Summary
Despite the recognition of Pseudomonas aeruginosa is an opportunistic pathogen, no vaccine against this bacteria have come to market. This review describes the current state-of-the-art in vaccinology for this bacterium. This includes a discussion of those at risk for infection, the types of vaccines and the approaches for empirical and targeted antigen selection under development, as well as a perspective on where the field should go. In addition, the challenges in developing a vaccine for those individuals at risk are discussed.
Keywords: Pseudomonas aeruginosa, vaccine, lipopolysaccharide, flagella, alginate, Th17, neutrophil
The year 1970 was an important one in Pseudomonas aeruginosa vaccine history. Alexander and Fisher published an editorial in the American Journal of Surgery that claimed success of an lipopolysaccharide (LPS)-based vaccine at preventing mortality in burned patients (on a trial using historical controls) [1]. Lanyi published the first description of the serologic differences in flagella typing [2]. Bartell and colleagues also published one of the first descriptions of the chemical composition of the protective slime antigen (later known as alginate) that year [3]. The Beatles song “Long and winding road” was also released in 1970, perhaps a premonition of the difficulties in achieving a broadly protective vaccine for human use despite many attempts to make vaccines based on these 3 important antigens: LPS, flagella, and alginate. This review will focus on newer approaches in vaccine development for P. aeruginosa. We refer the reader to other excellent recent reviews on vaccines for P. aeruginosa [4] and [5].
P. aeruginosa continues to cause serious infections in humans, particularly in the critically ill [6], the immunocompromised [7,8], those with burn wounds [9] or combat-related wound infections [10,11], and those with cystic fibrosis [12]. It is also one of the most frequently isolated pathogens from contact lens-associated bacterial keratitis [13]. The multitude of intrinsic and acquired resistance mechanisms contributes to the intractable nature of many of these infections. A recent population-based study of P. aeruginosa bacteremia in Canada shed light on the overall incidence of serious P. aeruginosa infections [14]. This study reported an exponential increased risk of bacteremia after age 60, with a remarkably high annual incidence (per 100,000) of 10 in ages 60–69, 20 in ages 70–79, and 35 in ages >80, and an overall mortality rate of 29%. About a third of these cases of bacteremia were classified as having a pulmonary source. This incidence is on par with that of invasive methicillin-resistant Staphylococcus aureus (MRSA) infections in the US in 2011, which was recently estimated to be 26 per 100,000 [15]. Based on the 2010 census, the US Census Bureau predicts that the US population age ≥65 will more than double between 2012 and 2060, from 43.1 million to 92.0 million, suggesting that the P. aeruginosa infections will become increasingly prevalent in the coming years.
Pulmonary infections caused by P. aeruginosa generally dichotomize between acute pneumonia, usually associated with mechanical ventilation (so-called ventilator-associated pneumonia [VAP], which falls under the rubric of healthcare-associated pneumonia) and the chronic pneumonia of cystic fibrosis (CF). Based on data from the National Healthcare Safety Network from 2009–2010 [16], P. aeruginosa was the most commonly isolated Gram-negative pathogen and the second most common pathogen overall in the setting of VAP, accounting for 11% of cases. Of the P. aeruginosa VAP isolates, 33% were fluoroquinolone-resistant, 30% were carbapenem-resistant, and 18% were multidrug resistant [16]. It is estimated that there are 300,000 cases of hospital-acquired pneumonia each year in the United States [17]. Other epidemiologic data suggest that 10–20% of adults receiving more than 48 hours of mechanical ventilation will develop VAP [18]. Compared to similar patients without VAP, patients with VAP are about twice as likely to die, require approximately 6 days more of ICU-level care, and incur $10,000 additional hospital costs [18]. Notably, VAP due to P. aeruginosa has a particularly high attributable mortality [19].
P. aeruginosa is also a major cause of combat-related wound infections. A recent review of the Joint Theater Trauma Registry (JTTR) for infections among more than 16,000 Iraq and Afghanistan combat casualties noted that infections were mostly due to Gram-negative bacteria (48%), most commonly involving wounds (27%) or lungs (15%), and 25% of the Gram-negatives were P. aeruginosa [10]. A review of all trauma casualties evacuated from the Iraqi theatre to a U.S. Navy hospital ship in 2003, 56 (27%) of 211 patients met criteria for infection, with Pseudomonas species causing 14% of these infections, most of which were wound infections [11]. There was high antibiotic resistance in these P. aeruginosa strains, with 56% being tobramycin-resistant and 37% ceftazidime-resistant [11]. Remarkably, in 2005, almost 50% of P. aeruginosa isolates from the ICU at Walter Reed Army Medical Center in Washington, DC were imipenem-resistant [20]. Together, these studies suggest that P. aeruginosa is a major cause of combat-related wound infections that are difficult to treat due to high antibiotic resistance and also associated with high morbidity.
Although CF is an autosomal-recessive genetic disorder, with about 1,000 new cases diagnosed each year and approximately 30,000 patients suffering from its complications in the U.S. alone, chronic lung infection with P. aeruginosa accounts for most of the morbidity and mortality associated with the disease [21]. Although emerging microbiome data suggest that many other bacterial species are present in CF sputum, most of which can be cultured using advanced techniques [22], it should be noted that when lung tissue from autopsies or lung transplants are examined, only traditional CF pathogens such as P. aeruginosa are found by either culture or DNA analysis [23]. Results using samples from oropharyngeal swabs [24] and expectorated sputum that must come through the oropharynx during collection should be interpreted with caution.
One might ask who would be immunized with a P. aeruginosa vaccine. Candidates to receive such a vaccine would be relatively easy to identify. It is becoming increasingly clear that as the population ages and spends more time in healthcare settings, the risk for nosocomial infection goes up. While some obvious populations can be identified (preoperative patients scheduled for major surgery, soldiers, police, firefighters, wearers of extended-use contact lenses, CF patients), it might be reasonable to consider clinical trials involving vaccination of everyone over 60 (or whatever is epidemiologically defined as “elderly”). An effective vaccine given to individuals with an increased risk for hospitalization due merely to age could be very cost effective. Other clinical trial designs could mirror those of Intercell’s ongoing OprF/I vaccine trial (clinicaltrials.gov, NCT01563263) that is vaccinating adults soon after admission to ICUs (which is predicated on rapid immune response, as was observed in healthy volunteers [25]). Overall, considering the problem of hospital-acquired infections, the prominent role of P. aeruginosa in this setting, the unrelenting problem of antibiotic resistance, and an aging population, a vaccine against P. aeruginosa is urgently needed.
LPS-based vaccines
It is well established from animal studies that antibodies to the LPS O antigen mediate high-level immunity to P. aeruginosa infections [26]. However, for many of the O antigens, protective epitopes are poorly immunogenic, while non-protective O antigen epitopes often elicit the best immune responses after active vaccination [27]. P. aeruginosa strains possessing LPS O antigen are currently classified into 20 serotypes based on the chemical composition of the O antigen, with each serotype possessing subtype strains having subtle variations, leading to over 30 subtypes [28]. A heptavalent O antigen vaccine prepared from LPS of seven different serotypes called Pseudogen (Parke Davis and Co.) showed efficacy in nonrandomized trials among adult cancer and burn patients in preventing fatal P. aeruginosa infections [29,30], but these vaccines were limited by toxicity, and studies in patients with leukemia and cystic fibrosis showed no benefit [31,32]. Although O antigen-based vaccines can elicit antibodies that are protective in animal models, this protection is generally seen only when the strains used to isolate the vaccine antigen are used in the challenge studies [33,34]. Broad-based protection against other strains, even subtypes within the same serotype, is not reliably generated [35,36]. With these observations in mind, an O antigen-based vaccine would need to be more than 30-valent to cover all strains, or about 10-valent to cover the most common serotypes seen clinically. However, when related purified O antigens from strains within the same serotype are combined, the immune response in mice to each individual component is diminished [35]. Furthermore, a hyperimmune globulin prepared from plasma of humans immunized with an octavalent P. aeruginosa O antigen conjugate vaccine (conjugated to P. aeruginosa exotoxin A) did not protect critically ill adults against P. aeruginosa infections after passive administration [37].
P. aeruginosa vaccines for CF patients
The susceptibility of CF patients to chronic pulmonary infection with P. aeruginosa is multifactorial [38], including failure of clearance do to lack of CFTR-mediated bacterial internalization by epithelial cells [39], defective mucosal immunity [38], abnormally viscous secretions that impair mucociliary clearance [40], and, as demonstrated recently in the CF pig model, abnormal pH of the airway surface liquid [41]. While the P. aeruginosa strains associated with pulmonary deterioration among CF patients usually have a mucoid phenotype due to overproduction of the exopolysaccharide alginate [42,43], the early isolates from younger children with CF are typically non-mucoid [44]. Another distinction of the P. aeruginosa isolates recovered in the early versus late stages of cystic fibrosis is that the early, non-mucoid isolates are lipopolysaccharide (LPS)-smooth and serologically typeable, while the late, mucoid isolates are LPS-rough and non-typeable [45]. LPS-rough strains lack the O antigen polysaccharide (also known as O side chain) attached to the LPS core.
Pier and colleagues showed in the mid-1980s that the small minority (<5%) of older CF patients who have escaped chronic P. aeruginosa infection have high levels of serum opsonic antibody directed against alginate [46]. Immunotherapeutic strategies that provide systemic opsonic antibodies to alginate can protect mice and rats from lung infection with mucoid P. aeruginosa encased in agar beads [47]. Alginate, however, is poorly immunogenic in humans [48], possibly because of pre-existing non-opsonic alginate-specific antibodies, which prevent the production of opsonic antibodies in animal models [49]. The role of such antibodies in the protection against initial colonization is not clear, especially in light of the fact that the early P. aeruginosa isolates from young CF patients are nearly always non-mucoid in vitro [44]. However, there is increasing evidence that non-mucoid isolates produce alginate in vivo [50]. Notably, an engineered human IgG1 monoclonal antibody to the mannuronic acid epitopes of alginate can protect mice against acute lethal pneumonia caused by non-mucoid, LPS-smooth strains [51].
A number of vaccines against P. aeruginosa have been tested in patients with CF, with little success to date. Indeed, none was deemed to be efficacious in a recent Cochrane review [52]. Notable among these is a bivalent flagella vaccine, which showed a small but statistically significant (P=0.05) reduction in P. aeruginosa infection in a large randomized trial led by the late Gerd Döring [53]. The vaccine clearly seemed to work, as only strains with flagella types not included in the vaccine were isolated from patients after vaccination. Unfortunately, the company involved in preparation of this vaccine (IMMUNO, Vienna, Austria) stopped production before the end of the trial [4] and, to our knowledge, no other company to date has taken over production of the bivalent flagella vaccine or any other version of a multivalent flagella vaccine. This clinical trial highlights the challenges of clinical studies evaluating P. aeruginosa infection CF patients who are usually undergoing frequent and sometimes continuous treatment with antibiotics.
A conjugate vaccine of flagellin coupled to polymannuronic acid (PMA, which contains the epitope recognized by the opsonic monoclonal antibody to alginate, described above) elicited opsonic antibodies against mucoid but not nonmucoid strains of P. aeruginosa, and rabbit antisera to this PMA-flagellin vaccine showed improved clearance of both mucoid and nonmucoid strains in a murine respiratory infection model [54]. Importantly, antibodies elicited by the PMA-flagellin vaccine did not neutralize the Toll-like receptor 5 (TLR5)-activating activity of flagellin, an important part of innate immunity flagellated microbial pathogens [54]. Cryz and co-workers assessed whether LPS-specific antibodies could prevent P. aeruginosa colonization of children with CF after active immunization with an octavalent O-antigen-exotoxin A conjugate vaccine. Although studies with historical controls were promising [55,56], later unpublished results from a prospective trial of this vaccine in Europe did not show a delay in colonization, so this vaccine was also abandoned.
Live-attenuated vaccines
P. aeruginosa mutants having a deletion of the aroA gene, which is required for the synthesis of aromatic amino acids, have proven to be both highly attenuated and immunogenic in animal models [57–59]. The Priebe, Pier, and Goldberg groups have shown that nasal immunization of mice with live-attenuated aroA deletion (ΔaroA) mutants of P. aeruginosa is highly protective against acute lethal pneumonia caused by P. aeruginosa strains having the same LPS O antigen as the vaccine (called serotype-homologous strains); but passive transfer of antibody was only effective at protecting recipient mice when a highly virulent variant of the parental strain was used (allowing a relatively low challenge dose). Passive transfer did not protect against a less virulent variant, while active immunization did, thus suggesting that cellular immunity also played a prominent role in protective immunity. Surprisingly, a P. aeruginosa ΔaroA vaccine strain based on strain PA14 (called PA14ΔaroA) protected against acute lethal pneumonia caused by LPS-heterologous P. aeruginosa strains in the absence of serum opsonic antibodies, suggesting a T cell effect [59]. Additional experiments showed that in immunized wild-type mice, the cytokine IL-17 was produced by a greater number of lung T cells, could be found at higher levels in bronchoalveolar lavage fluid (BALF), and was associated with more rapid recruitment of neutrophils to the airways when compared with control-immunized mice who succumbed following challenge. Depletion of IL-17 before challenge of immunized mice, or absence of the IL-17 receptor, abrogated the protective efficacy of the vaccine [59].
A brief digression about IL-17 is warranted here. Growing evidence suggests that a distinct lineage of IL-17-secreting T helper cells called Th17 cells [60,61] plays an important role in innate and adaptive antibacterial host defense in the lung [62–65] as well as in autoimmunity [66]. IL-17 is a proinflammatory cytokine expressed primarily by activated memory CD4+ T cells and is the prototypic member of a family of cytokines named IL-17A-F (where IL-17A is what is typically denoted IL-17, and IL-17E is IL-25) [67]. TGF-β is critical for commitment to Th17 development by upregulating IL-23R expression, thereby conferring responsiveness to IL-23 [68]. An inflammatory milieu (particularly the proinflammatory cytokine IL-6, with amplification by IL-1β and TNF-α) is also required [69]. While IL-23 is key to the survival and expansion of Th17 cells, it is not required for the initiation of Th17 responses [70]. Recent data in murine experimental autoimmune encephalomyelitis models show that Th17 cells can also secrete GM-CSF [71,72]. These ThGM-CSF cells are particularly inflammatory in murine experimental autoimmune encephalomyelitis models [71,72].
The action of IL-17 in the lung centers on neutrophil recruitment via induction of CXC chemokine secretion (KC and MIP-2 in rodents, GRO and IL-8 in humans) along with granulopoietic factors (such as G-CSF) that lead to increased bone marrow production and/or prolonged survival of neutrophils [62]. In studies of innate immunity to Klebsiella pneumoniae, Kolls and co-workers found that IL-17 receptor (IL-17R)-deficient mice were highly susceptible in an acute pneumonia model, with increased mortality and bacterial dissemination associated with delayed neutrophil recruitment to the lung as well as lower levels of G-CSF and MIP-2 in the knockout mice [64,73]. Vaccination of mice with K. pneumoniae outer membrane proteins protected mice from pneumonia in a Th17-dependent manner [65].
Of note, CF patients typically have high levels of IL-17 in BALF, particularly during pulmonary exacerbations [74,75], and they have also been found to have infiltration of the airway submucosa with Th17 lymphocytes [76]. These findings contrast with reports from the 1970s showing that T cell responses to gentamicin-killed P. aeruginosa were absent in the peripheral blood of CF patients with advanced lung disease despite strong responses to mitogens and to S. aureus, Group A Streptococcus, and Hemophilus influenzae [77,78]. An intrinsic T cell defect in CF is suggested by the findings that CF CD4 cells have abnormal Cl− conductance correctable by CFTR transfection [79]. Whether the high IL-17 found in the CF airways is the cause of the inflammatory lung disease that is a hallmark of CF [38] or just the result of chronic infection remains to be determined.
In other studies of live-attenuated ΔaroA vaccines in a neutropenic pneumonia model using mice made neutropenic by either cyclophosphamide or an anti-neutrophil monoclonal antibody (RB6-8C5), Kamei et al. [80] found higher numbers of monocytes/macrophages in the lungs after infection that correlated with a higher proportion of CD4 T cells secreting both IL-17 and GM-CSF in the lungs and spleens. They went on to show that CD4 T cell-secreted GM-CSF was critical for protection against pneumonia caused by LPS-heterologous strains in neutropenic mice [80]. Antibody-mediated blockade of GM-CSF or CD4 depletion led to diminished recruitment of monocytes/macrophages to the airways and diminished protection [80]. Thus, P. aeruginosa live-attenuated vaccines can be protective even when a key mediator of immunity, the neutrophil [81], is deficient.
In a different study, Kamei et al. [82] showed that a multivalent live-attenuated vaccination approach induced effector CD4 T cells as well as opsonic antibodies directed not only against multiple O antigens but also against the more conserved the LPS core and other surface proteins, all of which contributed to protection against acute lethal pneumonia in mice. Thus, successful vaccine candidates for P. aeruginosa will likely need to induce multiple immune effectors rather than just opsonic antibody to the LPS O antigen. Preliminary results with a live-attenuated vaccine based on the serotype O11 strain PA103, which required disruption of the exoU gene in addition to deletion of the aroA gene to achieve adequate attenuation, show extension of protection to serotype O11 strains, which had not been previously achieved [83].
Live-attenuated P. aeruginosa vaccines have also shown good protective efficacy against experimental corneal infections, with a broader spectrum of protection against LPS-heterologous strains compared to lung infections [84], likely related to the lower challenge doses needed to achieve a infection in the scratch-injured cornea infection model as well as the different pathogenesis of these infections whereby epithelial uptake of P. aeruginosa is a critical step in the infection process [85]. Interestingly, neutralization of IL-17 or absence of the IL-17 receptor leads to better outcomes in this corneal infection model [86], again highlighting that different mediators of immunity are needed in different sites of infection.
The Goldberg group constructed an LPS-based vaccine consisting of the O antigen from P. aeruginosa serotype O11 expressed on an attenuated strain of Salmonella typhimurium. Strains in this P. aeruginosa serotype are common clinically and have a high prevalence of cytotoxicity so are associated with a high mortality rate [87]. This live-attenuated vaccine elicited serotype-specific antibodies, and oral immunization of mice provided greater protection from acute P. aeruginosa pneumonia than intraperitoneal immunization [88]. Intranasal administration of this vaccine was more effective against acute pneumonia and could also protect against P. aeruginosa infections after burns or eye injury [89] and against pneumonia in immunocompromised mice made leukopenic after vaccination using either cyclophosphamide or an anti-neutrophil Gr1 monoclonal antibody (RB6-8C5) [90]. Administration of immune sera to immunocompromised mice via the intranasal route at the time of infection also led to improved survival.
Outer membrane proteins
Wu et al. [91] recently identified protective protein components of the live-attenuated vaccine described above (PA14ΔaroA) using a Th17-based reverse vaccinology strategy with a library of 258 PA outer-membrane and secreted proteins [92]. The proteins were produced with an in vitro transcription and translation system and used to stimulate splenocytes from mice immunized with PA14ΔaroA. The screen identified four Th17-stimulating proteins, and His-tagged purified version of three proteins from the library (OprL, PopB, and FpvA) stimulated IL-17 production in PA14ΔaroA-immune splenocytes. PopB was selected for further study since it was highly soluble, has been found in all P. aeruginosa strains tested [93], is a known virulence factor [94] as a structural component of the type III secretion system, and, unlike FpvA [95] and OprL [96], is highly conserved among PA strains, including sequential CF isolates [97]. Intranasal immunization of mice with PopB mixed with the beta-D-glucan curdlan, a known Th17 adjuvant [98–100], elicited strong Th17 responses and conferred IL-17-dependent, antibody-independent protection from lethal PA pneumonia in the absence of opsonophagocytic antibodies [91].
Other P. aeruginosa vaccine strategies have focused on the outer membrane proteins (OMPs) OprF [101–105] and OprI [106–108], which are antigenically related among all serotypes [109], as well as OprF/I fusion proteins [102,110]. Initial studies of OMPs as vaccines were confounded by LPS contamination of OMP preparations but did show evidence of serotype-heterologous protection with a purified OprF vaccine in addition to the homologous protection elicited by contaminating LPS [111,112]. Adenoviral vectors expressing an OprF peptide that possesses both B and T cell epitopes have shown great promise in animal models (recently reviewed in refs. [5,113]). Hughes et al. used synthetic peptides of OprF conjugated to KLH to immunize mice intranasally and found significant protection against acute pneumonia, although only 1 challenge strain was assessed [114]. An OprF DNA vaccine administered intradermally has also demonstrated protection in a mouse model of chronic pulmonary infection [115]. Using recombinant OprF/I fusion proteins, von Specht et al. showed that active and passive vaccination could protect neutropenic and SCID mice, respectively, against challenge doses 1000-fold above the LD50 [116,117]. OprF/I fusion proteins mixed with flagellins have also shown promise in murine models and were shown to be immunogenic in nonhuman primates [118,119]. Recombinant OprI [108,120] and an OprF/I fusion protein [102] have been shown to be well tolerated in human trials. The OprF/I fusion protein vaccine [25] is currently in phase II/III clinical trials (clinicaltrials.gov, NCT01563263) in which adults are vaccinated soon after admission to ICUs. This approach requires a rapid immune response, as was observed in healthy volunteers [25]. This OprF/I vaccine induces opsonic antibodies [25] as well as antibodies that inhibit IFN-γ binding to PA [110] (interfering with a virulence mechanism [121]), and, via the OprF329-342 epitope, it should induce IFN-γ+ CD4 and CD8 responses [122].
Passive immunotherapies
P. aeruginosa infection is often associated with patients with either an immune system compromise, injuries, or other underlying dysfunctions such as CF. In this patient population, the time or the function immune system necessary to develop an effective response to an active vaccination may not be achievable. Because of this, there is an effort to develop antibody-based (particularly humanized monoclonal) immunotherapies to eliminate P. aeruginosa after infection or, in select populations, to prevent infection. At the moment, none of these immunotherapies has been approved by the US FDA or other similar regulatory bodies outside the US. This portion of this review will describe the state-of-the-art with respect to these approaches and where we might go from here.
Anti-PcrV immunotherapy
PcrV is a surface-expressed, needle-tip protein component of the type III secretion apparatus of P. aeruginosa [123]. Early studies with polyclonal rabbit antisera to PcrV showed protection in various acute and chronic mouse models of infection (recently reviewed in [123]). In addition to providing protection, this polyclonal antibody was shown to block the translocation of type III secreted effectors into mammalian cells [124]. Because of this effect, in 2001, Shime et al. [125] compared the effectiveness of this rabbit IgG to PcrV and F(ab′)2 fragments prepared from the rabbit antisera. The anti-PcrV F(ab′)2 had comparable therapeutic effects in a number of different assays, indicating that the majority of the effectiveness was due to blocking the interaction with host cells rather than oposonophagocytsosis. Following this study, a specific mAb to PcrV (Mab166) was developed [126] and human Fab fragments that bound to the same epitope were subsequently generated [127].
An investigational, PEGylated [128] engineered human Fab′ reagent that recognized the same epitope has been developed by KaloBios Pharmaceutics, Inc. (South San Francisco, CA). This reagent, KB001, was tested for in a phase 2a study to determine safety and pharmacokinetics in 39 mechanically ventilated patients colonized with P. aeruginosa [129]. While almost all the patients in two treatment (3 mg/kg or 10 mg/kg of KB001 delivered as a single intravenous infusion) and the placebo groups had adverse events, there was no difference in the number or severity between the groups. By some criteria the patients receiving treatment fared better than the placebo group: 33–46% of the KB001 treated patients were alive and P. aeruginosa infection free compared to only 20% of the untreated patients. However this difference was not statistically significant. Somewhat surprisingly and distinct from what had been observed in animal models, there was no decrease in bacterial burden of any of the KB001-treated patients. On the other hand, none of the patients developed anti-KB001 antibodies during the trial suggesting that multiple doses could be given if necessary without excess inflammation or complement deposition due to the treatment with the Fab reagent.
KB001 has also been tested in a phase 1/2 randomized, double blind, placebo-controlled, single-dose, dose escalation study in CF patients infected with P. aeruginosa [130] (clinicaltrials.gov NCT00638365). Twenty-seven CF patients that were chronically infected were assigned to groups. There were no deaths or severe adverse events detected in this trial. The pharmacokinetics were similar between the 3 mg/kg and 10 mg/kg treatment groups. While not statistically significant, there was a consistent trend at 28 days towards KB001 dose-dependent reductions in sputum myeloperoxidase, IL-8, and IL-1β, and, in the 10 mg/kg group, there were significantly lower sputum neutrophil counts and neutrophil elastase levels compared to the placebo group. Similar to the mechanically ventilated patients described above [129], there was no statistical decrease in P. aeruginosa burden in the treated vs. untreated patients and further no improvement in pulmonary function [130]. The investigators leading this study suggest that the single dose is safe but that repeated doses and a longer-term study may be needed to observe statistically significant improved outcomes. This reagent has been modified to facilitate the PEGylation stop in the production process (KaloBios web site) and a study to determine whether this new product (KB001-A) will increase the time-to-need for antibiotic treatment in CF patients is currently recruiting participants (clinicaltrials.gov NCT01695343). It is speculated that since this reagent does not target essential functions that resistance to this non-antibiotic may not readily emerge.
Anti-P. aeruginosa IgY
Two different clinical trials are underway using a daily mouthwash containing antibodies to P. aeruginosa produced from eggs. Vaccinated hens produce specific IgY antibodies that are transferred to the egg yolk in large quantities. IgY antibodies have an advantage that they do not activate the complement system, bind human Fc receptors, or cross-react with human antibodies [131]. The anti-P. aeruginosa IgY antibodies appear to inhibit attachment of P. aeruginosa to epithelial cells [132]. Since eggs are a standard food source, there should be no adverse effects of this therapy, except for potential allergies.
One study sponsored by Immunsystem AB that is ongoing, but not recruiting participants (clinicaltrials.gov NCT00633191), is based on the initial studies of Kollberg et al.[132]. Hens were immunized with two different formaldehyde-fixed P. aeruginosa strains (PAO1 and Habs1). After the initial immunization the hens were given 3 boosters and eggs were collected. Antibodies were purified and CF patients gargled 50 mg of antibodies per day. This study had a small sample size, but showed a trend towards a slightly later time of acquisition of a positive P. aeruginosa culture compared to the time for patients that did not receive the treatment. These patients have continued with this treatment for 10 years and a follow-up study continues to suggest a delay in the time for first positive P. aeruginosa culture and later time until chronic infection occurs compared to those not treated [133]. Notably, the control subjects in these studies were from another country. A Phase III double-blind, placebo-controlled study to evaluate the safety and efficacy of anti-P. aeruginosa IgY antibodies to prevent acquisition of P. aeruginosa in CF patients is being sponsored by Mukoviszidose Institut gGmbH (clinicaltrials.gov NCT01455675). The plan is to enroll approximately 180 CF patients that are free of P. aeruginosa as they enter the study (i.e., not chronically colonized). Half of these patients will gargle with 70 mL of avian polyclonal anti-P. aeruginosa IgY antibodies (50 mg) every night for two minutes (for up to 24 months). The other half will gargle with the same solution without IgY. The primary outcome of the study will be the time to first isolation of P. aeruginosa as detected in sputum, throat swab, or endolaryngeal suction. The estimated date for collection of the primary outcome measurements is September 2014. Conclusions about the true efficacy of these IgY antibodies await the results of this well-controlled trial.
Anti-O11 mAb
As mentioned, LPS O antigen is generally considered the dominant protective antigen for P. aeruginosa. Kenta Biotech (Bern, Switzerland) developed a human mAb, KBPA101 (panobacumab) to the P. aeruginosa serotype O11 O polysaccharide. This antibody was generated by immunizing a volunteer with the early octovalent O-polysaccharide-toxin A conjugate vaccine (Aerugen) [134,135] followed by enriching B cells from peripheral bloods, which were immortalized. Cells producing antibodies against P. aeruginosa LPS serotype O11 (one of the more common serotypes in infection) were detected and hybridomas were produced and purified IgM/κ was formulated [136]. This monoclonal antibody, KBPA101, had opsonophagocytotic activity specifically against P. aeruginosa serotype O11. In preclinical studies using mouse models of infection, this antibody provide protection against burn wound when administer prophylactically 4 hours prior to infection as well as 4 hours post infection. It was also was injected into mice 2 hours prior to intratracheal challenge with a P. aerugionsa serotype O11 strain [136]. In this context, the mice given the mAb cleared the bacteria more readily. In follow up studies [137], mice were infected and then 4 hours later treated intravenously with KBPA101. A reduction in bacterial load was observed during infection with a serotype O11 strain with a slight decrease in lung injury; as expected no reduction was observed against a non-serotype O11 strain indicating the specificity of the response. Further, this antibody delayed death due to acute serotype O11 infection, but did not provide complete protection, suggesting that this reagent will likely need to be given in conjunction with appropriate antibiotic therapy. Importantly this antibody was found to be non-toxic in rabbits [136].
A safety and pharmacokinetics Phase 1 study of KBPA101 has been tested in healthy volunteers [138]. More recently [139], a multicenter, open-label Phase 2a study of this vaccine was performed (clinicaltrials.gov NCT00851435). Three doses of KBPA101 were given every 72 hours to a total of 18 patients with confirmed nosocomial pneumonia (15 with VAP and 3 with hospital-acquired pneumonia) due to serotype O11 P. aeruginosa. This treatment was well tolerated by patients receiving all 3 doses (among the 18 patients, 1 was excluded because the bacteria was misidentified as being serotype O11, 1 died, and 3 had serious adverse events that were evaluated to be unrelated to the administration of the vaccine). Among the 13 patients treated per protocol, 11 resolved the infection and 2 had a recurrence. The authors of this small study declared the safety of this approach and the potential clinical efficacy [139]. While P. aeruginosa serotype O11 is prominent in infection, there are a total of ~10 serotypes associated with disease [87]. Therefore, in addition to rapid diagnosis to determine whether infection is due to serotype O11, a panel of mAb directed to each of these serotypes will need to be available to combat all acute P. aeruginosa infections. Towards that end, Kenta Biotech has also developed a second mAb, this one against serotype O1 (KBPA104) (Kenta web site); however, additional reports on this reagent have not been published. Other human mAbs have been developed to multiple serotypes of LPS [140,141], but no human trials have been reported. As mentioned LPS rough mutants (that do not make the O antigen) emerge during chronic P. aeruginosa infections; mAb directed at the O antigen would therefore be ineffective in this situation, but may be useful during the initial stages of infection. These chronic isolates also are generally mucoid due to the overproduction of alginate. To target these chronic infecting isolated, human mAb to alginate [51,142] has shown efficacy in mouse models of lung and eye infections with both mucoid (alginate overproducing) as well as non-mucoid (non-alginate overproducing) [51,143], but to date protective studies in humans have not been reported.
Anti-Psl
More recently an unbiased approach was taken to identify new serotype-independent antigens for immunotherapy development, using blood from patients exposed to or recovering from P. aeruginosa infection. These samples were used to construct a phage display library of cloned scFv. This library and a control library made from blood of uninfected individuals were selected for binding to P. aeruginosa. Among the clones that were obtained were, most were not reactive with different O antigen serotype strains suggesting serotype-specificity. Those that were serotype-independent were selected and used for the generation of human IgG1 antibodies; these were also tested for in vitro opsonophagocytic activity. Among the group of antibodies that showed pronounced activity, one was further characterized. Using reactivity with mutants with known defects as a screen, this antibody was found to be specific for the polysaccharide Psl. Psl is a biofilm associated extracellular polysaccharide that is appears to block neutrophil phagocytosis. It is composed of repeating pentasaccharide units of D-mannose, D-glucose, and L-rhamnose [144]. Using this anti-Psl mAb, Cam-003, revealed that Psl was expressed on clinical isolates in vitro and in vivo. Most importantly when given intraperitoneally 24 hours prior to intranasal infection with various strains of P. aeruginosa, Cam-003 provided protection and reduced bacterial burden in the organs of mice in a concentration dependent manner. Similar results were obtained when immunized mice were infected in the scratch-injured cornea or after burns. In both cases, Cam-003 was effective at diminishing the infection [145]. Recently the epitopes of this mAb and other reacting with Psl have been mapped [146].
Synthesis of vaccine strategies
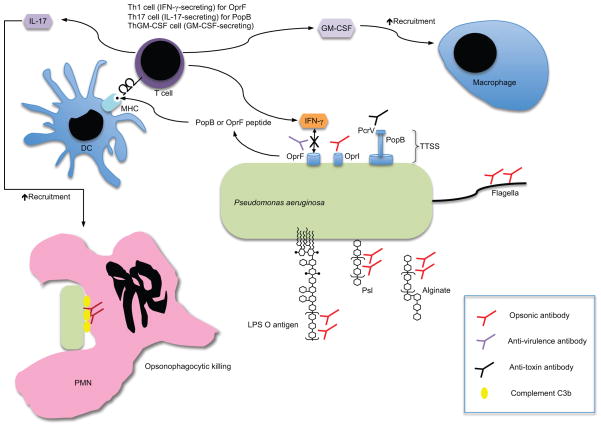
Figure 1 shows key immune mechanisms of selected vaccines for P. aeruginosa. Important humoral immune responses include enhancement of opsonophagocytosis (opsonic antibody, shown to be important for LPS O antigen, alginate, Psl, flagella, and OprI), disruption of the type III secretion system (anti-toxin antibody, shown to be important for PcrV), and interference with binding of OprF to IFN-γ (anti-virulence antibody). T cell mechanisms of protection include IFN-γ secretion (OprF), IL-17 secretion (PopB and live-attenuated vaccines), and GM-CSF secretion (live-attenuated vaccines), which will act in concert with opsonophagocytic antibodies to enhance bacterial clearance. It is likely that an effective vaccine against P. aeruginosa will need to incorporate many, if not all, of these immune mechanisms.
Fig. 1.
Key immune mechanisms of selected vaccines for Pseudomonas aeruginosa. Important antibody functions include enhancement of opsonophagocytosis (opsonic antibody, shown to be important for LPS O antigen, alginate, Psl, flagella, and OprI), disruption of the type III secretion system (anti-toxin antibody, shown to be important for PcrV), and interference with binding of OprF to IFN-γ (anti-virulence antibody). T cell mechanisms of protection include IFN-γ secretion (OprF), IL-17 secretion (PopB and live-attenuated vaccines), and GM-CSF secretion (live-attenuated vaccines), which will act in concert with opsonophagocytic antibodies to enhance bacterial clearance.
Expert Commentary and Five-year View
Much progress has been made in recent years towards an effective P. aeruginosa vaccine for use in humans, with the OprF/I vaccine showing great promise as an active vaccine (perhaps eventually combined with a Th17-stimulating antigen such as PopB) and the anti-PcrV Fab′ as a passive anti-virulence prophylactic/therapeutic (perhaps eventually combined with opsonophagocytic monoclonal antibodies to alginate, Psl, and/or O antigens). Major challenges to the development of a broadly protective vaccine for P. aeruginosa remain, however. A large part of this challenge relates to the diverse virulence mechanisms of the microbe itself. Another perhaps larger part centers on the host defects that characterize humans who get P. aeruginosa infections, whether CF patients, cancer patients with neutropenia, burned or wounded patients with impaired barriers and devitalized tissue, or ventilated patients with foreign bodies (endotracheal tubes) and impaired lung clearance mechanisms. Many of these host defects are not modeled effectively in animals, so reliable preclinical data are hard or impossible to obtain. New genomic and transcriptomic techniques relying on next-generation sequencing and applied to patient samples will likely provide valuable data in this regard over the next 5 years. In many types of P. aeruginosa infections, differentiation between colonization and infection is often difficult and leads to challenges with design of clinical trials to test vaccine candidates. These trials are even more confounded by concurrent antibiotic treatment, which can mask a potential vaccine effect. The results of the ongoing OprF/I vaccine trial in critically ill adults will provide key insights as to whether active vaccination in this setting can elicit protective immune effectors. It is likely that effective vaccines for P. aeruginosa will need to be tailored for specific types of infections in specific patient populations. Until then, the long and winding road in the quest for a P. aeruginosa vaccine will continue, bolstered by the results of new genomic and transcriptomic techniques focusing on both the microbe and the host.
Key issues.
Pseudomonas aeruginosa is known as important pathogen causing life-threatening infections in immunocompromised individuals and those with cystic fibrosis. More recently it has been gaining recognition as a nosocomial pathogen in the elderly and in combat-related wounds suggesting the appropriate target population to vaccinate.
Historically antigens for vaccine development such as LPS, alginate, and flagella have been selected empirically, but more recently high-throughput approaches have been utilized.
Despite years of effort, to date, no P. aeruginosa vaccine is available.
Passive immunotherapy may be advantageous for treating P. aeruginosa in patients unable to mount an effective immune response or after infection.
Issues with performing an adequate clinical trial are also discussed.
Footnotes
Financial disclosures:
JBG is supported by grants from the NIH and the Cystic Fibrosis Foundation. She has no competing interests. GPP is supported by grants from the NIH, the Cystic Fibrosis Foundation, and the William F. Milton Fund of Harvard Medical School.
Contributor Information
Gregory P. Priebe, Email: gregory.priebe@childrens.harvard.edu, Associate Professor of Anesthesia, Harvard Medical School, Division of Critical Care Medicine, Dept. of Anesthesiology, Perioperative and Pain Medicine, Boston Children’s Hospital, Division of Infectious Diseases, Dept. of Medicine, Boston Children’s Hospital, Division of Infectious Diseases, Dept. of Medicine, Brigham and Women’s Hospital, 181 Longwood Ave, Boston, MA 02115, Tel: 617-525-2663, Fax: 617-525-2510
Joanna B. Goldberg, Email: joanna.goldberg@emory.edu, Professor, Department of Pediatrics, Division of Pulmonology, Allergy/Immunology, Cystic Fibrosis and Sleep, Emory University School of Medicine, Rollins Research Center, 1510 Clifton Road NE, Suite 3009, Atlanta, GA 30322, Tel: 404-727-6760, Fax: 404-727-8250
References
* of interest;
** of considerable interest
- 1.Alexander JW, Fisher MW. Vaccination for Pseudomonas aeruginosa. Am J Surgery. 1970;120(4):512. doi: 10.1016/s0002-9610(70)80019-8. [DOI] [PubMed] [Google Scholar]
- 2.Lanyi B. Serological properties of Pseudomonas aeruginosa. II. Type-specific thermolabile (flagellar) antigens. Acta Microbiol Acad Sci Hung. 1970;17(1):35–48. [PubMed] [Google Scholar]
- 3.Bartell PF, Orr TE, Chudio B. Purification and chemical composition of the protective slime antigen of Pseudomonas aeruginosa. Infect Immun. 1970;2:543–548. doi: 10.1128/iai.2.5.543-548.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Döring G, Pier GB. Vaccines and immunotherapy against Pseudomonas aeruginosa. Vaccine. 2008;26(8):1011–1024. doi: 10.1016/j.vaccine.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Worgall S. 40 years on: have we finally got a vaccine for Pseudomonas aeruginosa? Future Microbiol. 2012;7(12):1333–1335. doi: 10.2217/fmb.12.106. [DOI] [PubMed] [Google Scholar]
- 6.Gaynes R, Edwards JR. Overview of nosocomial infections caused by Gram-negative bacilli. Clin Infect Dis. 2005;41(6):848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 7.Thirumala R, Ramaswamy M, Chawla S. Diagnosis and management of infectious complications in critically ill patients with cancer. Crit Care Clin. 2010;26(1):59–91. doi: 10.1016/j.ccc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Carratala J, Roson B, Fernandez-Sevilla A, Alcaide F, Gudiol F. Bacteremic pneumonia in neutropenic patients with cancer: causes, empirical antibiotic therapy, and outcome. Arch Intern Med. 1998;158(8):868–872. doi: 10.1001/archinte.158.8.868. [DOI] [PubMed] [Google Scholar]
- 9.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19(2):403–434. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray CK, Wilkins K, Molter NC, et al. Infections complicating the care of combat casualties during operations Iraqi Freedom and Enduring Freedom. J Trauma. 2011;71(1 Suppl):S62–73. doi: 10.1097/TA.0b013e3182218c99. [DOI] [PubMed] [Google Scholar]
- 11.Petersen K, Riddle MS, Danko JR, et al. Trauma-related infections in battlefield casualties from Iraq. Ann Surg. 2007;245(5):803–811. doi: 10.1097/01.sla.0000251707.32332.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folkesson A, Jelsbak L, Yang L, et al. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat Rev Microbiol. 2012;10(12):841–851. doi: 10.1038/nrmicro2907. [DOI] [PubMed] [Google Scholar]
- 13.Driebe WT., Jr Present status of contact lens-induced corneal infections. Ophthalmol Clin North Am. 2003;16(3):485–494. viii. doi: 10.1016/s0896-1549(03)00052-x. [DOI] [PubMed] [Google Scholar]
- 14.Parkins MD, Gregson DB, Pitout JD, Ross T, Laupland KB. Population-based study of the epidemiology and the risk factors for Pseudomonas aeruginosa bloodstream infection. Infection. 2010;38(1):25–32. doi: 10.1007/s15010-009-9145-9. [DOI] [PubMed] [Google Scholar]
- 15.Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173(21):1970–1978. doi: 10.1001/jamainternmed.2013.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;34(1):1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 17.McEachern R, Campbell GD. Hospital-acquired pneumonia: epidemiology, etiology, and treatment. Infect Dis Clin North Am. 1998;12(3):761–779. doi: 10.1016/s0891-5520(05)70209-9. [DOI] [PubMed] [Google Scholar]
- 18.Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33(10):2184–2193. doi: 10.1097/01.ccm.0000181731.53912.d9. [DOI] [PubMed] [Google Scholar]
- 19.Rello J, Rue M, Jubert P, et al. Survival in patients with nosocomial pneumonia: impact of the severity of illness and the etiologic agent. Crit Care Med. 1997;25(11):1862–1867. doi: 10.1097/00003246-199711000-00026. [DOI] [PubMed] [Google Scholar]
- 20.Aronson NE, Sanders JW, Moran KA. In harm’s way: infections in deployed American military forces. Clin Infect Dis. 2006;43(8):1045–1051. doi: 10.1086/507539. [DOI] [PubMed] [Google Scholar]
- 21.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168(8):918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 22.Sibley CD, Grinwis ME, Field TR, et al. Culture enriched molecular profiling of the cystic fibrosis airway microbiome. PLoS One. 2011;6(7):e22702. doi: 10.1371/journal.pone.0022702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goddard AF, Staudinger BJ, Dowd SE, et al. Direct sampling of cystic fibrosis lungs indicates that DNA-based analyses of upper-airway specimens can misrepresent lung microbiota. Proc Natl Acad Sci USA. 2012;109(34):13769–13774. doi: 10.1073/pnas.1107435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madan JC, Koestler DC, Stanton BA, et al. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. MBio. 2012;3(4) doi: 10.1128/mBio.00251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *25.Westritschnig K, Hochreiter R, Wallner G, Firbas C, Schwameis M, Jilma B. A randomized, placebo-controlled phase I study assessing the safety and immunogenicity of a Pseudomonas aeruginosa hybrid outer membrane protein OprF/I vaccine (IC43) in healthy volunteers. Hum Vaccin Immunother. 2013;10(1) doi: 10.4161/hv.26565. Testing of a hybrid outer member protein vaccine in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher MW, Devlin HB, Gnabski F. New immunotype schema for Pseudomonas aeruginosa based on protective antigens. J Bacteriol. 1969;98:835–836. doi: 10.1128/jb.98.2.835-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pier GB. Promises and pitfalls of Pseudomonas aeruginosa lipopolysaccharide as a vaccine antigen. Carbohydr Res. 2003;338(23):2549–2556. doi: 10.1016/s0008-6215(03)00312-4. [DOI] [PubMed] [Google Scholar]
- 28.Knirel YA. Polysaccharide antigens of Pseudomonas aeruginosa. CRC Crit Rev Microbiol. 1990;17:273–304. doi: 10.3109/10408419009105729. [DOI] [PubMed] [Google Scholar]
- 29.Alexander JW, Fisher MW, MacMillan BG. Immunological control of Pseudomonas infection in burn patients: a clinical evaluation. Arch Surgery. 1971;102(1):31–35. doi: 10.1001/archsurg.1971.01350010033008. [DOI] [PubMed] [Google Scholar]
- 30.Young LS, Meyer RD, Armstrong D. Pseudomonas aeruginosa vaccine in cancer patients. Ann Intern Med. 1973;79(4):518–527. doi: 10.7326/0003-4819-79-4-518. [DOI] [PubMed] [Google Scholar]
- 31.Haghbin M, Armstrong D, Murphy ML. Controlled prospective trial of Pseudomonas aeruginosa vaccine in children with acute leukemia. Cancer. 1973;32(4):761–766. doi: 10.1002/1097-0142(197310)32:4<761::aid-cncr2820320405>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 32.Pennington JE, Reynolds HY, Wood RE, Robinson RA, Levine AS. Use of a Pseudomonas aeruginosa vaccine in pateints with acute leukemia and cystic fibrosis. Am J Med. 1975;58(5):629–636. doi: 10.1016/0002-9343(75)90498-2. [DOI] [PubMed] [Google Scholar]
- 33.Cryz SJ, Jr, Furer E, Germanier R. Protection against fatal Pseudomonas aeruginosa burn wound sepsis by immunization with lipopolysaccharide and high-molecular-weight polysaccharide. Infect Immun. 1984;43(3):795–799. doi: 10.1128/iai.43.3.795-799.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pier GB, Thomas D, Small G, Siadak A, Zweerink H. In vitro and in vivo activity of polyclonal and monoclonal human immunoglobulins G, M, and A against Pseudomonas aeruginosa lipopolysaccharide. Infect Immun. 1989;57(1):174–179. doi: 10.1128/iai.57.1.174-179.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatano K, Pier GB. Complex serology and immune response of mice to variant high-molecular-weight O polysaccharides isolated from Pseudomonas aeruginosa serogroup O2 strains. Infect Immun. 1998;66(8):3719–3726. doi: 10.1128/iai.66.8.3719-3726.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hatano K, Boisot S, DesJardins D, Wright DC, Brisker J, Pier GB. Immunogenic and antigenic properties of a heptavalent high-molecular-weight O-polysaccharide vaccine derived from Pseudomonas aeruginosa. Infect Immun. 1994;62(9):3608–3616. doi: 10.1128/iai.62.9.3608-3616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donta ST, Peduzzi P, Cross AS, et al. Immunoprophylaxis against Klebsiella and Pseudomonas aeruginosa infections. The Federal Hyperimmune Immunoglobulin Trial Study Group. J Infect Dis. 1996;174(3):537–543. doi: 10.1093/infdis/174.3.537. [DOI] [PubMed] [Google Scholar]
- 38.Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat Med. 2012;18(4):509–519. doi: 10.1038/nm.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pier GB, Grout M, Zaidi TS, et al. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science. 1996;271(5245):64–67. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goss CH, Ratjen F. Update in cystic fibrosis 2012. Am J Respir Crit Care Med. 2013;187(9):915–919. doi: 10.1164/rccm.201301-0184UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pezzulo AA, Tang XX, Hoegger MJ, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487(7405):109–113. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen SS, Hoiby N, Espersen F, Koch C. Role of alginate in infection with mucoid Pseudomonas aeruginosa in cystic fibrosis. Thorax. 1992;47:6–13. doi: 10.1136/thx.47.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parad RB, Gerard CJ, Zurakowski D, Nichols DP, Pier GB. Pulmonary outcome in cystic fibrosis is influenced primarily by mucoid Pseudomonas aeruginosa infection and immune status and only modestly by genotype. Infect Immun. 1999;67(9):4744–4750. doi: 10.1128/iai.67.9.4744-4750.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burns JL, Gibson RL, McNamara S, et al. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J Infect Dis. 2001;183(3):444–452. doi: 10.1086/318075. [DOI] [PubMed] [Google Scholar]
- 45.Hancock REW, Mutharia LM, Chan L, Darveau RP, Speert DP, Pier GB. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: A class of serum-sensitive, non-typeable strains deficient in lipopolysaccharide O side-chains. Infect Immun. 1983;42:170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pier GB, Saunders JM, Ames P, et al. Opsonophagocytic killing antibody to Pseudomonas aeruginosa mucoid exopolysaccharide in older noncolonized patients with cystic fibrosis. New Engl J Med. 1987;317(13):793–798. doi: 10.1056/NEJM198709243171303. [DOI] [PubMed] [Google Scholar]
- 47.Pier GB, Small GJ, Warren HB. Protection against mucoid Pseudomonas aeruginosa in rodent models of endobronchial infections. Science. 1990;249(4968):537–540. doi: 10.1126/science.2116663. [DOI] [PubMed] [Google Scholar]
- 48.Pier GB, DesJardin D, Grout M, et al. Human immune response to Pseudomonas aeruginosa mucoid exopolysaccharide (alginate) vaccine. Infect Immun. 1994;62(9):3972–3979. doi: 10.1128/iai.62.9.3972-3979.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pier GB, Takeda S, Grout M, Markham RB. Immune complexes from immunized mice and infected cystic fibrosis patients mediate murine and human T cell killing of hybridomas producing protective, opsonic antibody to Pseudomonas aeruginosa. J Clin Invest. 1993;91(3):1079–1087. doi: 10.1172/JCI116265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bragonzi A, Worlitzsch D, Pier GB, et al. Nonmucoid Pseudomonas aeruginosa expresses alginate in the lungs of patients with cystic fibrosis and in a mouse model. J Infect Dis. 2005;192(3):410–419. doi: 10.1086/431516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *51.Pier GB, Boyer D, Preston M, et al. Human monoclonal antibodies to Pseudomonas aeruginosa alginate that protect against infection by both mucoid and nonmucoid strains. J Immunol. 2004;173(9):5671–5678. doi: 10.4049/jimmunol.173.9.5671. Recognition of the importance of alginate expressed by P. aeruginosa even during acute infections. [DOI] [PubMed] [Google Scholar]
- 52.Johansen HK, Gotzsche PC. Vaccines for preventing infection with Pseudomonas aeruginosa in cystic fibrosis. Cochrane Database Syst Rev. 2013;6:CD001399. doi: 10.1002/14651858.CD001399.pub3. [DOI] [PubMed] [Google Scholar]
- 53.Doering G, Meisner C, Stern M. A double-blind randomized placebo-controlled phase III study of a Pseudomonas aeruginosa flagella vaccine in cystic fibrosis patients. Proc Natl Acad Sci USA. 2007;104(26):11020–11025. doi: 10.1073/pnas.0702403104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campodonico VL, Llosa NJ, Bentancor LV, Maira-Litran T, Pier GB. Efficacy of a conjugate vaccine containing polymannuronic acid and flagellin against experimental Pseudomonas aeruginosa lung infection in mice. Infect Immun. 2011;79(8):3455–3464. doi: 10.1128/IAI.00157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lang AB, Rudeberg A, Schoni MH, Que JU, Furer E, Schaad UB. Vaccination of cystic fibrosis patients against Pseudomonas aeruginosa reduces the proportion of patients infected and delays time to infection. Pediatr Infect Dis J. 2004;23(6):504–510. doi: 10.1097/01.inf.0000129688.50588.ac. [DOI] [PubMed] [Google Scholar]
- 56.Cryz SJ, Jr, Lang A, Rudeberg A, et al. Immunization of cystic fibrosis patients with a Pseudomonas aeruginosa O-polysaccharide-toxin A conjugate vaccine. Behring Inst Mitt. 1997;(98):345–349. [PubMed] [Google Scholar]
- 57.Priebe GP, Brinig MM, Hatano K, et al. Construction and characterization of a live, attenuated aroA deletion mutant of Pseudomonas aeruginosa as a candidate intranasal vaccine. Infect Immun. 2002;70(3):1507–1517. doi: 10.1128/IAI.70.3.1507-1517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Priebe GP, Meluleni GJ, Coleman FT, Goldberg JB, Pier GB. Protection against fatal Pseudomonas aeruginosa pneumonia in mice after nasal immunization with a live, attenuated aroA deletion mutant. Infect Immun. 2003;71(3):1453–1461. doi: 10.1128/IAI.71.3.1453-1461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Priebe GP, Walsh RL, Cederroth TA, et al. IL-17 is a critical component of vaccine-induced protection against lung infection by lipopolysaccharide-heterologous strains of Pseudomonas aeruginosa. J Immunol. 2008;181(7):4965–4975. doi: 10.4049/jimmunol.181.7.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 61.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13(2):139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 62.Kolls JK, Kanaly ST, Ramsay AJ. Interleukin-17: an emerging role in lung inflammation. Am J Respir Cell Mol Biol. 2003;28(1):9–11. doi: 10.1165/rcmb.2002-0255PS. [DOI] [PubMed] [Google Scholar]
- 63.Ye P, Garvey PB, Zhang P, et al. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25(3):335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 64.Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin-17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194(4):519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen K, McAleer JP, Lin Y, et al. Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity. 2011;35(6):997–1009. doi: 10.1016/j.immuni.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177(1):566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 67.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of TH17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 69.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 71.Codarri L, Gyulveszi G, Tosevski V, et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12(6):560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 72.El-Behi M, Ciric B, Dai H, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12(6):568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Happel KI, Zheng M, Young E, et al. Cutting Edge: Roles of Toll-Like Receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003;170(9):4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McAllister F, Henry A, Kreindler JL, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol. 2005;175(1):404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tiringer K, Treis A, Fucik P, et al. A Th17- and Th2-skewed cytokine profile in cystic fibrosis lungs represents a potential risk factor for Pseudomonas aeruginosa infection. Am J Respir Crit Care Med. 2013;187(6):621–629. doi: 10.1164/rccm.201206-1150OC. [DOI] [PubMed] [Google Scholar]
- 76.Tan HL, Regamey N, Brown S, Bush A, Lloyd CM, Davies JC. The Th17 pathway in cystic fibrosis lung disease. Am J Respir Crit Care Med. 2011;184(2):252–258. doi: 10.1164/rccm.201102-0236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sorensen RU, Stern RC, Polmar SH. Cellular immunity to bacteria: Impairment of in vitro lymphocyte responses to Pseudomonas aeruginosa in cystic fibrosis patients. Infect Immun. 1977;18(3):735–740. doi: 10.1128/iai.18.3.735-740.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sorensen RU, Stern RC, Polmar SH. Lymphocyte responsiveness to Pseudomonas aeruginosa in cystic fibrosis: Relationship to status of pulmonary disease in sibling pairs. J Pediatr. 1978;93(2):201–205. doi: 10.1016/s0022-3476(78)80496-x. [DOI] [PubMed] [Google Scholar]
- 79.Krauss RD, Bubien JK, Drumm ML, et al. Transfection of wild-type CFTR into cystic fibrosis lymphocytes restores chloride conductance at G1 of the cell cycle. EMBO J. 1992;11(3):875–883. doi: 10.1002/j.1460-2075.1992.tb05125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kamei A, Wu W, Traficante DC, et al. Collaboration between macrophages and vaccine-induced CD4+ T cells confers protection against lethal Pseudomonas aeruginosa pneumonia during neutropenia. J Infect Dis. 2013;207(1):39–49. doi: 10.1093/infdis/jis657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koh AY, Priebe GP, Ray C, Van Rooijen N, Pier GB. Inescapable need for neutrophils as mediators of cellular innate immunity to acute Pseudomonas aeruginosa pneumonia. Infect Immun. 2009;77(12):5300–5310. doi: 10.1128/IAI.00501-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kamei A, Coutinho-Sledge YS, Goldberg JB, Priebe GP, Pier GB. Mucosal vaccination with a multivalent, live-attenuated vaccine induces multifactorial immunity against Pseudomonas aeruginosa acute lung infection. Infect Immun. 2011;79(3):1289–1299. doi: 10.1128/IAI.01139-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu W, Huang J, Traficante DC, Pier GB, Lory S, Priebe GP. Construction and evaluation of live-attenuated vaccines for Pseudomonas aeruginosa serogroup O11 strains. American Society for Microbiology Meeting; San Francisco, CA. Jun, 2012. p. abstract 2339. [Google Scholar]
- 84.Zaidi TS, Priebe GP, Pier GB. A live-attenuated Pseudomonas aeruginosa vaccine elicits outer membrane protein-specific active and passive protection against corneal infection. Infect Immun. 2006;74(2):975–983. doi: 10.1128/IAI.74.2.975-983.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zaidi TS, Lyczak J, Preston M, Pier GB. Cystic fibrosis transmembrane conductance regulator-mediated corneal epithelial cell ingestion of Pseudomonas aeruginosa is a key component in the pathogenesis of experimental murine keratitis. Infect Immun. 1999;67(3):1481–1492. doi: 10.1128/iai.67.3.1481-1492.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zaidi TS, Zaidi T, Pier GB, Priebe GP. Topical neutralization of interleukin-17 during experimental Pseudomonas aeruginosa corneal infection promotes bacterial clearance and reduces pathology. Infect Immun. 2012;80(10):3706–3712. doi: 10.1128/IAI.00249-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Faure K, Shimabukuro D, Ajayi T, Allmond LR, Sawa T, Wiener-Kronish JP. O-Antigen serotypes and type III secretory toxins in clinical isolates of Pseudomonas aeruginosa. J Clin Microbiol. 2003;41(5):2158–2160. doi: 10.1128/JCM.41.5.2158-2160.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.DiGiandomenico A, Rao J, Goldberg JB. Oral vaccination of BALB/c mice with Salmonella enterica Serovar Typhimurium expressing Pseudomonas aeruginosa O antigen promotes increased survival in an acute fatal pneumonia model. Infect Immun. 2004;72(12):7012–7021. doi: 10.1128/IAI.72.12.7012-7021.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DiGiandomenico A, Rao J, Harcher K, et al. Intranasal immunization with heterologously expressed polysaccharide protects against multiple Pseudomonas aeruginosa infections. Proc Natl Acad Sci USA. 2007;104(11):4624–4629. doi: 10.1073/pnas.0608657104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scarff JM, Goldberg JB. Vaccination against Pseudomonas aeruginosa pneumonia in immunocompromised mice. Clin Vaccine Immunol. 2007;15(2):367–375. doi: 10.1128/CVI.00419-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **91.Wu W, Huang J, Duan B, et al. Th17-stimulating protein vaccines confer protection against Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2012;186(5):420–427. doi: 10.1164/rccm.201202-0182OC. Defines new P. aeruginosa antigens for vaccine development that elicit Th17 immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Montor WR, Huang J, Hu Y, et al. Genome-wide study of Pseudomonas aeruginosa outer membrane protein immunogenicity using self-assembling protein microarrays. Infect Immun. 2009;77(11):4877–4886. doi: 10.1128/IAI.00698-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feltman H, Schulert G, Khan S, Jain M, Peterson L, Hauser AR. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology. 2001;147(Pt 10):2659–2669. doi: 10.1099/00221287-147-10-2659. [DOI] [PubMed] [Google Scholar]
- 94.Koh AY, Mikkelsen PJ, Smith RS, et al. Utility of in vivo transcription profiling for identifying Pseudomonas aeruginosa genes needed for gastrointestinal colonization and dissemination. PLoS One. 2010;5(12):e15131. doi: 10.1371/journal.pone.0015131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smith EE, Sims EH, Spencer DH, Kaul R, Olson MV. Evidence for diversifying selection at the pyoverdine locus of Pseudomonas aeruginosa. J Bacteriol. 2005;187(6):2138–2147. doi: 10.1128/JB.187.6.2138-2147.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pirnay JP, Bilocq F, Pot B, et al. Pseudomonas aeruginosa population structure revisited. PLoS One. 2009;4(11):e7740. doi: 10.1371/journal.pone.0007740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smith EE, Buckley DG, Wu Z, et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci USA. 2006;103(22):8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kankkunen P, Teirila L, Rintahaka J, Alenius H, Wolff H, Matikainen S. (1,3)-beta-glucans activate both dectin-1 and NLRP3 inflammasome in human macrophages. J Immunol. 2010;184(11):6335–6342. doi: 10.4049/jimmunol.0903019. [DOI] [PubMed] [Google Scholar]
- 99.Zygmunt BM, Rharbaoui F, Groebe L, Guzman CA. Intranasal immunization promotes Th17 immune responses. J Immunol. 2009;183(11):6933–6938. doi: 10.4049/jimmunol.0901144. [DOI] [PubMed] [Google Scholar]
- 100.Leibundgut-Landmann S, Gross O, Robinson MJ, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8(6):630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 101.Brennan FR, Jones TD, Gilleland LB, et al. Pseudomonas aeruginosa outer-membrane protein F epitopes are highly immunogenic in mice when expressed on a plant virus. Microbiology. 1999;145(Pt 1):211–220. doi: 10.1099/13500872-145-1-211. [DOI] [PubMed] [Google Scholar]
- 102.Mansouri E, Gabelsberger J, Knapp B, et al. Safety and immunogenicity of a Pseudomonas aeruginosa hybrid outer membrane protein F-I vaccine in human volunteers. Infect Immun. 1999;67(3):1461–1470. doi: 10.1128/iai.67.3.1461-1470.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Matthews-Greer JM, Robertson DE, LBG, Gilleland HE., Jr Pseudomonas aeruginosa outer membrane protein F produced in Escherichia coli retains vaccine efficacy. Curr Microbiol. 1990;20:171–175. [Google Scholar]
- 104.Krause A, Whu WZ, Qiu J, et al. RGD capsid modification enhances mucosal protective immunity of a non-human primate adenovirus vector expressing Pseudomonas aeruginosa OprF. Clin Exp Immunol. 2013;173(2):230–241. doi: 10.1111/cei.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sharma A, Krause A, Xu Y, Sung B, Wu W, Worgall S. Adenovirus-based vaccine with epitopes incorporated in novel fiber sites to induce protective immunity against Pseudomonas aeruginosa. PLoS One. 2013;8(2):e56996. doi: 10.1371/journal.pone.0056996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Toth A, Schodel F, Duchene M, et al. Protection of immunosuppressed mice against translocation of Pseudomonas aeruginosa from the gut by oral immunization with recombinant Pseudomonas aeruginosa outer membrane protein I expressing Salmonella dublin. Vaccine. 1994;12(13):1215–1221. doi: 10.1016/0264-410x(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 107.Duchene M, Barron C, Schweizer A, von Specht BU, Domdey H. Pseudomonas aeruginosa outer membrane lipoprotein I gene: molecular cloning, sequence, and expression in Escherichia coli. J Bacteriol. 1989;171(8):4130–4137. doi: 10.1128/jb.171.8.4130-4137.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Finke M, Duchene M, Eckhardt A, Domdey H, von Specht B-U. Protection against experimental Pseudomonas aeruginosa infection by recombinant P. aeruginosa lipoprotein I expressed in Escherichia coli. Infect Immun. 1990;58:2241–2244. doi: 10.1128/iai.58.7.2241-2244.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mutharia LM, Nicas TI, Hancock RE. Outer membrane proteins of Pseudomonas aeruginosa serotype strains. J Infect Dis. 1982;146(6):770–779. doi: 10.1093/infdis/146.6.770. [DOI] [PubMed] [Google Scholar]
- 110.Ding B, von Specht BU, Li Y. OprF/I-vaccinated sera inhibit binding of human interferon-gamma to Pseudomonas aeruginosa. Vaccine. 2010;28(25):4119–4122. doi: 10.1016/j.vaccine.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 111.Gilleland HE, Jr, Parker MG, Matthews JM, Berg RD. Use of a purified outer membrane protein F (porin) preparation of Pseudomonas aeruginosa as a protective vaccine in mice. Infect Immun. 1984;44(1):49–54. doi: 10.1128/iai.44.1.49-54.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fox CW, Campbell GD, Jr, Anderson WM, Zavecz JH, Gilleland LB, Gilleland HE., Jr Preservation of pulmonary function by an outer membrane protein F vaccine. A study in rats with chronic pulmonary infection caused by Pseudomonas aeruginosa. Chest. 1994;105(5):1545–1550. doi: 10.1378/chest.105.5.1545. [DOI] [PubMed] [Google Scholar]
- 113.Sharma A, Krause A, Worgall S. Recent developments for Pseudomonas vaccines. Hum Vaccin. 2011;7(10):999–1011. doi: 10.4161/hv.7.10.16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hughes EE, Gilleland HE., Jr Ability of synthetic peptides representing epitopes of outer membrane protein F of Pseudomonas aeruginosa to afford protection against P. aeruginosa infection in a murine acute pneumonia model. Vaccine. 1995;13(18):1750–1753. doi: 10.1016/0264-410x(95)00166-x. [DOI] [PubMed] [Google Scholar]
- 115.Worgall S, Kikuchi T, Singh R, Martushova K, Lande L, Crystal RG. Protection against Pseudomonas aeruginosa chronic lung infection in mice by genetic immunization against outer membrane protein F (OprF) of P. aeruginosa. Infect Immun. 2001;69(7):4521–4527. doi: 10.1128/IAI.69.7.4521-4527.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.von Specht BU, Knapp B, Muth G, et al. Protection of immunocompromised mice against lethal infection with Pseudomonas aeruginosa by active or passive immunization with recombinant P. aeruginosa outer membrane protein F and outer membrane protein I fusion proteins. Infect Immun. 1995;63(5):1855–1862. doi: 10.1128/iai.63.5.1855-1862.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Knapp B, Hundt E, Lenz U, et al. A recombinant hybrid outer membrane protein for vaccination against Pseudomonas aeruginosa. Vaccine. 1999;17(13–14):1663–1666. doi: 10.1016/s0264-410x(98)00420-4. [DOI] [PubMed] [Google Scholar]
- 118.Weimer ET, Lu H, Kock ND, Wozniak DJ, Mizel SB. A fusion protein vaccine containing OprF epitope 8, OprI, and type A and B flagellins promotes enhanced clearance of nonmucoid Pseudomonas aeruginosa. Infect Immun. 2009;77(6):2356–2366. doi: 10.1128/IAI.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Weimer ET, Ervin SE, Wozniak DJ, Mizel SB. Immunization of young African green monkeys with OprF epitope 8-OprI-type A- and B-flagellin fusion proteins promotes the production of protective antibodies against nonmucoid Pseudomonas aeruginosa. Vaccine. 2009;27(48):6762–6769. doi: 10.1016/j.vaccine.2009.08.080. [DOI] [PubMed] [Google Scholar]
- 120.von Specht BU, Lucking HC, Blum B, Schmitt A, Hungerer KD, Domdey H. Safety and immunogenicity of a Pseudomonas aeruginosa outer membrane protein I vaccine in human volunteers. Vaccine. 1996;14(12):1111–1117. doi: 10.1016/0264-410x(96)00054-0. [DOI] [PubMed] [Google Scholar]
- 121.Wu L, Estrada O, Zaborina O, et al. Recognition of host immune activation by Pseudomonas aeruginosa. Science. 2005;309(5735):774–777. doi: 10.1126/science.1112422. [DOI] [PubMed] [Google Scholar]
- 122.Worgall S, Krause A, Rivara M, et al. Protection against P. aeruginosa with an adenovirus vector containing an OprF epitope in the capsid. J Clin Invest. 2005;115:1281–1289. doi: 10.1172/JCI23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sato H, Frank DW. Multi-functional characteristics of the Pseudomonas aeruginosa type III needle-tip protein, PcrV; comparison to orthologs in other Gram-negative bacteria. Front Microbiol. 2011;2:142. doi: 10.3389/fmicb.2011.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sawa T, Yahr TL, Ohara M, et al. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat Med. 1999;5(4):392–398. doi: 10.1038/7391. [DOI] [PubMed] [Google Scholar]
- 125.Shime N, Sawa T, Fujimoto J, et al. Therapeutic administration of anti-PcrV F(ab′)(2) in sepsis associated with Pseudomonas aeruginosa. J Immunol. 2001;167(10):5880–5886. doi: 10.4049/jimmunol.167.10.5880. [DOI] [PubMed] [Google Scholar]
- 126.Frank DW, Vallis A, Wiener-Kronish JP, et al. Generation and characterization of a protective monoclonal antibody to Pseudomonas aeruginosa PcrV. J Infect Dis. 2002;186(1):64–73. doi: 10.1086/341069. [DOI] [PubMed] [Google Scholar]
- **127.Baer M, Sawa T, Flynn P, et al. An engineered human antibody Fab fragment specific for Pseudomonas aeruginosa PcrV antigen has potent antibacterial activity. Infect Immun. 2009;77(3):1083–1090. doi: 10.1128/IAI.00815-08. Efficacy of anti-PcrV is due to inhibiting effector translocation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chapman AP. PEGylated antibodies and antibody fragments for improved therapy: a review. Adv Drug Deliv Rev. 2002;54(4):531–545. doi: 10.1016/s0169-409x(02)00026-1. [DOI] [PubMed] [Google Scholar]
- 129.Francois B, Luyt CE, Dugard A, et al. Safety and pharmacokinetics of an anti-PcrV PEGylated monoclonal antibody fragment in mechanically ventilated patients colonized with Pseudomonas aeruginosa: a randomized, double-blind, placebo-controlled trial. Crit Care Med. 2012;40(8):2320–2326. doi: 10.1097/CCM.0b013e31825334f6. [DOI] [PubMed] [Google Scholar]
- 130.Milla CE, Chmiel JF, Accurso FJ, et al. Anti-PcrV antibody in cystic fibrosis: A novel approach targeting Pseudomonas aeruginosa airway infection. Pediatr Pulmonol. 2013 Sep 9; doi: 10.1002/ppul.22890. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carlander D, Kollberg H, Wejaker PE, Larsson A. Peroral immunotherapy with yolk antibodies for the prevention and treatment of enteric infections. Immunol Res. 2000;21(1):1–6. doi: 10.1385/IR:21:1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kollberg H, Carlander D, Olesen H, Wejaker PE, Johannesson M, Larsson A. Oral administration of specific yolk antibodies (IgY) may prevent Pseudomonas aeruginosa infections in patients with cystic fibrosis: a phase I feasibility study. Pediatr Pulmonol. 2003;35(6):433–440. doi: 10.1002/ppul.10290. [DOI] [PubMed] [Google Scholar]
- 133.Nilsson E, Larsson A, Olesen HV, Wejaker PE, Kollberg H. Good effect of IgY against Pseudomonas aeruginosa infections in cystic fibrosis patients. Pediatr Pulmonol. 2008;43(9):892–899. doi: 10.1002/ppul.20875. [DOI] [PubMed] [Google Scholar]
- 134.Cryz SJ, Jr, Furer E, Sadoff JC, Germanier R. A polyvalent Pseudomonas aeruginosa O-polysaccharide-toxin A conjugate vaccine. Antibiot Chemother. 1987;39:249–255. doi: 10.1159/000414350. [DOI] [PubMed] [Google Scholar]
- 135.Cryz SJ, Jr, Sadoff JC, Furer E. Octavalent Pseudomonas aeruginosa O-polysaccharide-toxin A conjugate vaccine. Microb Pathog. 1989;6(1):75–80. doi: 10.1016/0882-4010(89)90010-7. [DOI] [PubMed] [Google Scholar]
- 136.Horn MP, Zuercher AW, Imboden MA, et al. Preclinical in vitro and in vivo characterization of the fully human monoclonal IgM antibody KBPA101 specific for Pseudomonas aeruginosa serotype IATS-O11. Antimicrob Agents Chemother. 2010;54(6):2338–2344. doi: 10.1128/AAC.01142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Secher T, Fauconnier L, Szade A, et al. Anti-Pseudomonas aeruginosa serotype O11 LPS immunoglobulin M monoclonal antibody panobacumab (KBPA101) confers protection in a murine model of acute lung infection. J Antimicrob Chemother. 2011;66(5):1100–1109. doi: 10.1093/jac/dkr038. [DOI] [PubMed] [Google Scholar]
- 138.Lazar H, Horn MP, Zuercher AW, et al. Pharmacokinetics and safety profile of the human anti-Pseudomonas aeruginosa serotype O11 immunoglobulin M monoclonal antibody KBPA-101 in healthy volunteers. Antimicrob Agents Chemother. 2009;53(8):3442–3446. doi: 10.1128/AAC.01699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *139.Lu Q, Rouby JJ, Laterre PF, et al. Pharmacokinetics and safety of panobacumab: specific adjunctive immunotherapy in critical patients with nosocomial Pseudomonas aeruginosa O11 pneumonia. J Antimicrob Chemother. 2011;66(5):1110–1116. doi: 10.1093/jac/dkr046. Safety of passive antibodies to P. aeruginosa serotype O11. [DOI] [PubMed] [Google Scholar]
- 140.Hemachandra S, Kamboj K, Copfer J, Pier G, Green LL, Schreiber JR. Human monoclonal antibodies against Pseudomonas aeruginosa lipopolysaccharide derived from transgenic mice containing megabase human immunoglobulin loci are opsonic and protective against fatal Pseudomonas sepsis. Infect Immun. 2001;69(4):2223–2229. doi: 10.1128/IAI.69.4.2223-2229.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lai Z, Kimmel R, Petersen S, et al. Multi-valent human monoclonal antibody preparation against Pseudomonas aeruginosa derived from transgenic mice containing human immunoglobulin loci is protective against fatal Pseudomonas sepsis caused by multiple serotypes. Vaccine. 2005;23(25):3264–3271. doi: 10.1016/j.vaccine.2005.01.088. [DOI] [PubMed] [Google Scholar]
- 142.Zaidi T, Bajmoczi M, Golan DE, Pier GB. Disruption of CFTR-dependent lipid rafts reduces bacterial levels and corneal disease in a murine model of Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2008;49(3):1000–1009. doi: 10.1167/iovs.07-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zaidi TS, Zaidi T, Pier GB. Role of neutrophils, MyD88-mediated neutrophil recruitment, and complement in antibody-mediated defense against Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2010;51(4):2085–2093. doi: 10.1167/iovs.09-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Byrd MS, Sadovskaya I, Vinogradov E, et al. Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Mol Microbiol. 2009;73(4):622–638. doi: 10.1111/j.1365-2958.2009.06795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **145.DiGiandomenico A, Warrener P, Hamilton M, et al. Identification of broadly protective human antibodies to Pseudomonas aeruginosa exopolysaccharide Psl by phenotypic screening. J Exp Med. 2012;209(7):1273–1287. doi: 10.1084/jem.20120033. Novel approach to recognize serotype independent antigens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li H, Mo KF, Wang Q, Stover CK, Digiandomenico A, Boons GJ. Epitope mapping of monoclonal antibodies using synthetic oligosaccharides uncovers novel aspects of immune recognition of the Psl exopolysaccharide of Pseudomonas aeruginosa. Chemistry. 2013 doi: 10.1002/chem.201302916. [DOI] [PubMed] [Google Scholar]