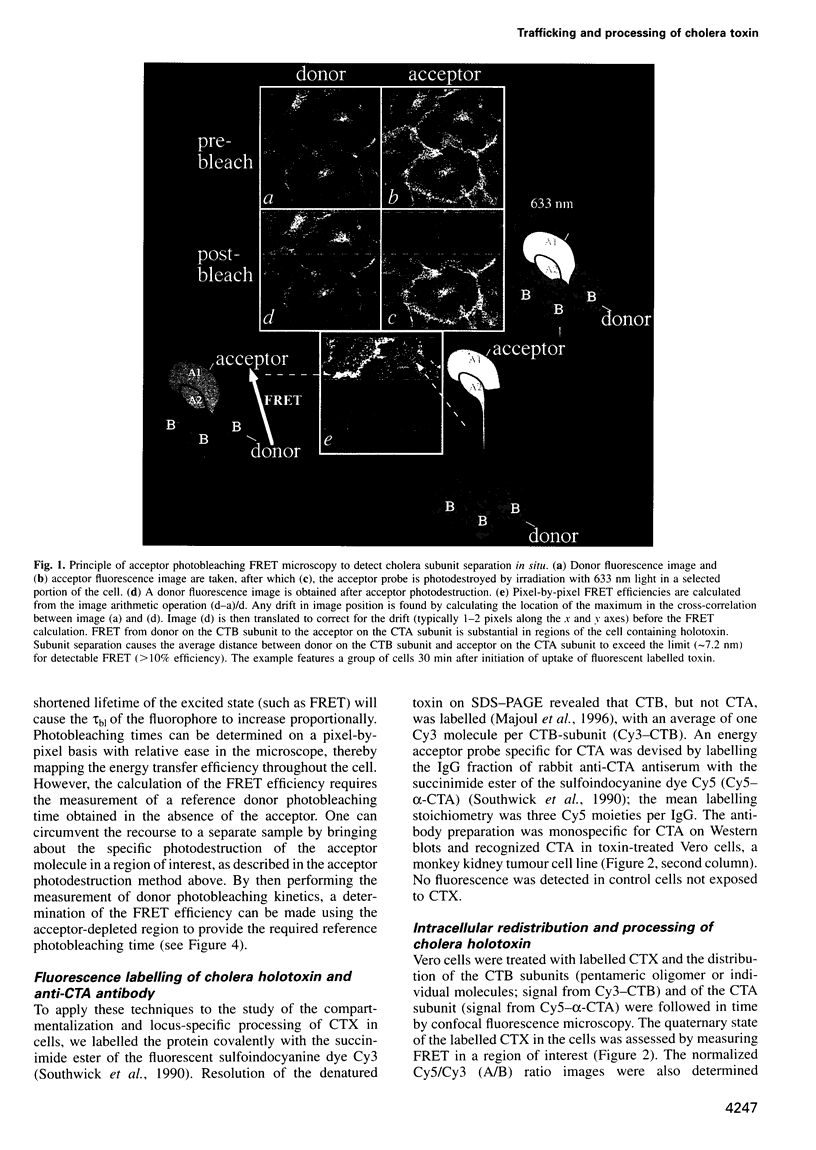
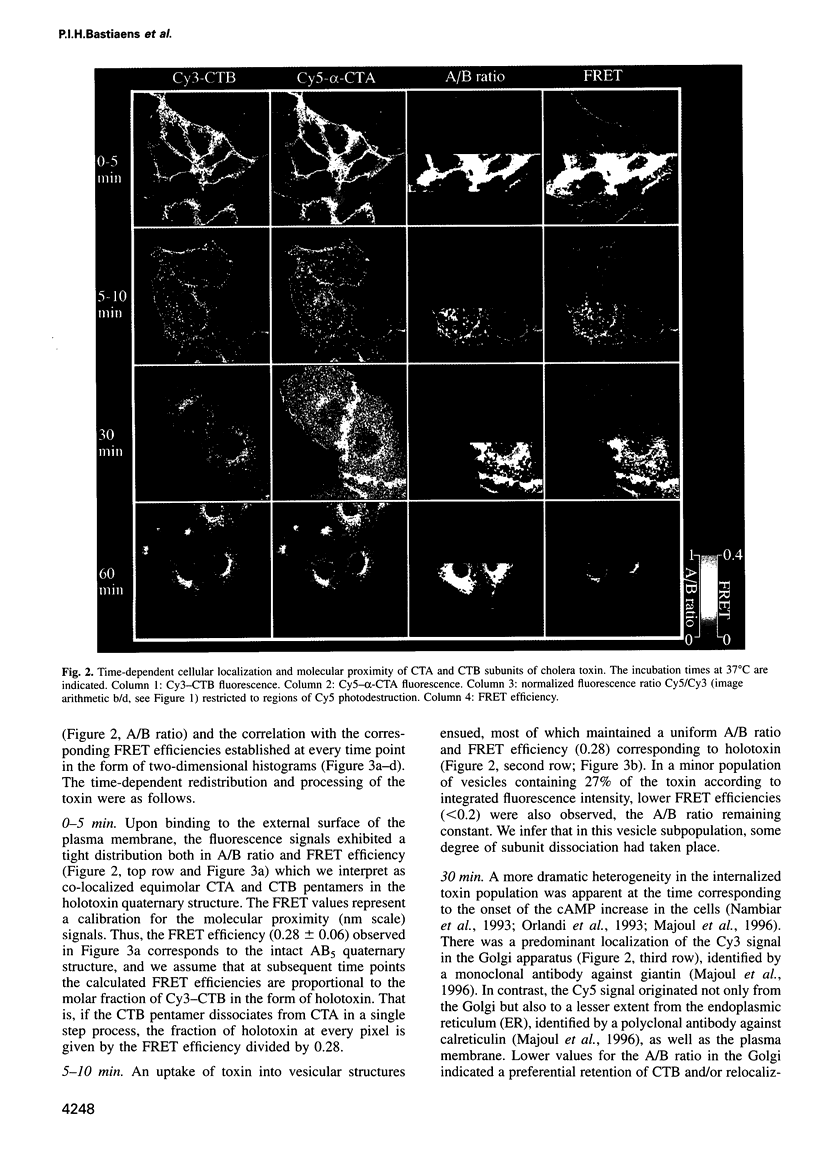
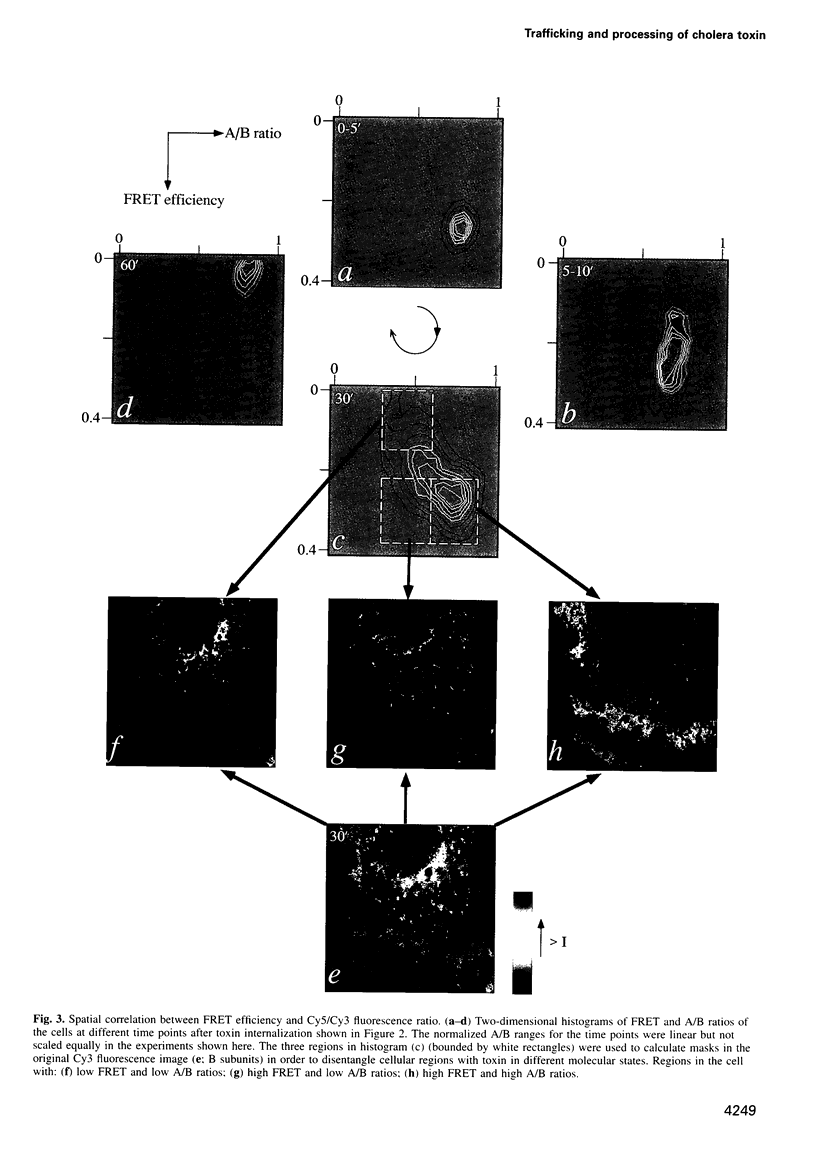
Abstract
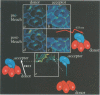
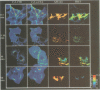
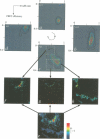
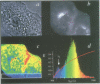
The subcellular localization and corresponding quaternary state of fluorescent labelled cholera toxin were determined at different time points after exposure to living cells by a novel form of fluorescence confocal microscopy. The compartmentalization and locus of separation of the pentameric B subunits (CTB) from the A subunit (CTA) of the toxin were evaluated on a pixel-by-pixel (voxel-by-voxel) basis by measuring the fluorescence resonance energy transfer (FRET) between CTB labelled with the sulfoindocyanine dye Cy3 and an antibody against CTA labelled with Cy5. The FRET efficiency was determined by a new technique based on the release of quenching of the Cy3 donor after photodestruction of the Cy5 acceptor in a region of interest within the cell. The results demonstrate vesicular transport of the holotoxin from the plasma membrane to the Golgi compartment with subsequent separation of the CTA and CTB subunits. The CTA subunit is redirected to the plasma membrane by retrograde transport via the endoplasmic reticulum whereas the CTB subunit persists in the Golgi compartment.
Full text
PDF
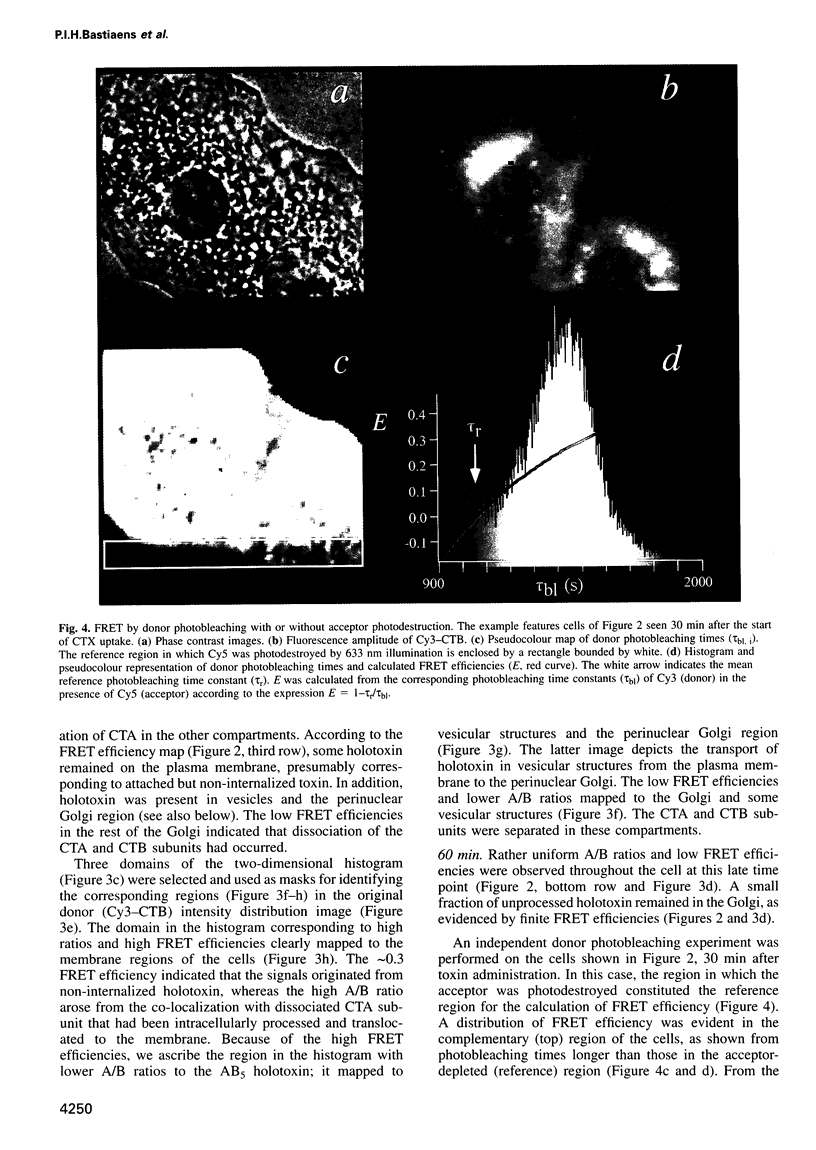
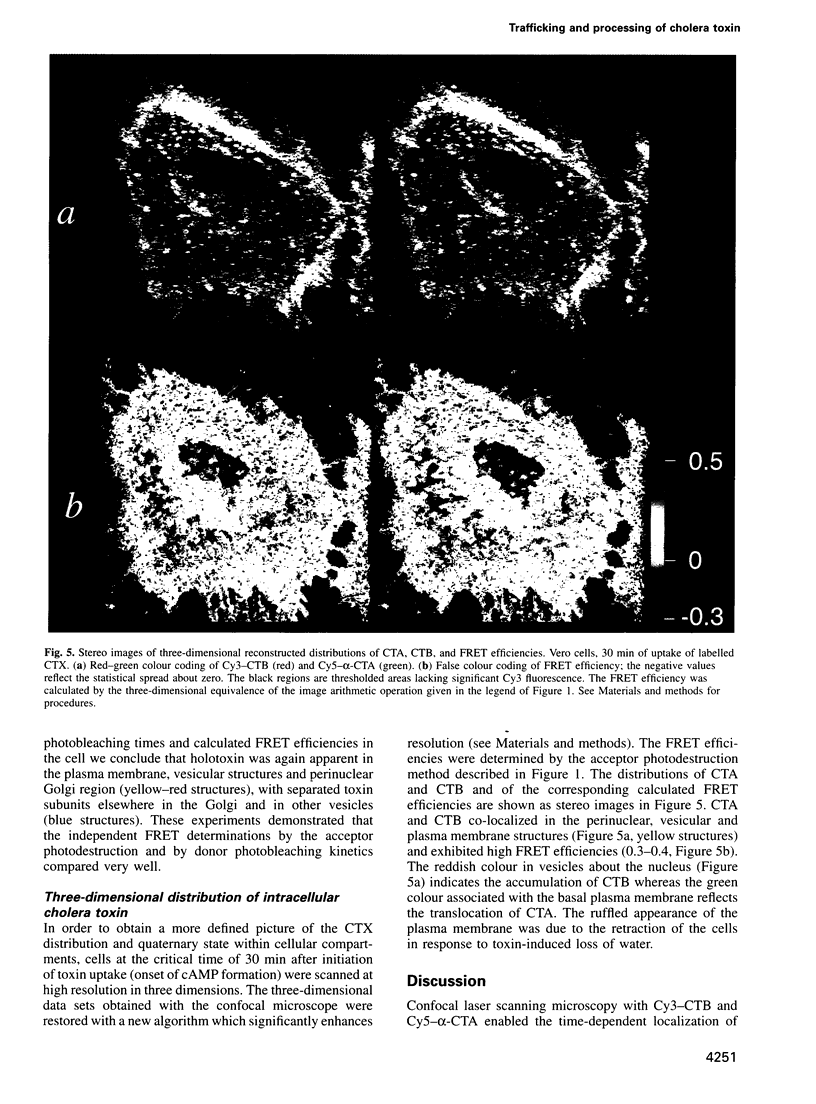


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carrington W. A., Lynch R. M., Moore E. D., Isenberg G., Fogarty K. E., Fay F. S. Superresolution three-dimensional images of fluorescence in cells with minimal light exposure. Science. 1995 Jun 9;268(5216):1483–1487. doi: 10.1126/science.7770772. [DOI] [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg R. M. Fluorescence resonance energy transfer. Curr Opin Biotechnol. 1995 Feb;6(1):103–110. doi: 10.1016/0958-1669(95)80016-6. [DOI] [PubMed] [Google Scholar]
- Field M., Rao M. C., Chang E. B. Intestinal electrolyte transport and diarrheal disease (1). N Engl J Med. 1989 Sep 21;321(12):800–806. doi: 10.1056/NEJM198909213211206. [DOI] [PubMed] [Google Scholar]
- Gadella T. W., Jr, Jovin T. M. Oligomerization of epidermal growth factor receptors on A431 cells studied by time-resolved fluorescence imaging microscopy. A stereochemical model for tyrosine kinase receptor activation. J Cell Biol. 1995 Jun;129(6):1543–1558. doi: 10.1083/jcb.129.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway T. S., van Heyningen S. Binding of NAD+ by cholera toxin. Biochem J. 1987 May 15;244(1):225–230. doi: 10.1042/bj2440225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M. Mechanism of action of cholera toxin. Adv Cyclic Nucleotide Res. 1977;8:85–118. [PubMed] [Google Scholar]
- Gill D. M. The arrangement of subunits in cholera toxin. Biochemistry. 1976 Mar 23;15(6):1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- Heyningen S Van Cholera toxin: interaction of subunits with ganglioside GM1. Science. 1974 Feb 15;183(4125):656–657. doi: 10.1126/science.183.4125.656. [DOI] [PubMed] [Google Scholar]
- Lencer W. I., Moe S., Rufo P. A., Madara J. L. Transcytosis of cholera toxin subunits across model human intestinal epithelia. Proc Natl Acad Sci U S A. 1995 Oct 24;92(22):10094–10098. doi: 10.1073/pnas.92.22.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majoul I. V., Bastiaens P. I., Söling H. D. Transport of an external Lys-Asp-Glu-Leu (KDEL) protein from the plasma membrane to the endoplasmic reticulum: studies with cholera toxin in Vero cells. J Cell Biol. 1996 May;133(4):777–789. doi: 10.1083/jcb.133.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J., Collier R. J., Romig W. R. Enzymic activity of cholera toxin. II. Relationships to proteolytic processing, disulfide bond reduction, and subunit composition. J Biol Chem. 1979 Jul 10;254(13):5855–5861. [PubMed] [Google Scholar]
- Orlandi P. A., Curran P. K., Fishman P. H. Brefeldin A blocks the response of cultured cells to cholera toxin. Implications for intracellular trafficking in toxin action. J Biol Chem. 1993 Jun 5;268(16):12010–12016. [PubMed] [Google Scholar]
- Parton R. G., Joggerst B., Simons K. Regulated internalization of caveolae. J Cell Biol. 1994 Dec;127(5):1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton R. G. Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J Histochem Cytochem. 1994 Feb;42(2):155–166. doi: 10.1177/42.2.8288861. [DOI] [PubMed] [Google Scholar]
- Sofer A., Futerman A. H. Cationic amphiphilic drugs inhibit the internalization of cholera toxin to the Golgi apparatus and the subsequent elevation of cyclic AMP. J Biol Chem. 1995 May 19;270(20):12117–12122. doi: 10.1074/jbc.270.20.12117. [DOI] [PubMed] [Google Scholar]
- Southwick P. L., Ernst L. A., Tauriello E. W., Parker S. R., Mujumdar R. B., Mujumdar S. R., Clever H. A., Waggoner A. S. Cyanine dye labeling reagents--carboxymethylindocyanine succinimidyl esters. Cytometry. 1990;11(3):418–430. doi: 10.1002/cyto.990110313. [DOI] [PubMed] [Google Scholar]
- Spangler B. D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992 Dec;56(4):622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D., Carpentier J. L., Sawano F., Gorden P., Orci L. Ligands internalized through coated or noncoated invaginations follow a common intracellular pathway. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7957–7961. doi: 10.1073/pnas.84.22.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Bacskai B. J., Adams S. R. FRET for studying intracellular signalling. Trends Cell Biol. 1993 Jul;3(7):242–245. doi: 10.1016/0962-8924(93)90124-j. [DOI] [PubMed] [Google Scholar]