Abstract
A 47-year-old man with a history of recurrent pleomorphic adenoma of the right lacrimal gland presented with rapid onset of a swelling in the right orbit. Initial imaging with CT showed that the swelling was grossly involving the extraocular muscles. Hence we had a suspicion of malignant transformation, so a radical approach in the form of right orbital exenteration with anterior skull base resection and temporalis muscle reconstruction was taken. Postoperative histology revealed epithelial–myoepithelial carcinoma, with immunopositivity for epithelial and myoepithelial components. Adjuvant radiation of 60 Gy was given with three-dimensional CT-based planning. This case portrays the importance of adjuvant treatment in recurrent pleomorphic adenoma and chance of malignant transformation in rare histologies.
Background
Epithelial–myoepithelial carcinoma (EMC) is a rare low-grade malignant neoplasm of the salivary gland accounting for 1% of all tumours. Histologically, it is characterised by biphasic morphology of ductal and myoepithelial cells. EMC most commonly arises from the parotid gland. EMC is extremely rare in the lacrimal gland, with only five cases reported in the English literature to date.1–5 We report a case of EMC of the lacrimal gland arising from a recurrent pleomorphic adenoma. We reviewed the literature for the management of this rare entity.
Case presentation
A 47-year-old man presented to our multidisciplinary tumour clinic in October 2014, with a 3-month history of decreasing vision of the right eye and a slowly progressive, painless swelling of the right orbit. Two years earlier, he had undergone exploratory orbitotomy with wide local excision for a similar swelling. The histopathological examination at the time had revealed pleomorphic adenoma of the lacrimal gland. The patient was asymptomatic for about a year after which the swelling recurred, and he underwent wide excision at another hospital. No details of the surgery were available and no adjuvant therapy was given then. The patient remained symptom free for about 8 months prior to the present evaluation, when painless progressive proptosis developed in the right orbit. Other symptoms included increased discharge, redness and loss of vision in the right eye. On physical examination, performance status was Eastern Cooperative Oncology Group (ECOG) 1. Local examination revealed a firm 3×2 cm non-tender mass over the superolateral quadrant of the right orbit, associated with diplopia and diminution of vision. Best-corrected visual acuity was 0/6 in the right eye and 6/6 in the left eye, as evaluated with Snellen's chart. Right eye ocular movements were restricted. There were no palpable cervical lymph nodes. Systemic work up did not reveal any abnormalities.
Investigations
A CT of the face and neck revealed a well-circumscribed heterogeneously enhancing mass in the right lacrimal fossa with displacement of globe inferiorly (figure 1). The mass was causing expansion of the orbit with destruction and remodelling of the lateral wall of the right orbit involving the medial, superior rectus and all oblique muscles. No significant lymph nodes were seen. A clinical stage CT4aN0 M0 stage IVA was made according to AJCC (American Joint Committee on Cancer 2010).
Figure 1.
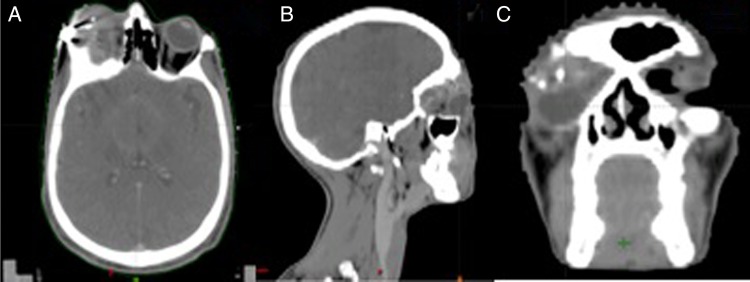
Contrast-enhanced CT of face and neck revealing well-circumscribed heterogeneously enhancing mass in the right lacrimal fossa with displacement of globe inferiorly. Mass was causing expansion of orbit with destruction and remodelling of lateral wall of right orbit involving medial, superior rectus and all oblique muscles.
Treatment
In view of gross disease involvement of the extraocular muscles, radical orbital exenteration and anterior skull base resection with temporalis muscle reconstruction was carried out. A postoperative gross specimen of the globe showed a 3×3 cm tumour grossly involving the extraocular muscles (figure 2A, B). Histopathological examination revealed EMC of lacrimal gland with myoepithelial cells showing immunopositivity for smooth muscle antigen (SMA), S-100, epithelial membrane antigen (EMA) and cytokeratin. All resected margins were free of tumour (figures 3 and 4).
Figure 2.
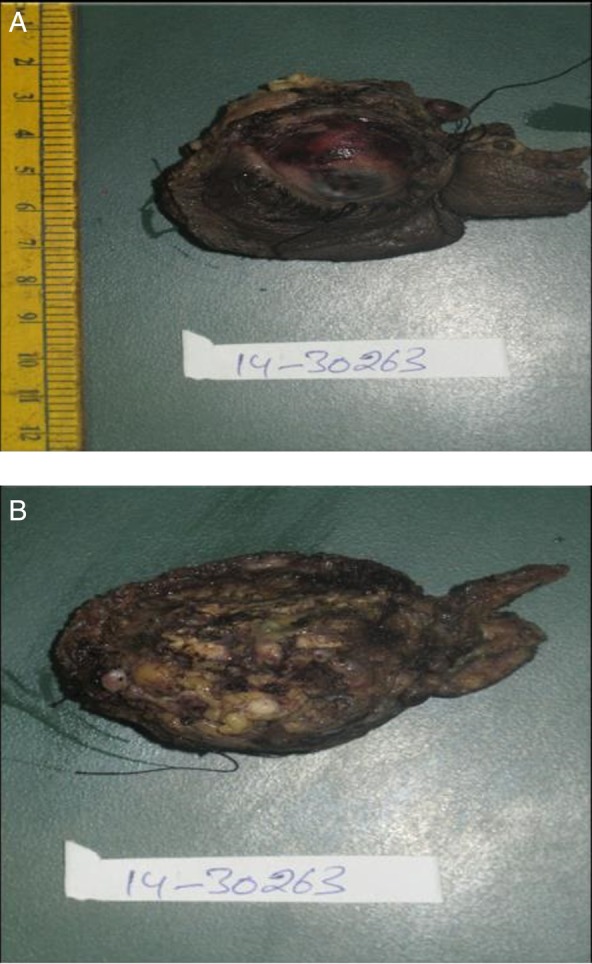
(A and B) Gross specimen of globe with a 3×3 cm tumour grossly involving extraocular muscles.
Figure 3.
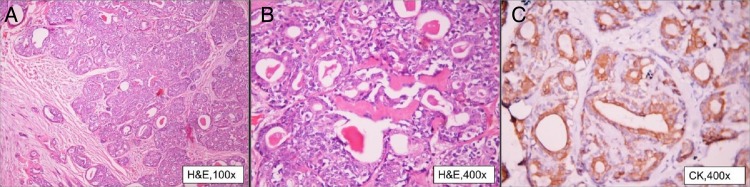
(A) Photomicrograph showing a tumour with infiltrating borders and tumour cells arranged in sheets (×10). (B) Tumour is composed of bilayered tubules (×20). (C) Tubular structures are lined by ductal cells and surrounded by an outer layer of clear cells (myoepithelial cells) (×40) (all H&E stain).
Figure 4.
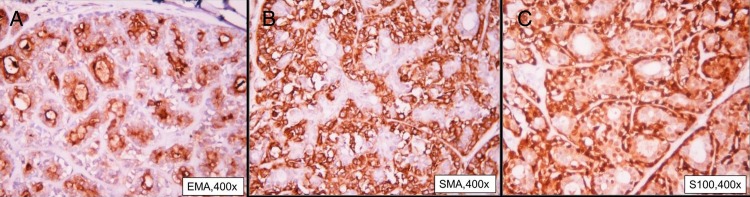
(A–C) Immunopositivity for epithelial membrane antigen, smooth muscle antigen and S-100, respectively.
A final diagnosis of EMC of lacrimal gland was made based on histological and immunohistochemistry findings. Adjuvant radiotherapy was planned in view of the recurrent nature of the disease. Linac based three-dimensional (3D) conformal radiation (Elekta Medical Systems Crawley, UK) was planned for the right orbit. Postoperative radiotherapy was given at a dose of 60 Gy in 30 fractions over 6 weeks with superior and inferior vertex fields, and 6 MV photons. The entire orbit was contoured to form the clinical target volume and 5 mm margin was added to form the planning target volume (PTV). Radiation dose to surrounding organs and normal organs at risk were within acceptable limits.
3D conformal radiotherapy planning achieved dose maximum (Dmax) to PTV up to 67.87 Gy and dose minimum to 43.17 Gy. Dose limits to organs at risk included Dmax to the left eye as 2.4 Gy, left optic nerve 2.8 Gy, optic chiasma 54 Gy, spinal cord 21.7 Gy and brainstem 23 Gy (figure 5).
Figure 5.
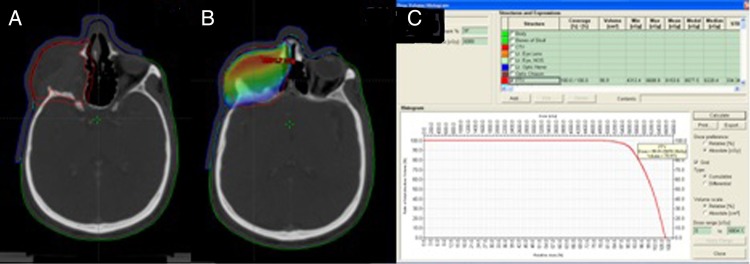
(A) Clinical target volume (CTV) included entire preoperative tumour volume with 5 mm margin. Planning target volume (PTV) was generated with 5 mm margin to CTV. (B and C) Dose colour wash and dose volume histogram of 95% of PTV receiving 57.11 Gy.
Outcome and follow-up
Acute toxicity included grade 2 skin reaction, which settled 2 weeks after completion of radiation therapy. At 6 months follow-up in our clinic, no long-term side effects were observed.
Discussion
Tumours of the lacrimal glands represent approximately 10% of all orbital masses. The epithelial tumours of the lacrimal gland constitute 28% of all lacrimal gland tumours.6 7 The most common epithelial tumours are pleomorphic adenomas, adenoid cystic carcinomas and adenocarcinoma. Lacrimal and salivary gland tumours are comparable in terms of morphology and clinical characteristics. Pleomorphic adenoma is the most common benign tumour of the lacrimal gland, constituting 65% of all epithelial tumours. Malignant transformation may be seen in less than 10% of cases.8 EMC is a rare malignant tumour of the salivary and submandibular gland, accounting for only 1% of all salivary gland tumours. Being a low-grade malignancy, it is associated with good survival rates in the salivary gland, however, the outcome in the lacrimal gland is not clear from the existing literature.5 EMC may develop as primary myoepithelial carcinoma or more rarely from malignant transformation of pre-existing pleomorphic adenoma, as in the case reported here.1–5 It is extremely rare for EMC malignant transformation to occur in a pleomorphic adenoma, as in this patient. A similar case was reported by Gonçalves et al,5 where EMC of the lacrimal gland was observed 14 years after complete excision of a pleomorphic adenoma.
Histological diagnosis of EMC is a challenge. Diagnostic criteria for EMC include myoepithelial differentiation, increased mitotic activity in higher power field, KI-67 >10%, perineural invasion and necrosis. They are found to be indicators of aggressive biology.9 10 Immunochemical methods are helpful for establishing the diagnosis. In the case reported, diagnosis was confirmed by positive SMA, S-100, cytokeratin and EMA. Lacrimal gland EMC is a therapeutic challenge due to complex orbital anatomy limiting complete surgical resection; adjuvant therapy may be beneficial to achieve superior local control. However, this rare clinical entity does not have standard management guidelines in the existing literature. In the case reported, the patient underwent radical orbital exenteration and anterior skull base resection followed by radiation therapy. Such an approach has also been followed in other reported cases.4 5 Patients with high-risk histopathological features that include close margins or positive margins may be considered for adjuvant radiation therapy for improved recurrence-free rates. The role of adjuvant radiation therapy to improve local control in malignant epithelial neoplasms of the lacrimal gland is also emphasised by Skinner et al.11 It was observed that patients who did not receive adjuvant radiation therapy developed local recurrence earlier as compared to the group who received radiation.
Newer radiotherapy techniques, which include intensity modulated radiotherapy and stereotactic radiotherapy, deliver radiation with high precision. Proton beam therapy provides uniform dose coverage of the target and, unlike photon beams, has no exit dose. These techniques provide a superior conformity index and improved orbital bone and brain sparing, and, therefore, reduce the late side effects significantly.
Primary nodal drainage for lacrimal apparatus is through the intraparotid and preauricular lymph nodes. Secondary nodal drainage for the lacrimal sac is the submaxillary nodal basin. In case series reported, to date, only 9% of cases had nodal failures, with none being isolated failures. Therefore, currently, elective nodal irradiation is not recommended.11
Learning points.
Pleomorphic adenomas may undergo malignant transformation in rare histologies such as epithelial myoepithelial carcinoma.
In unresectable disease involving extraocular muscles with poor chances of vision preservation, radical surgery and exenteration followed by adjuvant radiation to the entire orbit may achieve improved local control.
Complete evaluation and total excision of pleomorphic adenoma are necessary, and adjuvant radiation therapy may be planned appropriately, as these tumours tend to have a long course with multiple recurrences.
Footnotes
Contributors: BPV was responsible for review of the literature, drafting and revising the manuscript. SP was involved in conception, review of the literature, patient evaluation and drafting, revision and final approval of the manuscript. AGV participated in histopathological examination and revision of the manuscript. BC was responsible for patient evaluation and manuscript revision.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Singh G, Sharma MC, Agarwal S et al. Epithelial-myoepithelial carcinoma of the lacrimal gland: a rare case. Ann Diagn Pathol 2012;16:292–7. 10.1016/j.anndiagpath.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 2.Ostrowski ML, Font RL, Halpern J et al. Clear cell epithelial-myoepithelial carcinoma arising in pleomorphic adenoma of the lacrimal gland. Ophthalmology 1994;101:925–30. 10.1016/S0161-6420(94)31236-X [DOI] [PubMed] [Google Scholar]
- 3.Chan WM, Liu DTL, Lam LYM et al. Primary epithelial-myoepithelial carcinoma of the lacrimal gland. Arch Ophthalmol 2004;122:1714–17. 10.1001/archopht.122.11.1714 [DOI] [PubMed] [Google Scholar]
- 4.Wiwatwongwana D, Berean KW, Dolman PJ et al. Unusual carcinomas of the lacrimal gland: epithelial-myoepithelial carcinoma and myoepithelial carcinoma. Arch Ophthalmol 2009;127:1054–6. 10.1001/archophthalmol.2009.195 [DOI] [PubMed] [Google Scholar]
- 5.Gonçalves ACP, de Lima PP, Monteiro MLR. Epithelial-myoepithelial carcinoma of the lacrimal gland 14 years after en bloc resection of a pleomorphic lacrimal gland adenoma. Ophthal Plast Reconstr Surg 2014. [DOI] [PubMed] [Google Scholar]
- 6.Shields CL, Shields JA, Eagle RC et al. Clinicopathologic review of 142 cases of lacrimal gland lesions. Ophthalmology 1989;96:431–5. 10.1016/S0161-6420(89)32873-9 [DOI] [PubMed] [Google Scholar]
- 7.Rootman J. Diseases of orbit—a multidisciplinary approach. 2nd edn Philadelphia, PA: Lippincott, 2003. [Google Scholar]
- 8.Weis E, Rootman J, Joly TJ et al. Epithelial lacrimal gland tumors: pathologic classification and current understanding. Arch Ophthalmol 2009;127:1016–28. 10.1001/archophthalmol.2009.209 [DOI] [PubMed] [Google Scholar]
- 9.el-Naggar A, Batsakis JG, Luna MA et al. DNA content and proliferative activity of myoepitheliomas. J Laryngol Otol 1989;103:1192–7. 10.1017/S0022215100111326 [DOI] [PubMed] [Google Scholar]
- 10.Nagao T, Sugano I, Ishida Y et al. Salivary gland malignant myoepithelioma: a clinicopathologic and immunohistochemical study of ten cases. Cancer 1998;83:1292–9. [DOI] [PubMed] [Google Scholar]
- 11.Skinner HD, Garden AS, Rosenthal DI et al. Outcomes of malignant tumors of the lacrimal apparatus: the University of Texas MD Anderson Cancer Center experience. Cancer 2011;117:2801–10. 10.1002/cncr.25813 [DOI] [PubMed] [Google Scholar]


