table 1.
Pre- and post-operative characteristics of patients with absence or presence of lymph nodes in PAFP
| LN absent in PAFP | LN present in PAFP | P-value | |
|---|---|---|---|
| (N = 7891) | (N = 909) | ||
| Age, years: mean (SD) | 62.7 (7.5) | 62.9 (7.7) | 0.413 |
| BMI, kg/m2 :mean (SD) | 27.9 (3.7) | 27.9 (4.2) | 0.814 |
| PSA, ng/ml: mean (SD) | 8.84 (12.32) | 10.00 (24.62) | 0.107 |
| Categorical PSA, ng/ml: % | 0.814 | ||
| 0-3.9 | 20.0 | 22.0 | |
| 4-9.9 | 58.3 | 56.2 | |
| 10-20 | 15.1 | 14.0 | |
| >20 | 6.7 | 7.8 | |
| Biopsy GS: % | <0.001 | ||
| 6-7 | 82.8 | 78.3 | |
| 8-10 | 17.2 | 21.7 | |
| Pathologic GS: % | 0.307 | ||
| 6-7 | 83.8 | 82.1 | |
| 8-10 | 16.7 | 17.9 | |
| Pathologic T stage: % | 0.659 | ||
| T2≥ | 67.5 | 66.8 | |
| T3≤ | 32.5 | 33.2 | |
| Pathologic N stage: % | 0.001 | ||
| N0/Nx | 96.0 | 93.5 | |
| N1 | 4.0 | 6.5 | |
| Margin status: % | <0.001 | ||
| Negative | 82.3 | 78.1 | |
| Positive | 17.7 | 21.9 | |
PAFP, Prostate anterior fat pad; LN, Lymph node; BMI, Body mass index; PSA, Prostate-specific antigen; GS, Gleason score
Table 2 lists the clinicopathologic results of the patients with metastatic disease to the LNs stratified by location. Group 1 had isolated metastasis to the pelvic LNs (PLNs) (n = 206). Group 2 had metastatic disease limited to the PAFP LNs (n = 63). Group 3 involved disease both in the pelvic and PAFP LNs (n = 25). Among the eighty-eight patients with metastasis to the PAFP LNs, eight (9.1 %) had low-risk disease based on the D’Amico criteria and sixty-three (71.6 %, Group 2) were up-staged as a result of the PAFP pathologic analysis. Compared to men with pelvic LNs metastasis only (group 1), patients with metastatic disease to the PAFP LNs (Group 2 and 3) had more aggressive features in biopsy and pathologic GS as well as pathologic stage.
Table 2.
Differences in clinicopathologic results among the 3 groups stratified by the location of positive lymph nodes.
| Group 1, N = 206 | Group 2, N = 63 | Group 3, N = 25 | P-value | |
|---|---|---|---|---|
| Age, years: mean (SD) | 63.3 (6.9) | 63.3 (7.4) | 64.4 (8.1) | 0.744 |
| PSA, ng/ml: mean (SD) | 21.6 (36.0) | 26.9 (85.5) | 37.3 (66.5) | 0.336 |
| BCR-free survival, months: mean (range) | 19.2 (0.7-77.7) | 21.6 (1.0-76.3) | 19.6 (2.6-60.0) | 0.163 |
| BCR: N (%) | 0.073 | |||
| No | 145 (70.4) | 35 (55.6) | 15 (60.0) | |
| Yes | 61 (29.6) | 28 (44.4) | 10 (40.0) | |
| D’Amico risk: N (%) | 0.009 | |||
| Low risk | 29 (14.1) | 7 (11.1) | 1 (4.0) | |
| Intermediate risk | 65 (31.6) | 17 (27.0) | 1 (4.0) | |
| High risk | 112 (54.4) | 39 (61.9) | 23 (92.0) | |
| Biopsy GS: N (%) | <0.001 | |||
| 6-7 | 116 (56.5) | 25 (41.0) | 5 (20.0) | |
| 8-10 | 89 (43.4) | 36 (59.0) | 20 (80.0) | |
| Pathologic GS: N (%) | 0.021 | |||
| 6-7 | 113 (54.9) | 29 (46.0) | 8 (32.0) | |
| 8-10 | 93 (45.1) | 34 (54.0) | 17 (68.0) | |
| Pathologic T stage: N (%) | 0.005 | |||
| T2 | 81 (39.3) | 18 (28.6) | 3 (12.0) | |
| T3a | 48 (23.3) | 22 (34.9) | 6 (24.0) | |
| T3b | 59 (28.6) | 19 (30.2) | 10 (40.0) | |
| T4 | 18 (8.7) | 4 (6.3) | 6 (24.0) | |
| Margin status: N (%) | 0.043 | |||
| Negative | 69 (33.5) | 37 (58.7) | 9 (36.0) | |
| Positive | 137 (66.5) | 26 (41.3) | 16 (64.0) | |
| Adjuvant therapy: N (%) | 0.012 | |||
| No | 165 (80.1) | 42 (66.7) | 16 (64.0) | |
| Yes | 41 (19.9) | 21 (33.3) | 9 (36.0) |
Group 1, Pelvic LN metastasis only; Group 2, PAFP LN metastasis only; Group 3, Both pelvic LN & PAFP LN metastasis; PSA, Prostate-specific antigen; BCR, Biochemical recurrence; GS, Gleason score
Adjuvant therapy, including androgen deprivation (ADT), radiation, and chemotherapy was performed more frequently in men with PAFP LN involvement. 63 out of 71 patients (88.7 %) who were given adjuvant therapy received ADT with or without radiation and chemotherapy. The remaining 8 patients (11.3 %) did not receive ADT and received radiation, chemotherapy, or both. The median BCR-free survival period for PLN+, PAFP+, and PAFP+/PLN+ were 19.2, 21.6, and 19.6 months, respectively. Currently, fifty patients with PAFP LN metastasis remain free of BCR.
In order to check whether PAFP LN+ was a surrogate for extracapsular extension (ECE+), survival analysis of those with simultaneous ECE+ and PAFP LN+ was compared with that of individuals with ECE- or PAFP LN-. Although the relative frequency of BCR seemed different, the Kaplan-Meier analysis revealed no differences between the two groups: 46.3 % of ECE+/PAFP LN+ group had BCR with the median BCR free survival time of 18.0 months. On the other hand, 30.0 % of ECE- or PAFP- group had BCR with the median BCR free survival time of 15.4 months (P = 0.287). To determine the anatomic location of the LNs with in PAFP, LN mapping was carried out at one institution as reported previously [17]. From the cohort of 300 men, the total number of LNs detected was 65 (Table 3). Of these, 44 (67.7 %) were located in the middle packet. The numbers of LNs found in the left and right segments were 11 (16.9 %) and 10 (15.4 %), respectively.
Table 3.
Location of lymph nodes within the PAFP
| Total # Patients | 300 |
|---|---|
| Number of Nodes Detected | 657 |
| Middle (# of Nodes) | 44 |
| Left (# of Nodes) | 11 |
| Right (# of Nodes) | 10 |
The Multivariate Cox regression model suggested that higher preoperative PSA was predictive of higher recurrence rates in all patients (HR 1.005; 95 % CI 1.000-1.009; P = 0.042) and in the subgroup of patients with adjuvant therapy (HR 1.009; 95 % CI 1.001-1.016; P = 0.019). In addition, PLN+, PAFP LN+, and PLN+/PAFP LN+ demonstrated comparable risks of developing BCR (Table 4).
Table 4.
Multivariate Cox regression analyses to identify predictors of biochemical recurrence
| Variables | HR | 95 % CI | P-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| All patients | ||||
| Age | 1.008 | .979 | 1.038 | .604 |
| Preoperative PSA | 1.005 | 1.000 | 1.009 | .042 |
| Post-operative GS (≤7 vs. ≥8) | 1.264 | .822 | 1.943 | .286 |
| Pathologic stage (T2 vs. T3) | .931 | .584 | 1.484 | .763 |
| Margin status (Negative vs. Positive) | 1.159 | .745 | 1.803 | .514 |
| Pelvic and PAFP LN metastasis status | ||||
| Group 1 | 1 | - | - | - |
| Group 2 | 1.335 | .821 | 2.169 | .244 |
| Group 3 | 1.288 | .639 | 2.594 | .479 |
| Patients without adjuvant therapy | ||||
| Age | 1.020 | .985 | 1.055 | .265 |
| Preoperative PSA | 1.003 | .996 | 1.009 | .423 |
| Post-operative GS (≤7 vs. ≥8) | 1.094 | .648 | 1.848 | .737 |
| Pathologic stage (T2 vs. T3) | 1.024 | .601 | 1.746 | .930 |
| Margin status (Negative vs. Positive) | 1.094 | .648 | 1.848 | .555 |
| Pelvic and PAFP LN metastasis status | ||||
| Group 1 | 1 | - | - | - |
| Group 2 | 1.350 | .754 | 2.418 | .312 |
| Group 3 | 1.064 | .406 | 2.792 | .899 |
| Patients with adjuvant therapy | ||||
| Age | .994 | .933 | 1.059 | .842 |
| Preoperative PSA | 1.009 | 1.001 | 1.016 | .019 |
| Post-operative GS (≤7 vs. ≥8) | 1.925 | .774 | 4.788 | .159 |
| Pathologic stage (T2 vs. T3) | .727 | .250 | 2.117 | .559 |
| Margin status (Negative vs. Positive) | 1.316 | .467 | 3.711 | .604 |
| Pelvic and PAFP LN metastasis status | ||||
| Group 1 | 1 | - | - | - |
| Group 2 | 1.633 | .600 | 4.446 | .337 |
| Group 3 | 2.592 | .857 | 7.842 | .092 |
HR, hazard ratio; CI, confidence interval; Group 1, Pelvic LN metastasis only; Group 2, PAFP LN metastasis only; Group 3, Both pelvic LN & PAFP LN metastasis
Kaplan-Meier curves were used to assess BCR according to the location of the metastatic LNs (Fig. 1). No statistically significant difference was found in the BCR when all three groups were compared (Fig. 1a). When stratified by the administration of adjuvant therapy, again no difference was observed among the three groups (Fig. 1b and c).
Fig. 1.
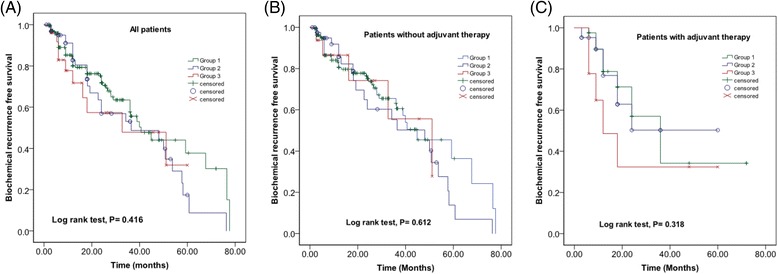
Kaplan-Meier curves for BCR-free survival according to the location of metastatic lymph nodes in (a) all patients (b) patients without adjuvant therapy, and (c) patients with adjuvant therapy
Discussion
Our international study spanning multiple institutions has demonstrated that in 8800 patients who underwent RP, the overall incidence of metastasis in the PAFP LNs was 0.93 %. Simultaneously, the rate of LNs detected within the PAFP was 10.3 %. LN mapping within the PAFP demonstrated that 67.7 % of the LNs were located in the middle packet. Of the 88 patients with PAFP LN metastasis, 63 were upstaged as a result of the PAFP pathologic evaluation.
When clinicopathologic features were analyzed between men with and without LN in the PAFP, patients with LNs in the PAFP more frequently had biopsy GS 8–10, N1 disease pathologically, and positive surgical margins. In comparison to the patients with isolated metastasis to the pelvic LNs, men with PAFP LNs metastasis had worse pathologic features. Yet, there was no significant difference in BCR free survival when the data were assessed based on the location of the metastasis (pelvic LN+, PAFP LN+, or pelvic LN+/PAFP LN+). Collectively, these observations suggest that the PAFP should be removed in all patients undergoing RP and that the oncologic implication of PAFP LN metastasis is equivalent to that of pelvic LN involvement in men with PCa.
Previously, our group reported on the detailed analysis of 40 patients with metastatic PCa to the PAFP LNs [17]. Because this original report was largely focused on the clinicopathologic features of men with PAFP LN metastasis, we designed the current study to assess the oncologic implications of PAFP LN involvement in men with PCa. To this end, we have increased the sample size to 9510 by increasing the number of participating institutions to thirteen. The study sites represent fourteen urologic surgeons from three different countries – USA, South Korea, and Taiwan.
A careful analysis of 8800 men after excluding patients with incomplete data revealed that men with LNs present in the PAFP were more likely to have aggressive disease as indicated by the higher frequency of biopsy GS 8–10 PCa, pathologic stage N1, and positive surgical margin. In a significantly smaller sample size of 356 men, Hansen et al. similarly reported that pathologic N1 disease was more frequently detected in patients who harbor LNs within the PAFP than those without LNs within the PAFP (21.1 % vs. 7 %, P = 0.02) [14]. In addition, it has been suggested that patients with LNs found in the PAFP were younger (60.5 vs. 65.0, P = 0.002) [13] while Jeong et al. noted that the mean preoperative PSA level was significantly higher in patients with LNs present in the PAFP (7.70 vs. 6.01, P = 0.039) [15]. But in the present study, age and PSA did not show any differences between the two groups.
Clinically, the current study demonstrated that the outcome of men with metastatic PCa to the PAFP LNs is similar to that of patients with pelvic LN metastasis. To assess the oncologic significance of PAFP LN metastasis in men with PCa, we have compared the outcome based on the location of the positive LNs (pelvic LN only, PAFP LN only, and pelvic LN+/PAFP LN+) in both Cox regression model as well as Kaplan-Meier survival analysis. Pathologic analysis revealed that men with PAFP LN involvement, regardless of the pelvic LN status, had more aggressive features. Nevertheless, BCR free survival duration was not significantly different among the three groups. More importantly, this lack of difference in BCR free survival period was present regardless of adjuvant therapy (P = 0.469). Moreover, among 88 patients with PAFP LN+, there were 67 patients who had simultaneous ECE+ and PAFP LN+, illustrating a high level of correlation. The risk of BCR in the above group was highly elevated although no statistical difference was found when compared to those with ECE- or PAFP-: (31/67) 46.3 % vs. (68/227) 30.0 %, respectively (P = 0.287). Taken together, these findings suggest that PCa patients with metastasis to the PAFP LNs should be treated as those with pelvic LN metastasis.
Finally, results of the present study provide multiple reasons for the PAFP removal and pathologic analysis in all men undergoing RP. First, the PAFP LNs are likely an independent and separate anatomic landing zone for PCa metastasis. In our group’s initial publication, we have reported that the LNs within the PAFP overwhelmingly mapped to the middle packet [17]. In this update, we have increased the sample size and carried out LN mapping in 300 patients. Again, a significant majority (67.7 %) of the LNs in PAFP were located in the middle packet. Accordingly, the detection of LNs within PAFP is not likely a result of an incomplete dissection of the obturator LNs. Second, the pathologic analysis of PAFP enhances the accuracy of staging. Of the 88 men with metastatic disease to the PAFP LNs, 63 were upstaged based on the PAFP LNs involvement. Third, there are no reliable pre-operative parameters that predict the PAFP LN metastasis. Although no preoperative imaging is currently recommended for the detection of PAFP LN metastasis and for guidance in removing PAFP LN, the added surgical step in the absence of imaging modality will not likely compromise the quality of surgical outcomes. In our aforementioned initial multi-institution study that analyzed forty patients with PAFP LN disease, only three had a low-risk disease defined by the D’Amico criteria pre-operatively. Based on this observation, we suggested that the pathologic analysis of PAFP may not be necessary in men with low-risk PCa. However in the current study, 9.1 % had low-risk disease. Accordingly, all PAFP specimens should be analyzed pathologically. Fourth, there may be a therapeutic effect of PAFP removal. Of the 88 men with PAFP LN metastasis, fifty remain free of BCR. Taken together with the minimal surgical morbidity of PAFP dissection, we now contend that the removal and pathologic examination be a standard procedure in all patients undergoing RP.
Notwithstanding the strength of the largest sample size to date on this topic, our study is not without weaknesses. First, the number of men with PAFP LN metastasis was only 88. Given this small number of event, it is entirely possible that there is a unique oncologic implication of PAFP LN metastasis that requires a larger sample size to uncover. Indeed in this cohort, PAFP LN involvement, regardless of the pelvic LN status, had more aggressive pathologic features. Second, additional follow-up is necessary to evaluate cancer-specific and overall survival. Third, BCR comparisons among pelvic LN+, PAFP LN+, and PAFP LN+/Pelvic LN+ groups were likely confounded because a greater proportion of men with PAFP LN+ with and without pelvic LN+ (group 2 and 3) received adjuvant therapy than the men with pelvic LN+ only (group 1) (P = 0.012) (Table 2). Because adjuvant therapy may lower BCR, adjuvant therapy-adjusted BCR in group 2 and 3, may in fact, be higher. Hence, this finding may further support the substantial BCR risk associated with PAFP metastasis. We plan to continue increasing the overall sample size and track the patients with PAFP LN metastasis to determine the long-term oncologic outcome. In the meantime, the present study provides the relative confidence that PCa patients with PAFP LN metastasis should be treated as those with pelvic LN disease.
Conclusions
Metastasis to the PAFP LNs and pelvic LNs had equivalent duration of BCR free survival. Because the PAFP is likely an anatomically independent and separate landing zone of PCa metastasis, the PAFP should be removed and analyzed in all men undergoing RP.
Acknowledgements
This work was supported by a grant from the National Cancer Institute (P30CA072720), generous grants from the Tanzman Foundation, and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C1642).
Abbreviations
- BCR
Biochemical recurrence
- ECE
Extracapsular extension
- GS
Gleason score
- LN
Lymph node
- PAFP
Prostatic anterior fat pad
- PCa
Prostate cancer
- PSA
Prostate-specific antigen
- PAFP+
Metastasis to prostatic anterior fat pad lymph node
- PNL+
Metastasis to pelvic lymph node
- PLN
Pelvic lymph node
- RARP
Robot-assisted radical prostatectomy
- RRP
Retropubic radical prostatectomy
Additional files
Ethics statement. docx. (DOCX 81 kb)
Institutional breakdown (Table S1). docx (DOCX 17 kb)
Footnotes
Young Suk Kwon and Yun-Sok Ha contributed equally to this work.
Competing interests
None of the contributing authors have any conflicts of interest, including specific financial interests and relationships and affiliation relevant to the subject matter or materials discussed in the manuscript.
Authors’ contributions
YSK and YSH reviewed the pertinent literature, analyzed the results, and drafted and edited the manuscript. IYK was responsible for the entire project. He designed the study concept, guided the study design, conducted data acquisition, and revised the manuscript critically for important intellectual content. PKM, AS, JSP, NP, IF, MM, DIL, EL, TP, KHR, TA, DS, HA, SKC, SP, SSJ, YCO, DE, VM, DA, KB, BY, NR, THK, TGK, DM, JH, and WJK collected data, analyzed data, and revised the manuscript. All authors read and approved the final manuscript.
Contributor Information
Young Suk Kwon, Email: yk411@cinj.rutgers.edu.
Yun-Sok Ha, Email: yunsokha@gmail.com.
Parth K. Modi, Email: modi.parth@gmail.com
Amirali Salmasi, Email: salmasi.armirali@gmail.com.
Jaspreet S. Parihar, Email: jaspreetparihar@gmail.com
Neal Patel, Email: patel.neal@gmail.com.
Izak Faiena, Email: faienaiz@rwjms.rutgers.edu.
Michael May, Email: mayms@rwjmslrutgers.edu.
David I. Lee, Email: david.lee@uphs.upenn.edu
Elton Llukani, Email: elton.llukani@uphs.upenn.edu.
Tuliao Patrick, Email: patrick@yuhs.ac.
Koon Ho Rha, Email: khrha@yuhs.ac.
Thomas Ahlering, Email: tahlerin@uci.edu.
Douglas Skarecky, Email: dwskarec@uci.edu.
Hanjong Ahn, Email: hiahn@amc.seoul.kr.
Seung-Kwon Choi, Email: cherynsong@amc.seoul.kr.
Sejun Park, Email: geheimnis79@hanmail.net.
Seong Soo Jeon, Email: seongsoo.jeon@samsung.com.
Yen-Chuan Ou, Email: ycou@vghtc.gov.tw.
Daniel Eun, Email: daniel.eun@tuhs.temple.edu.
Varsha Manucha, Email: varsha.manucha@tuhs.temple.edu.
David Albala, Email: dalbala@luc.edu.
Ketan Badani, Email: ketanbadani@gmail.com.
Bertram Yuh, Email: byuh@coh.org.
Nora Ruel, Email: nruel@coh.org.
Tae-Hwan Kim, Email: doctork@knu.ac.kr.
Tae Gyun Kwon, Email: tgkwon@knu.ac.kr.
Daniel Marchalik, Email: dan.marchalik@gmail.com.
Jonathan Hwang, Email: jonathan.j.hwang@medstar.net.
Wun-Jae Kim, Email: wjkim@chungbuk.ac.kr.
Isaac Yi Kim, Phone: 732 235 2043, Email: kimiy@cinj.rutgers.edu.
References
- 1.Briganti A, Blute ML, Eastham JH, Graefen M, Heidenreich A, Karnes JR, Montorsi F, Studer UE. Pelvic lymph node dissection in prostate cancer. Eur Urol. 2009;55(6):1251–1265. doi: 10.1016/j.eururo.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Briganti A, Larcher A, Abdollah F, Capitanio U, Gallina A, Suardi N, Bianchi M, Sun M, Freschi M, Salonia A, et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol. 2012;61(3):480–487. doi: 10.1016/j.eururo.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 3.Briganti A, Abdollah F, Nini A, Suardi N, Gallina A, Capitanio U, Bianchi M, Tutolo M, Passoni NM, Salonia A, et al. Performance characteristics of computed tomography in detecting lymph node metastases in contemporary patients with prostate cancer treated with extended pelvic lymph node dissection. Eur Urol. 2012;61(6):1132–1138. doi: 10.1016/j.eururo.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Joung JY, Cho I-C, Lee KH. Role of pelvic lymph node dissection in prostate cancer treatment. Korean J Urol. 2011;52(7):437–445. doi: 10.4111/kju.2011.52.7.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 6.Pagliarulo V, Hawes D, Brands FH, Groshen S, Cai J, Stein JP, Lieskovsky G, Skinner DG, Cote RJ. Detection of occult lymph node metastases in locally advanced node-negative prostate cancer. J Clin Oncol. 2006;24(18):2735–2742. doi: 10.1200/JCO.2005.05.4767. [DOI] [PubMed] [Google Scholar]
- 7.Daneshmand S, Quek ML, Stein JP, Lieskovsky G, Cai J, Pinski J, Skinner EC, Skinner DG. Prognosis of patients with lymph node positive prostate cancer following radical prostatectomy: long-term results. J Urol. 2004;172(6 Pt 1):2252–2255. doi: 10.1097/01.ju.0000143448.04161.cc. [DOI] [PubMed] [Google Scholar]
- 8.Heidenreich A, Varga Z, Von Knobloch R. Extended pelvic lymphadenectomy in patients undergoing radical prostatectomy: high incidence of lymph node metastasis. J Urol. 2002;167(4):1681–1686. doi: 10.1016/S0022-5347(05)65177-4. [DOI] [PubMed] [Google Scholar]
- 9.Bader P, Burkhard FC, Markwalder R, Studer UE. Disease progression and survival of patients with positive lymph nodes after radical prostatectomy. Is there a chance of cure? J Urol. 2003;169(3):849–854. doi: 10.1097/01.ju.0000049032.38743.c7. [DOI] [PubMed] [Google Scholar]
- 10.Ahlering TE, Eichel L, Edwards RA, Lee DI, Skarecky DW. Robotic radical prostatectomy: a technique to reduce pT2 positive margins. Urology. 2004;64(6):1224–1228. doi: 10.1016/j.urology.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 11.Kothari PS, Scardino PT, Ohori M, Kattan MW, Wheeler TM. Incidence, location, and significance of periprostatic and periseminal vesicle lymph nodes in prostate cancer. Am J Surg Pathol. 2001;25(11):1429–1432. doi: 10.1097/00000478-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Finley DS, Deane L, Rodriguez E, Vallone J, Deshmukh S, Skarecky D, Carpenter P, Narula N, Ornstein DK, Ahlering TE. Anatomic excision of anterior prostatic fat at radical prostatectomy: implications for pathologic upstaging. Urology. 2007;70(5):1000–1003. doi: 10.1016/j.urology.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 13.Yuh B, Wu H, Ruel N, Wilson T. Analysis of regional lymph nodes in periprostatic fat following robot-assisted radical prostatectomy. BJU Int. 2012;109(4):603–607. doi: 10.1111/j.1464-410X.2011.10336.x. [DOI] [PubMed] [Google Scholar]
- 14.Hansen J, Budaus L, Spethmann J, Schlomm T, Salomon G, Rink M, Haese A, Steuber T, Heinzer H, Huland H, et al. Assessment of rates of lymph nodes and lymph node metastases in periprostatic fat pads in a consecutive cohort treated with retropubic radical prostatectomy. Urology. 2012;80(4):877–882. doi: 10.1016/j.urology.2012.06.052. [DOI] [PubMed] [Google Scholar]
- 15.Jeong J, Choi EY, Kang DI, Ercolani M, Lee DH, Kim W-J, Kim IY. Pathologic implications of prostatic anterior fat pad. Urol Oncol. 2013;31(1):63–67. doi: 10.1016/j.urolonc.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Aning JJ, Thurairaja R, Gillatt DA, Koupparis AJ, Rowe EW, Oxley J. Pathological analysis of lymph nodes in anterior prostatic fat excised at robot-assisted radical prostatectomy. J Clin Pathol. 2014;67(9):787–791. doi: 10.1136/jclinpath-2014-202303. [DOI] [PubMed] [Google Scholar]
- 17.Kim IY, Modi PK, Sadimin E, Ha Y-S, Kim JH, Skarecky D, Cha DY, Wambi CO, Ou Y-C, Yuh B, et al. Detailed analysis of patients with metastasis to the prostatic anterior fat pad lymph nodes: a multi-institutional study. J Urol. 2013;190(2):527–534. doi: 10.1016/j.juro.2013.02.073. [DOI] [PubMed] [Google Scholar]


