Abstract
Hamsters are often used to determine the effects of various dietary ingredients on the development of cardiovascular disease (CVD). The study was conducted to obtain baseline data on CVD risk factors and mRNA expression of selected genes in hamsters fed a standard maintenance diet (STD) for 24 wk, beginning when animals were 7 wk old. Plasma triacylglycerol and aortic cholesteryl ester concentrations did not significantly change during the study. Total plasma cholesterol (75.9–127.9 mg/dL), LDL- (3.2–12.2 mg/dL), and HDL- (53.8–98.9 mg/dL) cholesterols increased over the 24wk study. Aortic total cholesterol increased from 9.72 to 12.20 μg/mg protein, whereas aortic cholesteryl ester, a measure of atherosclerosis development, was less than 0.18 μg/mg protein throughout the study. The expression of hepatic endothelin 1, peroxisome proliferator-activated receptor α , and hepatic cholesterol 7-α-hydroxylase mRNA did not change throughout the study, indicating that fatty acid β-oxidation and cholesterol metabolism remained consistent. The mRNA expression of ATP-binding cassette, subfamily B member 11 increased between wk 0 and 8 but then remained unchanged, suggesting increased requirements for cholesterol in early growth. These results indicate that the consumption of a STD does not increase atherosclerotic disease risk factors in golden Syrian hamsters through 31 wk of age.
Abbreviations: ABCB11, ATP-binding cassette subfamily B member 11; C, cholesterol; CVD, cardiovascular disease; CYP7A1, cholesterol 7-α-hydroxylase; HFHC, high-fat, high-cholesterol; PPARα,peroxisome proliferator-activated receptor α; STD, standard diet
Cardiovascular disease (CVD) is a leading cause of death in the United States,5 and although it has been extensively investigated, the mechanism of disease progression and the effects of various dietary components are incompletely understood. Atherosclerosis and hypertension are prominent factors in CVD development. Atherosclerosis development is generally asymptomatic and can begin as early as childhood but usually is not discovered until much later in life. Rodent models are frequently used to study atherosclerotic disease and the effects of various foods and drugs on atherosclerotic development.9 Golden Syrian hamsters are a common model used when evaluating atherosclerosis and CVD risk factors because they closely resemble humans in regard to the rate of hepatic cholesterol synthesis1 and because hamsters develop atherosclerotic disease in response to a diet high in saturated fat and cholesterol.29,36 The blood cholesterol distribution of hamsters that consume a high-fat, high-cholesterol (HFHC) diet is similar to that in humans,22 in that the major plasma cholesterol carrier becomes LDL-cholesterol (LDL-C).29 Unlike rats and mice but like humans, hamsters have cholesterol ester transport protein.14 In addition, hamsters have comparable bile acid metabolism to humans.22 Bile acids synthesized from cholesterol in the liver facilitate the absorption of cholesterol, dietary lipid, and fat-soluble vitamins from the intestinal lumen. The synthesis of the rate-limiting component for the conversion of cholesterol to bile acid, cholic acid, is controlled by the gene cholesterol 7-α-hydroxylase (CYP7A1).26 Studies of human liver cells30 and reports of human CYP7A1 deficiency32 suggest that the dysfunction of the gene may result in hypercholesterolemia. The protein ATP-binding cassette subfamily B member 11 (ABCB11) mediates biliary cholesterol excretion; this protein is the major bile salt export pump, which transports cholic acid from the hepatocytes to bile. Overexpression of ABCB11 promote hypercholesterolemia and obesity in mice.15 Peroxisome proliferator-activated receptor α (PPARα) is predominantly involved in fatty acid and lipid catabolism.28 In the liver, the activation of PPARα leads to a reduction in plasma triacylglycerols and the upregulation of hepatic apo A-I and A-II, leading to an increase in circulating HDL-C.31 Compounds that compete for PPARα binding sites have been reported to slow the progression of CVD.45,46 The protein endothelin 1 (ET1) is produced by a variety of cells and performs a range of functions important in hypertension, atherosclerosis, and cardiac disorders.18 In addition, ET1 is involved in pulmonary, liver, and renal diseases in rats, hamsters, and humans.17,23,27 In the liver, elevated levels of ET1 can cause cirrhosis, fibrogenesis, portal hypertension, and biliary obstruction.17 Hamsters as a research model have limited utility, as do all animal models, but overall they are an appropriate model for studying the effects of various diets on blood chemistry risk factors and the development of diet-induced atherosclerosis.29 To our knowledge, there are no published studies that evaluate the potential of age-associated effects associated with the use of male golden Syrian hamsters. A study in which 4 strains of golden Syrian hamsters (age, 5 to 8 wk) were fed a cholesterol-free diet for 3 wk revealed differences in plasma cholesterol concentrations, suggesting that genetic lines may differ regarding this marker.41 Some data have been published on total cholesterol and cholesterol distributions in hamsters consuming standard breeding diets, but information on the effect of age are limited by the relatively short duration (2 to 12 wk) of those studies.7,10,11,40-42,44 Other studies have used HFHC diets as the control diet to which components were added to evaluate the efficacy of the resulting diets in reducing CVD, but such studies complicate the evaluation of age-specific effects alone. In response to reviewer queries regarding the effect of age in a previous study using a HFHC control diet,39 the basis for the current study and our hypothesis was that the age of golden Syrian hamsters (to 31 wk) does not affect results in CVD studies. Furthermore, our use of a standard diet (STD) in the examination of various liver biomarkers, including cholesteryl ester, free cholesterol, total plasma cholesterol, and plasma cholesterol distribution, provided baseline data for future work with these animals.
Materials and Methods
Animals and diets.
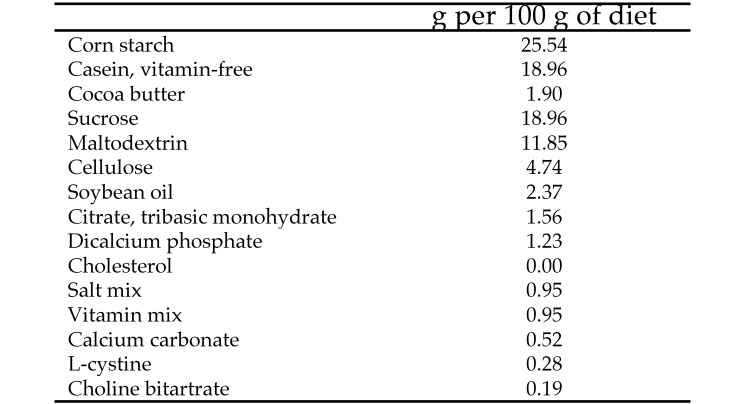
Male golden Syrian hamsters (Mesocricetus auratus) were obtained from Harlan (Indianapolis, IN) at approximately 6 wk old and weighted approximately 80 g when received. Hamsters were housed individually and maintained on a 12:12-h light:dark cycle in an environmentally controlled room at the North Carolina State University Biologic Research Facility (Raleigh, NC). The study was reviewed and approved by the IACUC of North Carolina State University and thus conformed to the Public Health Service Policy on Humane Care and Use of Laboratory Animals. Hamsters were acclimated for one week after arrival and then incrementally introduced to the STD diet (TestDiet, Richmond, IN), which was a purified diet containing 18.7% protein, 10.3% fat, and 71.0% carbohydrate (lipid at 9 kcal/g; carbohydrate and protein at 4 kcal/g; Figure 1). Hamsters were fed free choice, and clean water was provided. Body weight was measured every other week throughout the study.
Figure 1.
Composition of the standard diet.
Plasma and tissue collection.
At 0, 8, 12, 18, and 24 wk, 6 hamsters were fasted for 12 h and anesthetized with carbon dioxide. Hamsters were desanguinated by cardiocentesis, and blood was stored in 7% EDTA tubes. Plasma was separated after centrifugation at 1500 × g for 10 min at 4 °C and stored at –20 °C until analysis. Livers were collected, weighed, flash-frozen in liquid nitrogen, and stored at –80 °C until used for other analyses. Aortas were removed, cleaned, and stored in 10% formalin until analysis.
Plasma lipoprotein cholesterol and triacylglycerol analyses.
Analyses were performed at the Department of Pathology–Lipid Sciences (Wake Forest School of Medicine, Winston-Salem, NC) according to published methods.19 Briefly, total plasma cholesterol was measured, and lipoprotein particle distributions were determined by HPLC analysis. A 0.9% saline solution containing 0.01% EDTA and 0.01% azide was used at 0.4 mL/min on a Superose 6 10/300 column (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) for online cholesterol distributions. The effluent from the column was split, and half was mixed with total cholesterol reagent (Cholesterol H/P, Roche Diagnostics, Indianapolis, IN). Plasma lipoprotein cholesterol distribution concentrations were determined by size-exclusion chromatography. Plasma triacylglycerol concentrations were determined by using an enzymatic assay.13
Aortic cholesterol analysis.
Aortas were gently blotted to remove exterior formalin, weighed, and placed individually in 16 × 100-mm, screw-cap, round-bottom glass tubes containing chloroform–methanol (2:1, v/v) containing 20.5 µg of 5-α-cholestane as an internal standard, and the lipids were extracted. The lipid extract was separated by filtration, dried under N2 at 60 °C, and then dissolved in hexane. Free and total cholesterol were analyzed in 2 injections per sample on a DB17 (15 m × 0.53 mm [inner diameter] × 1 µm) gas–liquid chromatography column (J and W Scientific, Folsom, CA) at 250 °C and installed in a gas chromatograph (model 5890 GC, Hewlett-Packard, Houston, TX) equipped with an automatic injector (model 7673A, Hewlett-Packard) using online column injection and a flame-ionization detector. Cholesteryl ester was calculated as the difference between free and total cholesterol, as measured before and after saponification and reextraction of the nonsaponifiable sterol into hexane, multiplied by 1.67 (to correct for fatty acid loss).34 The tissue was digested and dissolved in 1 N NaOH, and total protein was determined by the Lowry protein assay.25
mRNA isolation.
Livers were powdered by using a Retsch Mixer Mill (model MM301, GlenMills, Clifton, NJ) under liquid nitrogen and stored at –80 °C until analysis. Total RNA was extracted on ice by using a MasterPure Complete DNA and RNA purification kit (Epicentre Biotechnologies, Madison, WI) with slight modifications to the manufacturer's protocol. RNA quality was measured in duplicate at wavelengths of 230, 260, 280, and 320 nm and was determined by using the 260:280 ratio. RNA quality was confirmed on a 1% agarose gel (SeaKem LE Agarose, Cambrex BioScience, Rockland, ME) with 1× Tris–acetate–EDTA buffer; GelStar stain (Lonza, Mapleton, IL) was used to visualize bands. Bands were imaged under UV light (Molecular Imager GelDoc XR, BioRad, Hercules, CA). Total RNA was calculated according to the absorbance at 260 nm × sample dilution factor × 40. The total RNA for each sample was normalized to 5 μg/μL and was transcribed to mRNA with an mRNA Only kit (Epicentre Biotechnologies) and incubated at 30 °C for 60 min in a thermocycler (model 5345 Mastercycler Gradient S PCR Thermal Cycler, Eppendorf, Westbury, NY).
Real-time RT–PCR analysis.
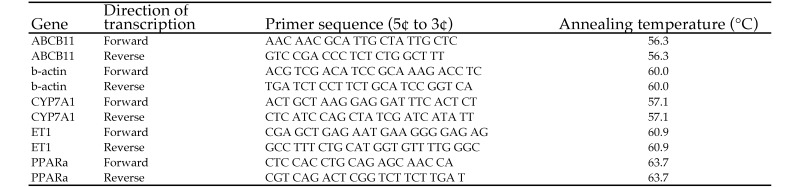
mRNA (20 μL) was converted to cDNA (High-Capacity cDNA Reverse Transcription Kit, Applied Biosystems, Carlsbad, CA) by using the following conditions: 25 °C for 10 min, 37 °C for 120 min, and then 85 °C for 5 min. Primers were synthesized (Operon, Huntsville, AL) according to published cDNA sequences, and the annealing temperatures were optimized (Figure 2). RT–PCR was performed by using the SsoAdvanced SYBR Green Supermix (Bio-Rad) at 95 °C for 5 s, then 40 cycles of 95 °C for 5 s followed by the optimal annealing temperature for 20 s. SYBR Green is a general double-stranded DNA intercalating dye that may result in the detection of nonspecific products, primers, dimers, and the amplicon of interest.3 A melting curve was performed to confirm the presence of cDNA; the conditions were: 95 °C for 15 s, 60 °C for 15 s, temperature ramp from 60 to 95 °C over 20 min, and 95 °C for 15 s. All samples were measured in duplicate, and there were 6 replicates per group. The relative change in expression for each gene of interest expression was calculated from real-time RT–PCR data by using the 2(–ΔΔCt) method.24
Figure 2.
Sequences and annealing temperatures of PCR primers
Statistical analysis.
All values are expressed as mean ± SEM. Time effects among observed means were evaluated by one-way ANOVA by using the Student t test for means separation (JMP10, SAS Institute, Cary, NC). Significance was defined at the 95% confidence level.
Results
Weight, cholesterol, and triglycerides.
In this 24-wk study, hamster body weight increased significantly (P < 0.05) from 0 to 6 wk but did not change between 8 and 24 wk (Table 1). Hamster liver weights significantly (P < 0.05) increased from 3.03 to 5.43 g over the 24-wk study (Table 1).
Table 1.
Body and hepatic weights and plasma and aortic components (mean ± SEM) of hamsters that consumed a standard diet over time
| 0 wk | 8 wk | 12 wk | 18 wk | 24 wk | P | |
| Body weight (g) | 83.45 ± 3.85b | 118.72 ± 3.85a | 122.70 ± 3.85a | 188.67 ± 3.85a | 126.60 ± 3.85a | <0.0001 |
| Hepatic weight (g) | 3.03 ± 0.37b | 3.72 ± 0.37b | 4.20 ± 0.37a,b | 4.28 ± 0.37a,b | 5.43 ± 0.37a | 0.0019 |
| Total plasma cholesterol (mg/dL) | 75.93 ± 10.25b | 109.55 ± 10.25a,b | 130.95 ± 10.25a | 109.11 ± 10.25a,b | 127.94 ± 10.25a | 0.0069 |
| Plasma triacylglycerol (mg/dL) | 114.00 ± 24.59 | 144.38 ± 24.59 | 74.17 ± 24.59 | 112.33 ± 24.59 | 171.33 ± 24.59 | 0.1176 |
| Plasma VLDL-cholesterol (mg/dL) | 18.86 ± 2.81a,b | 16.12 ± 2.81a,b | 7.73 ± 2.81b | 12.72 ± 2.81a,b | 20.07 ± 2.81a | 0.0324 |
| Plasma LDL-cholesterol (mg/dL) | 3.21 ± 1.88c | 17.65 ± 1.88a | 13.28 ± 1.88a,b | 7.05 ± 1.88b,c | 12.22 ± 1.88a,b | 0.0004 |
| Plasma HDL-cholesterol (mg/dL) | 53.87 ± 9.64a | 76.80 ± 9.64a,b | 104.08 ± 10.56a | 89.35 ± 9.64a,b | 98.99 ± 9.64a | 0.0059 |
| Plasma nonHDL-cholesterol (mg/dL) | 22.06 ± 3.90b | 38.41 ± 3.90a | 21.02 ± 3.90b | 19.77 ± 3.90b | 28.95 ± 3.90a,b | 0.0125 |
| Total aortic cholesterol (µg/mg protein) | 9.72 ± 0.41b | 10.56 ± 0.41a,b | 11.65 ± 0.41a | 12.19 ± 0.41a | 12.20 ± 0.41a | 0.0005 |
| Aortic free cholesterol (µg/mg protein) | 10.38 ± 0.39c | 10.89 ± 0.39b,c | 12.21 ± 0.39a,b | 12.12 ± 0.39a,b | 12.70 ± 0.39a | 0.0013 |
| Aortic cholesteryl ester (µg/mg protein) | 0.00 ± 0.08a | 0.02 ± 0.08a | 0.05 ± 0.08a | 0.18 ± 0.08a | 0.15 ± 0.08a | 0.4441 |
Within a row, values followed by the same letter are not significantly different (P < 0.05).
Total plasma cholesterol concentration ranged from 75.93 to 127.94 mg/dL over the course of the study (Table 1). Hamsters that consumed the STD diet continued to have low LDL-C concentrations over the course of the study, and at 24 wk, LDL-C was only 12.22 ± 1.88 mg/dL. VLDL-C concentrations were 18.86 mg/dL at 0 wk and 20.07 mg/dL at 24 wk in hamsters that consumed the STD diet (Table 1).
NonHDL-C did not significantly change (22.06 to 28.95 mg/dL) over 24 wk. HDL-C concentration increased between 0 and 8 wk in hamsters that consumed the STD diet then remained level at approximately 90 mg/dL. Triacylglycerol concentrations for hamsters that consumed the STD diet were not significantly different over the length of the study but ranged from 114.00 to 171.33 mg/dL (Table 1).
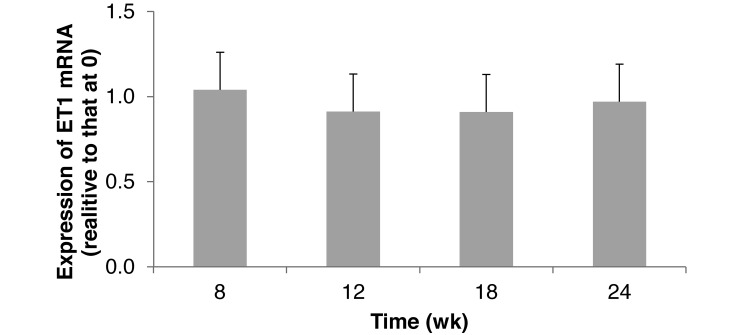
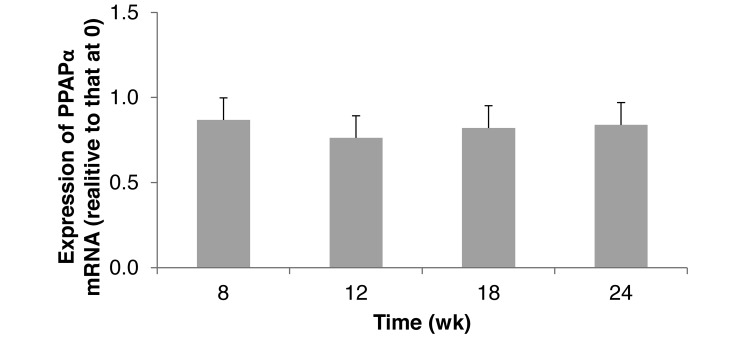
Hepatic ET1 gene expression did not significantly change over time, and levels at all sample times were comparable to that at 0 wk (Figure 3). PPARα gene expression was not significantly affected by age, indicating similar fatty acid and lipid metabolism from 8 to 24 wk. Hamsters at 0 wk had greater fatty acid β-oxidation in the liver than did older hamsters (Figure 4).
Figure 3.
Hepatic expression of endothelin 1 (ET1) mRNA in hamsters (n = 6) that consumed a standard diet for 24 wk. Means did not differ significantly (P > 0.05).
Figure 4.
Hepatic expression of peroxisome proliferator-activated receptor α (PPARα) mRNA in hamsters (n = 6) that consumed a standard diet for 24 wk. Means did not differ significantly (P > 0.05).
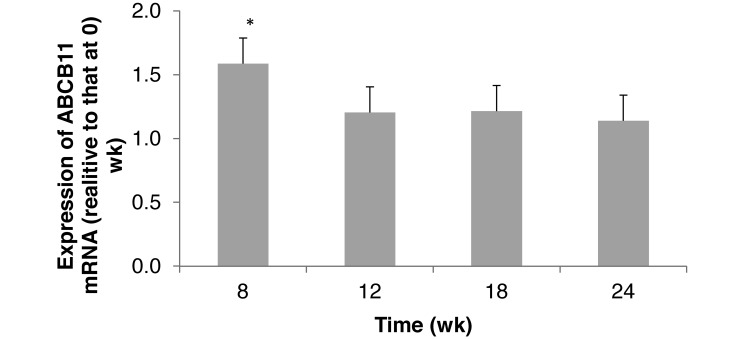
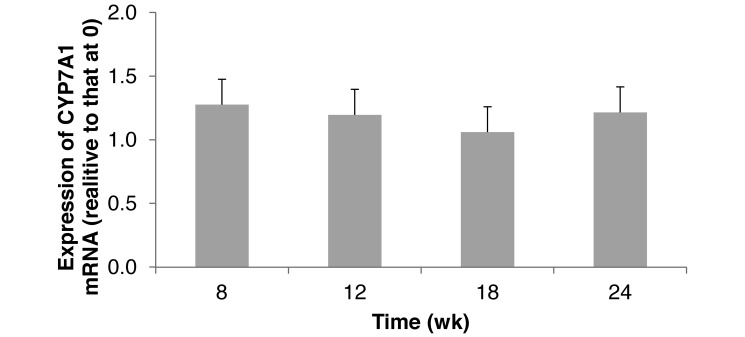
CYP7A1 gene expression did not change over time (Figure 5). ABCB11 expression in hamsters that consumed a STD diet was significantly (P < 0.05) greater at 8 wk than in older hamsters (12 to 24 wk; Figure 6). This increase may simply be due to normal growth of the animals. Hamster weight significantly (P < 0.05) changed from 0 to 8 wk (Table 1), indicating the time of most rapid weight gain and demand for nutrients.
Figure 5.
Hepatic expression of cholesterol 7-α-hydroxylase (CYP7A1) mRNA in hamsters (n = 6) that consumed a standard diet for 24 wk. Means did not differ significantly (P > 0.05).
Figure 6.
Hepatic expression of ATP-binding cassette subfamily B member 11 (ABCB11) mRNA in hamsters (n = 6) that consumed a standard diet for 24 wk. *, Value is significantly greater (P < 0.05) than those at other time points.
Aortic total cholesterol increased from 9.72 to 12.20 μg/mg protein, and aortic free cholesterol increased from 10.38 to 12.70 μg/mg protein over the 24-wk study (Table 1). The majority of aortic cholesterol was free cholesterol in hamsters that consumed the STD diet (Table 1); consequently concentrations of aortic cholesteryl ester (>0.18 μg/mg protein) did not suggest any development of atherosclerosis.
Discussion
Hamsters have an average life span of 1 to 3 y, an average respiratory rate of 74 breaths per minute, and a heart rate of about 332 bpm.12 This level of metabolism and relatively short life span suggest that age-related metabolic changes in relatively long-term studies (6 mo) with hamsters could affect data regarding CVD and their interpretation. The current study indicated that blood and tissue lipid chemistry as well as the expression of genes variously involved in inflammation, fatty acid metabolism, and cholesterol metabolism important to the development of atherosclerosis changed very little in male golden Syrian hamsters fed a standard diet through the age of 31 wk.
Because our current study is a single-treatment study with age as the variable, we discuss the data relative to published short-term studies and studies using various diets to compare and contrast the relatively low magnitude of metabolic changes that we noted in hamsters on the STD diet. The STD diet in this study contained 10.3% fat, 18.3% protein, and 71.0% carbohydrates. As anticipated, hamsters thrived well on this diet and gained and maintained weight. Chow diets and standard diets are the common breeding diets used in research,4 and both diets are low in fat and do not contain added cholesterol. Chow diets are unpurified grain- or cereal-based diets that can vary from batch to batch, whereas standard diets are purified diets, and the ingredient composition is highly defined. Hamsters fed a chow diet for 2 to 3 wk and for 5 to 11 wk did not have a significant change in body weight, but the initial ages of the hamsters were not reported.6,42,44 Weight gain or loss can affect or indicate progression of some metabolic diseases.48 Hamster body weight changed significantly from 0 to 8 wk (Table 1), at the time of most rapid weight gain and demand for nutrients. During rapid growth, the liver excretes cholic acid to bile, so that cholesterols can be reabsorbed and recycled for metabolic use. The single significantly higher level of ABCB11 mRNA expression occurred at 8 wk (Figure 6), supporting the process of cholesterol reabsorption and recycling for metabolic use.
Liver weight in hamsters has been reported to be 3.2 to 4.6 g at 3 wk of age,6,41 similar to the data from our study (3.0 to 5.4 g). Our hamsters were 7 wk old at the initiation of the study, with an average liver weight of 3.03 g. Liver weight did not significantly increase until 24 wk into the study. The liver weight of hamsters fed HF or HFHC diets20,39,47 were more than 200 times those in this STD study. Fatty livers generally suggest impaired metabolism, but the STD diet liver weight in hamsters changed little from 7 to 31 wk of age.
Total plasma cholesterol concentrations in the current study (75.9 to 127.9 mg/dL) did not significantly differ from 8 to 24 wk (Table 1) and were similar to concentrations (96 to 135 mg/dL) from hamsters that consumed a purified or chow diet for only 3 to 12 wk.7,10,11,40,42,44 Hamsters from 2 different breeding labs were fed a standard, nonpurified diet, and plasma lipids did not change over 12 wk in one strain but decreased in the other.10 The similarity of total plasma cholesterol concentrations in our study to published data and the lack of change during the study suggest that total plasma cholesterol is not an age-related CVD risk factor. These concentrations are contrasted against a total plasma cholesterol concentration of 1081.6 mg/dL when hamsters consumed a HFHC diet (40% fat, 0.5% cholesterol) diet for 24 wk.39 In that study, hamsters on the HFHC diet had significantly higher concentrations of aortic cholesteryl ester, an indicator of atherosclerosis, compared with those of hamsters on other treatments in the study that were designed to reduce the development of atherosclerosis.
Triacylglycerol concentrations in hamsters that consumed the STD diet were not significantly different over the length of the study but ranged from 114.0 to 171.3 mg/dL (Table 1). A triacylglycerol concentration of 219 mg/dL has been reported in hamsters after 6 wk on a chow diet.11 The high variability in triacylglycerol concentrations reported in that previous study11 occurred in our current study as well.
To reduce the risks for CVD, a high plasma HDL-C concentration is as important as is a low LDL-C concentration.2 The HDL-C concentration was 76.8 mg/dL at 8 wk on the STD diet and did not change significantly thereafter (Table 1). Comparatively, HDL-C in hamsters has been reported as 58 to 71 mg/dL after 12 wk on standard or chow diets.7,10,11,40-42,44 In our previously published work using a HFHC diet,39 HDL-C concentration was 53 mg/dL initially and increased to approximately 160 mg/dL after 24 wk, whereas LDL-C increased from 15 to 354 mg/dL and accompanied by the development of atherosclerosis. In younger hamsters that consumed a standard or chow diet nonHDL-C is reported to range from 25 to 67 mg/dL after 12 wk.10,11,40 Older hamsters (12 to 15 mo) that consumed a diet with a small amount of cholesterol were reported to be more susceptible to the detrimental effect of lipids on LDL-C metabolism than were younger animals (1 to 3 mo) on the same diet.35 However, in one study,33 LDL-C concentrations increased to about 125 mg/dL at 4 mo of age in hamsters on diets with low amounts of cholesterol and then gradually declined to approximately 80 mg/dL over the subsequent 15 mo, suggesting that age did not have an adverse effect on LDL-C metabolism. In our study, hamsters that consumed the STD had a significant increase in LDL-C between 0 and 8 wk but then concentrations were relatively similar for the duration of the study. However, hamsters that consumed a HFHC diet39 had an increase in circulating LDL-C accompanied by development of atherosclerosis over time, perhaps indicating an increase in the detrimental effects of LDL-C with increased age, as suggested in another report.35 Hamsters on a HFHC diet had a LDL-C concentration of 354.3 mg/dL and VLDL-C concentration of 553.7 mg/dL after 24 wk; however, LDL-C was only 47.6 mg/dL when peanuts were included in the HFHC diet.39 The LDL-C concentration of 12.2 mg/dL and the VLDL-C of 20.1 mg/dL that we noted in hamsters on the STD diet indicate a low risk for CVD.
Atherosclerosis risk can also be evaluated by examining changes in the expression of genes known to be important in specific metabolic processes related to the disease process. ET1 accumulation can cause liver cirrhosis, portal hypertension, and biliary obstruction and affect energy metabolism.17,18 Expression of the ET1 gene did not change over our 24-wk study, further confirming the lack of age-related changes that might affect the development of CVD in hamsters. Changes in PPARα expression have been linked to dysfunctions in fatty acid β-oxidation and atherosclerosis.28,37 The lack of change in PPARα gene expression during our current study suggests a decreased concern regarding fatty acid β-oxidation and no increase in the risk of atherosclerosis. Several studies have used PPARα gene expression as an indication of disease progression in hamsters fed a HFHC diet.8,20,33,37,43,47 To our knowledge, our current study is the first evaluation of hepatic ET1 and PPARα gene expression in hamsters on a STD diet.
The function of the CYP7A1 and ABCB11 genes is critical in cholesterol metabolism. CYP7A1 dysfunction is associated with hypercholesteremia because the shuttle of cholesterol to cholic acid is disrupted and therefore can increase oxidized LDL-C.30,32 ABCB11 encodes for a protein that transports cholate from hepatocytes to bile and affects biliary cholesterol excretion.15 Dysfunction of CYP7A1 or ABCB11 results in an increased risk for CVD. Except for the single increase in ABCB11 expression at 8 wk, which was likely in support of cholesterol reabsorption and recycling for metabolic use, the expression of these 2 genes appears to support other data indicating no increase in atherosclerosis risk factors.
Aortic cholesteryl ester accumulation is one of the first changes in arterial tissues during atherosclerotic development.38 It is common to measure atherosclerosis in hamsters because the morphology of the aortic foam cells and lesions in animals fed a HFHC diet is similar to human atherosclerotic lesion development.16,21,29 The aortic cholesteryl ester concentration did not increase significantly over the 24 wk of the current study and was comparable to that in hamsters that consumed a chow diet for 12 wk.10 However, hamsters consuming a HFHC diet had a cholesteryl ester concentration of 19.2 ± 2.9 μg/mg protein, which is indicative of the development of atherosclerosis.39
In conclusion, male golden Syrian hamsters are often used in feeding trials to investigate the effects of chemicals or foods on CVD or liver function. Normal aging during a 24-wk study (animal age at study end, 31 wk) involving hamsters fed a STD diet appears to add little to CVD risk factors normally evaluated during atherosclerotic development. The body weight, liver weight, plasma lipid distribution, aortic cholesterol distribution, and gene expression data in our current study can be used as baseline data for future studies. Comparisons of risk factors between hamsters fed a STD with those of given a HFHC diet39 indicate that normal ageing does not contribute to CVD risk factors and atherosclerosis.
Acknowledgments
The authors thank Mara Massel (North Carolina State University, Raleigh, NC) for RT–PCR training and assistance, Martha Wilson (Wake Forest University, Winston-Salem, NC) for GC and HPLC analyses, and Kay Coole, Linda Hester, and the Biological Research Facility Staff (North Carolina State University, Raleigh, NC) for daily animal care, and personnel of the USDA, ARS, Market Quality and Handling Research Unit for laboratory assistance.
References
- 1.Andersen JM, Cook LR. 1986. Regulation of gallbladder cholesterol concentration in the hamster. Role of hepatic cholesterol level. Biochim Biophys Acta Lipids Lipid Met 875:582–592. [DOI] [PubMed] [Google Scholar]
- 2.Assmann G, Gotto AM., Jr 2004. Atherosclerosis: evolving vascular biology and clinical implications. HDL cholesterol and protective factors in atheroscelerosis. Circulation. 109:8–14. [DOI] [PubMed] [Google Scholar]
- 3.Bahrami AR, Dickman MJ, Marin MM, Ashby JR, Brown PE, Conroy MJ, Fowler GJS, Rose JP, Sheikh QI, Yeung AT, Hornby DP. 2002. Use of fluorescent DNA-intercalating dyes in the analysis of DNA via ion-pair reversed-phase denaturing high-performance liquid chromatography. Anal Biochem 309:248–252. [DOI] [PubMed] [Google Scholar]
- 4.Borzelleca JF. 1992. The safety evaluation of macronutrient substitutes. Crit Rev Food Sci Nutr 32:127–139. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. [Internet]. 2013. CDC features: American Heart Month. [Cited 4 February 2013]. Available at: www.cdc.gov/features/americanheartmonth/
- 6.Chen J, Song W, Redinger RN. 1996. Effects of dietary cholesterol on hepatic production of lipids and lipoproteins in isolated hamster liver. Hepatology 24:424–434. [DOI] [PubMed] [Google Scholar]
- 7.Chien YL, Wu LY, Lee TC, Hwang LS. 2010. Cholesterol-lowering effect of phytosterol-containing lactic-fermented milk powder in hamsters. Food Chem 119:1121–1126. [Google Scholar]
- 8.Davis P, Valacchi G, Pagnin E, Shao Q, Gross HB, Calo L, Yokoyama W. 2006. Walnuts reduce aortic ET-1 mRNA levels in hamsters fed a high-fat, atherogenic diet. J Nutr 136:428–432. [DOI] [PubMed] [Google Scholar]
- 9.Dillard A, Matthan NR, Lichtenstein AH. 2010. Use of hamster as a model to study diet-induced atherosclerosis. Nutr Metab (Lond) 7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorfman SE, Smith DE, Osgood DP, Lichtenstein AH. 2003. Study of diet-induced changes in lipoprotein metabolism in 2 strains of golden Syrian hamsters. J Nutr 133:4183–4188. [DOI] [PubMed] [Google Scholar]
- 11.Dorfman SE, Wang S, Vega-López S, Jauhiainen M, Lichtenstein AH. 2005. Dietary fatty acids and cholesterol differentially modulate HDL cholesterol metabolism in golden Syrian hamsters. J Nutr 135:492–498. [DOI] [PubMed] [Google Scholar]
- 12.Feild K, Sibold A. 1999Important biological features, p 10–11. In: Suckow MA. The laboratory hamster and gerbil. New York (NY): CRC Press. [Google Scholar]
- 13.Fossati P, Prencipe L. 1982. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem 28:2077–2080. [PubMed] [Google Scholar]
- 14.Gaynor BJ, Sand T, Clark RW, Aiello RJ, Bamberger MJ, Moberly JB. 1994. Inhibition of cholesteryl ester transfer protein activity in hamsters alters HDL lipid composition. Atherosclerosis 110:101–109. [DOI] [PubMed] [Google Scholar]
- 15.Henkel AS, Kavesh MH, Kriss MS, Dewey AM, Rinella ME, Green RM. 2011. Hepatic overexpression of abcd11 promotes hypercholesterolemia and obesity in mice. Gastroenterology 141:1404–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahlon TS, Chow FI, Irving DW, Sayre RN. 1996. Cholesterol response and foam cell formation in hamsters fed 2 levels of saturated fat and various levels of cholesterol. Nutr Res 16:1353–1368. [Google Scholar]
- 17.Kakui S, Mawatari K, Ohnishi T, Niwa Y, Tanoue N, Harada N, Takahashi A, Izumi K, Nakaya Y. 2004. Localization of the 31-amino-acid endothelin 1 in hamster tissue. Life Sci 74:1435–1443. [DOI] [PubMed] [Google Scholar]
- 18.Khimji AK, Rockey DC. 2010. Endothelin—biology and disease. Cell Signal 22:1615–1625. [DOI] [PubMed] [Google Scholar]
- 19.Kieft KA, Bocan TM, Krause BR. 1991. Rapid online determination of cholesterol distribution among plasma lipoproteins after high-performance gel -filtration chromatography. J Lipid Res 32:859–866. [PubMed] [Google Scholar]
- 20.Kim H, Bartley GE, Rimando AM, Yokoyama W. 2010. Hepatic gene expression related to lower plasma cholesterol in hamsters fed high-fat diets supplemented with blueberry peels and peel extract. J Agric Food Chem 58:3984–3991. [DOI] [PubMed] [Google Scholar]
- 21.Kowala MC, Nunnari JJ, Durham SK, Nicolosi RJ. 1991. Doxazosin and cholestyramine similarly decrease fatty streak formation in the aortic arch of hyperlipidemic hamsters. Atherosclerosis 91:35–49. [DOI] [PubMed] [Google Scholar]
- 22.Kris-Etherton PM, Dietschy J. 1997. Design criteria for studies examining individual fatty acid effects on cardiovascular disease risk factors: human and animal studies. Am J Clin Nutr 65:1590S–159S6. [DOI] [PubMed] [Google Scholar]
- 23.Kuddus RH, Nalesnik MA, Subbotin VM, Rao AS, Gandhi CR. 2000. Enhanced synthesis and reduced metabolism of endothelin 1 (ET1) by hepatocytes—an important mechanism of increased endogenous levels of ET1 in liver cirrhosis. J Hepatol 33:725–732. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–ΔΔCt) method. Methods. 25:402–408. [DOI] [PubMed] [Google Scholar]
- 25.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. [PubMed] [Google Scholar]
- 26.Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. 2000. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell 6:507–515. [DOI] [PubMed] [Google Scholar]
- 27.Mayer DC, Leinwand LA. 1997. Sarcomeric gene expression and contractility in myofibroblasts. J Cell Biol 139:1477–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minnich A, Tian N, Byan L, Bilder G. 2001. A potent PPARα agonist stimulates mitochondrial fatty acid β-oxidation in liver and skeletal muscle. Am J Physiol Endocrinol Metab. 280:E270–E279. [DOI] [PubMed] [Google Scholar]
- 29.Nistor A, Bulla D, Filip A, Radu A. 1987. The hyperlipidemic hamster as a model of experimental atherosclerosis. Atherosclerosis 68:159–173. [DOI] [PubMed] [Google Scholar]
- 30.Pandak WM, Schwarz C, Hylemon PB, Mallonee D, Valerie K, Heuman DM, Fisher RA, Redford K, Vlahcevic KR. 2001. Effects of CYP7A1 overexpression on cholesterol and bile acid homeostasis. Am J Physiol Gastrointest Liver Physiol 281:G878–G889. [DOI] [PubMed] [Google Scholar]
- 31.Pineda Torra I, Gervois P, Staels B. 1999. Peroxisome proliferator-activated receptor α in metabolic disease, inflammation, atherosclerosis, and aging. Curr Opin Lipidol 10:151–159. [DOI] [PubMed] [Google Scholar]
- 32.Pullinger CR, Eng C, Salen G, Shefer S, Batta AK, Erickson SK, Verhagen A, Rivera CR, Mulvihill SJ, Malloy MJ, Kane JP. 2002. Human cholesterol-7α-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Invest 110:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rimando AM, Nagmani R, Feller DR, Yokoyama W. 2005. Pterostilbene, a new agonist for the peroxisome proliferator-activated receptor α isoform, lowers plasma lipoproteins and cholesterol in hypercholesterolemic hamsters. J Agric Food Chem 53:3403–3407. [DOI] [PubMed] [Google Scholar]
- 34.Rudel LL, Kelley K, Sawyer JK, Shah R, Wilson MD. 1998. Dietary monounsaturated fatty acids promote aortic atherosclerosis in LDL receptor-null, human ApoB100-overexpressing transgenic mice. Arterioscl Thromb Vasc Biol. 18:1818–1827. [DOI] [PubMed] [Google Scholar]
- 35.Shin H-W, Kim D, Lee Y, Yoo HS, Lee BJ, Kim JS, Jang S, Lim H, Lee Y, Oh S. 2009. Alteration of sphingolipid metabolism and pSTAT3 expression by dietary cholesterol in the gallbladder of hamsters. Arch Pharm Res 32:1253–1262. [DOI] [PubMed] [Google Scholar]
- 36.Spady DK, Dietschy JM. 1988. Interaction of dietary cholesterol and triglycerides in the regulation of hepatic low-density lipoprotein transport in the hamster. J Clin Invest 81:300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srivastava RAK. 2011. Evaluation of antiatherosclerotic activities of PPARα, PPARγ, and LXR agonists in hyperlipidemic atherosclerosis-susceptible F1B hamsters. Atherosclerosis 214:86–93. [DOI] [PubMed] [Google Scholar]
- 38.St Clair RW. 1976. Cholesteryl ester metabolism in atherosclerotic arterial tissue. Ann N Y Acad Sci 275:228–237. [DOI] [PubMed] [Google Scholar]
- 39.Stephens AM, Dean LL, Davis JP, Osborne JA, Sanders TH. 2010. Peanuts, peanut oil, and fat free peanut flour reduced cardiovascular disease risk factors and the development of atherosclerosis in Syrian golden hamsters. J Food Sci 75:H116–H122. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan MA, Duffy A, Dimarco N, Liepa G. 1985. Effects of various dietary animal and vegetable proteins on serum and biliary lipids and on gallstone formation in the hamster. Lipids 20:1–6. [DOI] [PubMed] [Google Scholar]
- 41.Trautwein EA, Liang J, Hayes KC. 1993. Plasma lipoproteins, biliary lipids, and bile acid profile differ in various strains of Syrian hamsters Mesocricetus auratus. Comp Biochem Physiol Comp Physiol 104:829–835. [DOI] [PubMed] [Google Scholar]
- 42.Tzang B-S, Yang S-F, Fu S-G, Yang H-C, Sun H-L, Chen Y-C. 2009. Effects of dietary flaxseed oil on cholesterol metabolism of hamsters. Food Chem 114:1450–1455. [Google Scholar]
- 43.Valeille K, Férézou J, Amsler G, Quignard-Boulange A, Parquet M, Gripois D, Dorovska-Taran V, Martin JC. 2005. A cis-9, trans-11-conjugated linoleic acid-rich oil reduces the outcome of atherogenic process in hyperlipidemic hamster. Am J Physiol Heart Circ Physiol 289:H652–H659. [DOI] [PubMed] [Google Scholar]
- 44.Vinson JA, Dabbagh YA. 1998. Effect of green and black tea supplementation on lipids, lipid oxidation, and fibrinogen in the hamster: mechanisms for the epidemiological benefits of tea drinking. FEBS Lett 433:44–46. [DOI] [PubMed] [Google Scholar]
- 45.Zambon A, Gervois P, Pauletto P, Fruchart J-C, Staels B. 2006. Modulation of hepatic inflammatory risk markers of cardiovascular diseases by PPARα activators: clinical and experimental evidence. Arterioscl Throm Vasc Biol. 26:977–986. [DOI] [PubMed] [Google Scholar]
- 46.Zandbergen F, Plutzky J. 2007. PPARα in atherosclerosis and inflammation. Biochim Biophys Acta 1771:972–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H, Bartley GE, Mitchell CR, Zhang H, Yokoyama W. 2011. Lower weight gain and hepatic lipid content in hamsters fed high fat diets supplemented with white rice protein, brown rice protein, soy protein, and their hydrolysates. J Agric Food Chem 59:10927–10933. [DOI] [PubMed] [Google Scholar]
- 48.Zhao Q, Zhang S. 2012. Obesity and cardiovascular diseases. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 34:431–436. [DOI] [PubMed] [Google Scholar]