Abstract
The study of normal and abnormal development typically requires precise embryonic staging. In mice, this task is accomplished through timed matings and the detection of a copulation plug. However, the presence of a plug is not a definitive indicator of true pregnancy, particularly in inbred mice, in which false-pregnancy rates have been reported to be 50% or higher, depending on the strain. This high rate poses considerable financial and animal use burdens because manipulation of the putative dam is often required before pregnancy can be confirmed by palpation or visual inspection. To address this problem, we examined weight gain in a population of 275 wildtype C57BL/6J mice (age, 12 wk or older) between the time of plug detection and during early embryogenesis (gestational days 7 to 10). In this population, assessing pregnancy according to the presence of a plug alone yielded a 37.1% false-positive rate. Pregnant mice gained an average of 3.49 g, whereas nonpregnant mice gained only 1.15 g. Beginning at gestational day 7.75, implementing an optimal weight-gain discrimination threshold of 1.75 g reduced the false-positive rate to 10.5%, without excluding any pregnant mice. These results were consistent with those from younger (age, 8 wk) wildtype C57BL/6J and FVB/NTac female mice, suggesting broad applicability of this method across age and strain. Our findings provide a simple and effective method for reducing animal use and study costs.
Abbreviation: GD, gestational day
Nearly all medical advancements over the last century can be attributed to foundational research conducted in animal models.15,16 Conserved morphogenesis and genetic tractability make mouse models powerful and widely used tools to study human development and disease.6 Studies that examine genetic and environmental influences on development require timed pregnancies for precise embryonic staging. The onset of pregnancy, typically referred to as gestational day 0 (GD0), is detected by the presence of a copulation plug. However, the presence of a plug is not definitive for predicting pregnancy. In fact, in some inbred strains, plug detection after overnight mating determines true pregnancy with less than 50% accuracy.9,18
Pregnancy can be confirmed by visual inspection by GD15 or earlier, depending on the strain and age of the dam and the number of previous pregnancies.18 Although palpation can be used as early as GD10, the associated stress to the dam might represent a confounding variable, particularly for teratology studies.2,13 Moreover, many studies require chemical, environmental, or surgical manipulation of the dam or harvesting of embryos at earlier stages.1,17,19 Mice that are manipulated but not pregnant typically cannot be reused, thus increasing the total number of animals required to retain adequate study populations as well as the financial cost of conducting research.
To address this problem, several methods, including urine and fecal-based hormone detection assays, have been examined.20 These tests currently are not available to the research community at large, and the costs and labor associated with these methods may limit their widespread implementation. However, evidence from related studies has suggested that pregnant mice gain more weight than do nonpregnant mice, promoting our hypothesis that weight gain can be used before other methods for discriminating pregnancy in inbred mouse strains.3-5,14 Here, we examined whether weight gain can be used to accurately discriminate pregnant from nonpregnant mice in a large population of wildtype C57BL/6J mice with copulation plugs. We also assessed the rate of weight increase in young pregnant and nonpregnant wildtype C57BL/6J and FVB/NTac female mice.
Materials and Methods
Animals.
This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals.11 All procedures involving animals were approved by the University of Wisconsin School of Veterinary Medicine IACUC. The University of Wisconsin-Madison is AAALAC-accredited. Wildtype C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Wildtype FVB/NTac mice were purchased from Taconic (Oxnard, CA). Mice were housed in sterile, disposable, ventilated cages prefilled with 1/8-in. corncob bedding (Innovive, San Diego, CA) with interior dimensions of 81 in.2 of floor space and 345 in.3 of living space. Male mice were housed individually, and 1 to 5 female mice were housed per cage. Female mice were transferred to the cage of a single male for mating periods. Rooms were maintained at 22 ±2 °C and 30% to 70% humidity on a 12:12-h light:dark cycle (lights on, 0600 to 1800). Mice were fed an irradiated commercial diet (2920X, Teklad Global Soy-Protein-Free, Extruded Rodent Diet, Harlan Laboratories, Indianapolis, IN). Cages were changed at least every 14 d. Enrichment included igloos (Innovive) for female mice and shredded paper (Enviro-Dri, Shepherd Specialty Papers, Richland, MI) for male and female mice. Water was provided in prefilled water bottles (Aquavive, Innovive). All animal manipulation was performed under SPF conditions, including the use of dedicated lab coats, Tyvex sleeves, gloves, and dedicated shoes or shoe covers. Mice were handled in animal transfer stations (Baker, Sanford, ME), with disinfectant solution (Virkon, DuPont, Wilmington, DE) preapplied to cabinet surfaces. Sentinel mice housed in randomly assigned positions within the mouse colony were sampled quarterly by tape test, pelt exams, and cecal exams. Throughout this study, all sentinel animals were negative for all tested pathogens including ectromelia virus, epizootic diarrhea of infant mice virus, lymphocytic choriomeningitis virus, mouse adenovirus 1 and 2, mouse hepatitis virus, mouse parvovirus (NS1 protein), minute virus of mice, Mycoplasma pulmonis, polyoma virus, pneumonia virus of mice, reovirus 3, Sendai virus, Theiler murine encephalomyelitis virus, murine norovirus, and Helicobacter spp.
Pregnancy discrimination according to weight gain in C57BL/6J female mice.
For this part of the study, 1 to 3 nulliparous wildtype female C57BL/6J mice (age, 3 to 9 mo) were placed in a cage with a single male C57BL/6J mouse (age, 12 wk to 1 y) for 1 to 2 h between 0800 and 2200. The beginning of the breeding period in which a copulation plug was found was assigned as GD0. After the detection of a copulation plug by visual examination and physical inspection by using a no. 34 ball-ended burnisher (SurgiDental, Deer Park, NY), putative dams were weighed (balance characteristics: capacity, 610 g; readability, 0.01 g; repeatability, 0.02 g; linearity, 0.02 g; model MXX-612, Denver Instruments, Bohemia, NY) and moved to a different cage; plugged dams were weighed only once more, at a randomly chosen time point between GD7.0 and GD10.0. Female mice lacking copulation plugs were returned to the breeding colony without being weighed. Pregnancy was confirmed either by inspection of uterine horns for embryos or fetuses or by the presence of a litter at 22 d after the mating period. Plugged but nonpregnant mice were returned to the breeding colony. A total of 275 female mice and 17 male mice were used for this part of the study.
Weight gain in pregnant and nonpregnant C57BL/6J and FVB/NTac female mice.
We next examined weight gain in pregnant and nonpregnant 8-wk-old, wildtype C57BL/6J and FVB/NTac female mice. Female mice (n = 1 or 2) were weighed and then placed in a cage overnight with a single male mouse (age, 2 to 6 mo) of the same strain, beginning at 1500. Putative pregnancy was determined by the presence of a copulation plug the following morning (approximately 0800), which was designated as GD0.5. Female mice were weighed, group-housed (n = 1 to 5 mice per cage) according to their copulation-plug status, and then reweighed daily between 0600 and 1100 from GD4.5 to GD11.5. Pregnancy was confirmed by the birth of a litter. For this part of the study, we used 28 C57BL/6J and 24 FVB/NTac female mice and 4 C57BL/6J and 4 FVB/NTac male mice.
For the entire study, a total of 352 mice were used. Lines of best fit were generated and statistical analyses performed by using Prism 6 (GraphPad Software, San Diego, CA). Statistical significance was defined as a P value of 0.05 or less.
Results
Pregnancy discrimination according to weight gain in C57BL/6J female mice.
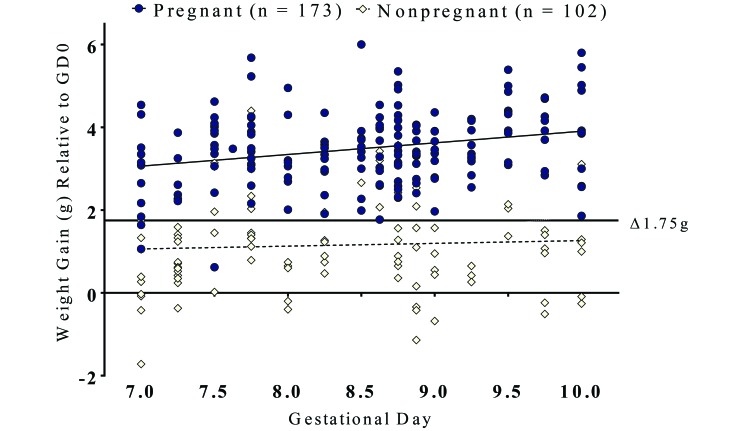
Of the 275 C57BL/6J female mice with copulation plugs, 173 mice were confirmed to be pregnant, yielding a 37.1% false-positive rate (Figure 1). From GD0 to between GD7 and GD10, pregnant mice gained an average of 3.49 g, whereas nonpregnant mice gained only 1.15 g. To distinguish between these 2 subpopulations, an optimal weight gain discrimination threshold of 1.75 g was identified to reduce the false-positive rate. Across the entire period of gestation that was sampled, applying this threshold produced a 12.8% false-positive rate and excluded only 3 mice (3.8%) that were pregnant. From GD7.75 to GD10, the use of the weight-gain threshold reduced the false-positive rate to 10.5%, without excluding any pregnant mice.
Figure 1.
Pregnancy discrimination according to weight gain in 275 wildtype C57BL/6J mice with copulation plugs. Lines of best fit (dashed) were applied to both pregnant and nonpregnant subpopulations. When applied after GD7.75, a 1.75-g cutoff (solid line) reduced the false-positive rate from 37.1% to 10.5% without excluding any pregnant animals.
Weight gain in pregnant and nonpregnant C57BL/6J and FVB/NTac female mice.
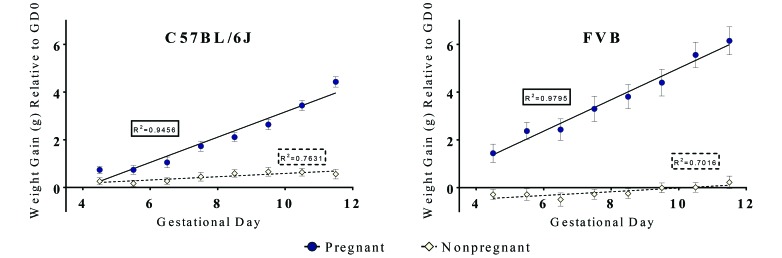
Pregnant and nonpregnant 8-wk-old C57BL/6J and FVB/NTac mice were weighed daily from GD4.5 to GD11.5 (Figure 2). Linear-regression analysis of these data demonstrated significantly (P < 0.0001) different slopes between pregnant and nonpregnant groups in both strains. Among those with copulation plugs, 4 of the 14 FVB/NTac mice and 3 of the 13 C57BL/6J were not pregnant (false-positive rates of 20% and 29%, respectively). Consistent with our evaluation of the 275 C57BL/6J mice, all 7 of these plugged but nonpregnant mice had gained less than 1.75 g by GD7.5. In addition, 2 C57BL/6J mice in which a plug was not detected were found to be pregnant, and both of these mice gained more than 1.75 g by GD7.5.
Figure 2.
Weight gain (g, mean ± SEM) in pregnant and nonpregnant 8-wk-old C57BL/6J (pregnant, n = 12; nonpregnant, n = 16) and FVB/NTac (pregnant, n = 10; nonpregnant, n = 14) mice. The linear regression slopes for pregnant mice are significantly (P < 0.0001) different from those for nonpregnant mice for both strains. For the C57BL/6J linear regression analysis, F = 72.48, DFn = 1, and DFd = 12. For the FVB/NTac linear regression analysis, F = 172.96, DFn = 1, and DFd = 12.
Discussion
Although the detection of a copulation plug is well known to be an unreliable indicator of pregnancy, false-pregnancy rates according to plug detection alone have not been well-documented. One report on commonly used strains stated a “67% success rate” for C57BL/6J mice, but whether this figure refers to pregnancy after a mating period or after plug detection is unclear.18 Here, in a large sample population (275 mice), we found that diagnosing pregnancy solely according to the detection of a copulation plug yielded a false-positive rate of 37.1% in C57BL/6J mice, the most commonly used inbred strain in biomedical research.10 We then demonstrated that discriminating pregnant from nonpregnant mice according to an optimal weight gain threshold of 1.75 g between GD7.75 and GD10.00 reduced the false-positive rate to 10.5% without excluding any pregnant mice (sensitivity, 89.5%; specificity, 100%). Between GD7.0 and GD10.0, the false-positive rate was 12.8%, and pregnancies were not identified in 3.75% of plugged female mice (sensitivity, 87.2%; specificity, 96.3%).
As done routinely in our laboratory and others’, C57BL/6J female mice (age, 12 wk or older) were mated for 1 to 2 h in the first experiment of the current study.7,8,12 However, investigators often use female mice as young as 8 wk and overnight mating periods to establish timed pregnancies.14 We therefore then evaluated 8-wk-old C57BL/6J and FVB/NTac mice to test the broader applicability of the described method and to examine weight gain in younger, nonpregnant animals. Linear-regression analysis of pregnant and nonpregnant groups yielded significantly different slopes, illustrating that pregnant mice gain weight more quickly than do nonpregnant mice.
In the current study, we used 2 common techniques for establishing timed pregnancies in mice, including both narrow (1 to 2 h) and overnight mating periods; in both cases, we used female mice that were at least 8 wk old. We did not examine weight gain in mice younger than 8 wk or in those that had been pregnant previously, because the use of such mice is relatively uncommon. In addition, although the method we describe here entails some stress to dams, the handling required to weigh mice is minimal compared with that required for their palpation. Another shortcoming of our study is that we examined only 2 inbred strains. Although both strains we chose are commonly used, additional study will be required to determine how broadly the described methodology can be applied across the many inbred mouse strains used in biomedical research.
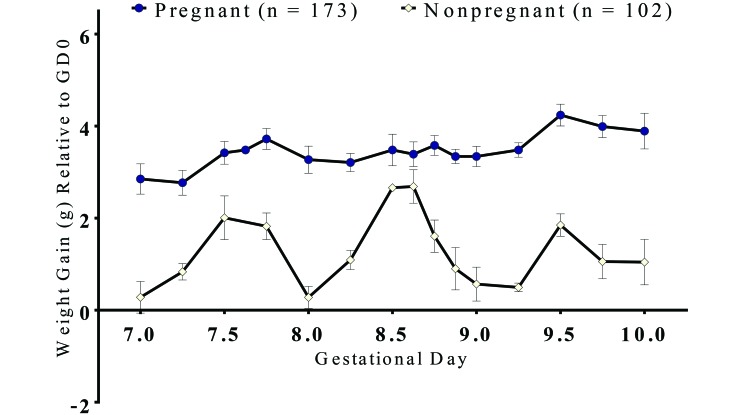
As illustrated in Figure 1, some plugged female mice gained more than 1.75 g but were not pregnant. These false positives cluster at the 0.5 and 0.75 GD time points, in which animals were weighed between 0800 and 1200, whereas those at 0.25 GD time points were weighed between 1400 and 1700. Animals at other time points were weighed between 0800 and 1800. Of the 25 false positives in this group, 22 occurred in mice weighed in the morning. This diurnal variation in weight, particularly in nonpregnant animals (Figure 3), is likely related to nocturnal feeding and subsequent weight loss during sleep throughout the light phase. This pattern may explain, at least in part, the higher false-positive rate in mice weighed in the morning and suggests that weight determination in the afternoon or evening may more reliably discriminate between pregnant and nonpregnant mice.
Figure 3.
Diurnal oscillation in weight (g, mean ± SEM) of plugged–pregnant and plugged–nonpregnant wildtype C57BL/6J mice. Weights from the population described in Figure 1 are shown as average values by gestational day for pregnant and nonpregnant mice.
This study demonstrates that weight gain can be used to reliably distinguish pregnant from nonpregnant female mice. The finding that weight gain is associated with pregnancy is congruous with a previous study in which 2 transgenic strains on the C57BL/6J background were weighed at GD7 and GD14.14 Although the weight change was significantly increased in pregnant compared with nonpregnant mice in both strains, the authors described interstrain differences in plug rates, pregnancy rates, weight gain during pregnancy, and the appearance of the plug. These outcomes may have resulted from the specific genetic differences between the examined strains. Here, we examined weight changes in wildtype C57BL/6J mice at fine temporal resolution throughout early postimplantation embryogenesis. This strategy enabled us to formulate a method to identify pregnancy in plugged female mice with greater specificity. In this population of 275 mice, application of the described method reduced the number of nonpregnant animals that would be used from 73 to 20, thus decreasing the total number of study animals required by nearly 20%. Consideration of diurnal weight oscillations as described may provide an additional strategy to better discriminate pregnancy. By providing a simple and reliable method for increasing the accuracy of pregnancy detection in inbred mice, the data presented here can be used to increase the efficiency and cost-effectiveness of research studies.
Acknowledgments
We thank Lydia Ansen-Wilson, Ruth Sullivan, Josh Everson, and Henry Kietzman for their ideas and helpful comments on the manuscript. This work was in part funded by R00DE022101-02 grant to RJL from the National Institute of Dental and Craniofacial Research/National Institutes of Health. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
References
- 1.Borchert A, Wang CC, Ufer C, Schiebel H, Savaskan NE, Kuhn H. 2006. The role of phospholipid hydroperoxide glutathione peroxidase isoforms in murine embryogenesis. J Biol Chem 281:19655–19664. [DOI] [PubMed] [Google Scholar]
- 2.Colomina MT, Albina ML, Domingo JL, Corbella J. 1997. Influence of maternal stress on the effects of prenatal exposure to methylmercury and arsenic on postnatal development and behavior in mice: a preliminary evaluation. Physiol Behav 61:455–459. [DOI] [PubMed] [Google Scholar]
- 3.Dewar AD. 1957. Body weight changes in the mouse during the oestrous cycle and pseudopregnancy. J Endocrinol 15:230–233. [DOI] [PubMed] [Google Scholar]
- 4.Dewar AD. 1957. The endocrine control of the extra-uterine weight gain of pregnant mice. J Endocrinol 15:216–229. [DOI] [PubMed] [Google Scholar]
- 5.Dewar AD. 1959. Observations on pseudopregnancy in the mouse. J Endocrinol 18:186–190. [DOI] [PubMed] [Google Scholar]
- 6.Diewert VM, Wang KY. 1992. Recent advances in primary palate and midface morphogenesis research. Crit Rev Oral Biol Med 4:111–130. [DOI] [PubMed] [Google Scholar]
- 7.Godin EA, Dehart DB, Parnell SE, O'Leary-Moore SK, Sulik KK. 2011. Ventromedian forebrain dysgenesis follows early prenatal ethanol exposure in mice. Neurotoxicol Teratol 33:231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godin EA, O'Leary-Moore SK, Khan AA, Parnell SE, Ament JJ, Dehart DB, Johnson BW, Allan Johnson G, Styner MA, Sulik KK. 2010. Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: effects of acute insult on gestational day 7. Alcohol Clin Exp Res 34:98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green EL. 1966. Biology of the laboratory mouse. New York (NY): Dover Publications. [Google Scholar]
- 10.Jackson Laboratory. [Internet] 2014. JAX Mice Database: 000664 C57BL/6J. [Cited 25 June 2014] Available at: http://jaxmice.jax.org/strain/000664.html.
- 11.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 12.Kietzman HW, Everson JL, Sulik KK, Lipinski RJ. 2014. The teratogenic effects of prenatal ethanol exposure are exacerbated by Sonic Hedgehog or GLI2 haploinsufficiency in the mouse. PLoS ONE 9:e89448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim R, Fedulov AV, Kobzik L. 2014. Maternal stress during pregnancy increases neonatal allergy susceptibility: role of glucocorticoids. Am J Physiol Lung Cell Mol Physiol 307:L141–L148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mader SL, Libal NL, Pritchett-Corning K, Yang R, Murphy SJ. 2009. Refining timed pregnancies in 2 strains of genetically engineered mice. Lab Anim (NY) 38:305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Research Council 1991. Science, medicine, and animals. Washington (DC): National Academies Press. [Google Scholar]
- 16.Royal Society (United Kingdom) 2004. The use of nonhuman animals in research: a guide for scientists. London (UK): Royal Society. [Google Scholar]
- 17.Schneider M, Vogt Weisenhorn DM, Seiler A, Bornkamm GW, Brielmeier M, Conrad M. 2006. Embryonic expression profile of phospholipid hydroperoxide glutathione peroxidase. Gene Expr Patterns 6:489–494. [DOI] [PubMed] [Google Scholar]
- 18.Silver LM. 1995. Mouse genetics: concepts and applications. New York (NY): Oxford University Press. [Google Scholar]
- 19.Ufer C, Wang CC. 2011. The roles of glutathione peroxidases during embryo development. Front Mol Neurosci 4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Washington I. [Internet]. 2013. Development of a urine-based pregnancy test in the mouse. Available at: http://www.aclam.org/Content/files/files/Forum2013/ACLAM_Forum_2013_Washington.pdf.