Abstract
Sexually mature zebrafish were housed as single male-female pairs with or without plastic vegetation for 1, 5, or 10 d for comparison of whole-body cortisol measured by radioimmunoassay. Individually housed male zebrafish were used as controls. In the fish that were pair-housed without vegetation (NVeg), one animal died in 5 of 24 pairs, and one animal was alive but wounded in an additional pair. No deaths or wounds occurred in the fish that were pair-housed with vegetation (Veg). Cortisol levels did not differ between the treatment groups on day 1. On day 5, cortisol values were higher in the Veg group than in the individually housed fish (P < 0.0005) and the NVeg fish (P = 0.004). On day 10, the relationships were inversed: cortisol levels had risen in the individually housed and NVeg groups and had fallen to baseline levels in the Veg group. Cortisol values on day 10 were lower in the Veg group than in the individually housed (P = 0.004) and NVeg (P = 0.05) groups. Cortisol levels in individually housed male zebrafish increased over time. Although this study did not demonstrate a reduction in cortisol levels associated with providing vegetation, this enrichment prevented injury and death from fighting. These findings show how commonly used housing situations may affect the wellbeing of laboratory zebrafish.
Abbreviations: NVeg, pair-housed without vegetation; Veg, pair-housed with vegetation
The use of zebrafish as animal models in research continues to expand and evolve rapidly. We are only beginning to understand what constitutes appropriate housing conditions for zebrafish in the laboratory setting. Their increased use heightens the need to define appropriate care of these animals.21 Studies of zebrafish in their natural habitat may guide the development of zebrafish husbandry standards regarding optimal housing conditions in the laboratory. In the wild, zebrafish are often found in shallow water, appear to prefer to spawn over gravel substrates, and typically associate in shoals.34,35 Both wild and captive zebrafish appear to prefer habitat complexity.3,9,19,31,35,36 Adding habitat complexity to zebrafish housing reduces aggression and improves reproduction.3,9 Zebrafish are a social species that prefers to associate in shoaling groups of varying size.4,12,14,29,34 Aggression has been observed when the fish are housed in low densities, particularly in single pairs.16 In our experience, providing habitat complexity to a single-pair breeding tank appears to reduce aggression and improve spawning. Although pair-housing is a common practice in the laboratory setting, the frequency and intensity of aggressive social interactions may negatively affect zebrafish wellbeing. We therefore wanted to determine whether the presence of habitat complexity with pair-housed zebrafish reduces cortisol levels compared with those of pair-housed fish in a barren tank.
Numerous studies have demonstrated that, when housed together, zebrafish establish dominant-subordinate relationships through aggressive interactions.20 The level of aggression appears to be influenced by habitat complexity, housing density, and food availability.20 These situations pose a problematic welfare situation, because pair-housing of zebrafish is an extremely common practice, particularly in genetic studies and other experiments in which the identity of the parents is critical. In these situations, the subordinate fish is subjected to chasing, biting, and even injury. In addition, the subordinate fish experiences reduced food intake and generally exhibits a stressed state, characterized by rapid opercular movement, blanched pigmentation, decreased appetite, and reduced exploration, when exposed to an aggressor.3,9,36 Chronic exposure to aggression from a dominant fish exerts negative performance, reproductive, and health effects on subordinate fish.9,15 Dominance has not been clearly associated with sex or body size.36 The dominance hierarchy within a tank of fish typically is established within a 5-d period, and this time frame has been used as the acclimation period for studies with group-housed zebrafish.15,20 Low-density pairings have exhibited increased aggressive behavior.33 Pair-housing was shown to be stressful in a study in which aggressive behavior was scored in male pairs; a marker for aggression, vasotocin, was measured.20 These findings were further supported and extended by other authors,15 who found that male–male and female–female pair-housing had significant negative physiologic effects on the subordinate fish.
Aside from the obvious potential welfare issue, stress can confound research outcomes by creating physiologic and immune status variability between subjects.10,15,23Although acute elevations of cortisol can be beneficial to animals for handling stressful situations, chronic elevations can be detrimental.9,15,24 Fish exposed to chronic stress have demonstrated reduced immunity and increased susceptibility to disease, poor reproductive capabilities, and decreased growth and survival.9,15 To have healthy subjects for research, it is important to determine the environmental factors that may be stressful when considering zebrafish husbandry in the laboratory setting.15 Cortisol levels in teleost fishes, including zebrafish, have been shown to positively correlate with other indicators of stress.1,2,6,24,27,30 Zebrafish exposed to a stressor have been shown to demonstrate a rise in cortisol.2,6,7,8,26,27,28,30 Cortisol extracted from pooled or individual whole-body samples has been quantified by using radioimmunoassay or ELISA and has been used to evaluate stress due to predators and crowding but not that associated with single male–female pair-housing in zebrafish.2,6,8,27,30
Adding habitat complexity to typically barren laboratory housing tanks has been shown to decrease aggressive interactions and resource monopolization in zebrafish.3,9 Previous studies have created habitat complexity through the addition of vertical-space-occupying objects such as clay pots, glass rods, and plastic vegetation.3,9,19,31,39 Here we sought to evaluate the effects of pair-housing of zebrafish either with or without habitat complexity in the form of plastic vegetation, which is an easily sanitized and readily accessible environmental enrichment option. Our hypothesis was that those pairs housed with habitat complexity would have improved survival and lower cortisol levels. The 3 time points we chose represent previously reported phases of zebrafish acclimation to housing: 1 d for the acute phase, 5 d for the acclimation phase, and 10 d for the chronic phase.20,39 Individually housed zebrafish were used as controls, because another study reported low basal cortisol levels for this housing condition.27 An opaque divider surrounded 3 sides (left, right, and back) of each tank to prevent visualization of other fish. A black background was chosen for the opaque divider, because this color was previously reported as a preferred background in zebrafish.5,22,28
Materials and Methods
Animals.
Wild-type AB/India hybrids18,38 were used. Fish were raised at 28.5 °C on a 14:10-h light:dark cycle and staged according to the time (hours or days) after fertilization. Fish were housed on a recirculating water system (Pent-air Aquatic Ecosystems, Sanford, NC). Adult fish were fed twice daily (TetraMin Tropical Flakes, Tetra Holdings, Blacksburg, VA, and Utah State Artemia, Ogden, UT). Experimental fish were kept at a housing density of 5 fish per liter in barren tanks until they reached approximately 5 mo of age. Water-quality testing for our facility involves testing nitrate, nitrite, and ammonia weekly, conductivity and temperature daily, and pH twice daily. All water-quality parameters were within normal limits during the course of this study.16
Experimental housing.
After reaching 5 mo of age, experimental zebrafish were placed into 1-L tanks (6 cm × 11 cm × 16 cm with approximately 10 cm of water depth) in 1 of 3 housing situations: individually housed male fish (controls); single male–female pair in a barren tank (NVeg group); and single male–female pair with plastic vegetation (Veg group; Chi Vine, Fluval, Quebec, Canada). The plastic vegetation added floated near the top of the tank but remained submerged within the vertical water column. Tanks were kept on the facility recirculating system, and opaque black dividers were placed between all tanks to prevent visualization of other zebrafish. Fish were fed on the same schedule as the rest of the facility (once in the morning and once in the afternoon). The experiment was performed in 2 runs. For each run, 3 tanks were set up for each treatment group (Veg, NVeg, and control) and time point (1, 5, and 10 d). However, deaths due to fighting occurred in the NVeg group during the first run, so additional tanks were set up to obtain, at minimum, 6 tanks for each group and time point. Zebrafish were sampled at 1, 5, and 10 d after experimental housing set-up. For sampling, fish were quickly removed from their tanks by using a net and immediately placed in an ice-water bath (1 to 4 °C). Sampling was performed at midday to avoid capturing fish during the potentially stressful mating and feeding periods (early morning and afternoon). All sampled fish were weighed, snap-frozen on dry ice, and kept in a –80 °C freezer until processing. All work was conducted in accordance with the Guide for the Care and Use of Laboratory Animals in an AAALAC-accredited institution.17All experimental procedures were approved by the Vanderbilt University IACUC.
Cortisol extraction.
Cortisol was extracted from the fish by a modification of a previous method.32 Briefly, fish were processed individually, with each fish being thawed, weighed, and cut into small pieces in a plastic weigh boat. The contents of the weigh boat were rinsed into a glass tube containing 2 mL PBS and then homogenized (Cyclone Vertishear, SP Scientific, Warminster, PA). The homogenizer was cleaned and rinsed with deionized water between samples. The homogenate was transferred to a 16 × 125-mm glass tube with a Teflon-lined cap. The 50-mL conical tube that held the original sample was rinsed with 5 mL ethyl ether, which was then transferred to the glass tube. The sample (homogenate and ethyl ether) was vortexed for 10 to 15 s, incubated at room temperature for 15 to 20 min, centrifuged at 2113 × g for 5 min, and placed in a –80 °C freezer for 10 min. The ether layer was transferred to a clean glass tube. The extraction was performed twice, and the resulting ether layers were combined and stored at –20°C.
To determine the efficiency and consistency of the extraction procedure, 5 fish were homogenized as described and 3H-cortisol (Cortisol, [1,2,6,7-3H(N)]-), [1,2,6,7-3H(N)], Perkin Elmer, Waltham, MA) was added. The homogenate was divided into 5 equal aliquots and cortisol was extracted as described. The amount of 3H-cortisol in the final extract was determined by liquid scintillation spectrometry. The extraction efficiency (mean ± 1 SD) was 58.3% ± 1.4%.
Cortisol radioimmunoassay.
Measurement of cortisol levels by radioimmunoassay (catalog no. TKCO1, Siemens, Munich, Germany) was performed by the Vanderbilt Hormone Assay Core. Zebrafish samples in diethyl ether were placed under the hood to evaporate the solvent. To solubilize the cortisol, 100 μL absolute ethanol was added to each tube and mixed thoroughly, and then 900μL cortisol buffer was added and vortexed well. Assays were performed in duplicate. Standards (range, 0.05 to 5 ng/mL) were prepared from the highest-concentration standard in the kit. In each test tube, 100 µL sample, 100 µL antibody (courtesy of Wendell Nicholson, Vanderbilt University, Nashville, TN), and 100 µL125I-cortisol (catalog no. TKCO1, Siemens) were added. After incubation at 4 °C for 24 h, we added 100 µL normal rabbit serum (catalog no. S20-100, Millipore, Billerica, MA) in 0.1% BSA PO4–EDTA buffer (dilution, 1:50) and 1 mL precipitating reagent (dilution, 1:50; catalog no. 40-GR30, goat antirabbit γ-globulin, Fitzgerald Industries International, Acton, MA; in 3% polyethylene glycol–PO4–EDTA buffer). The sample was mixed well, incubated for 30 min, centrifuged at 2543 × g for 30 min, decanted, and counted in a gamma counter. The sensitivity of the assay was 0.05 ng/mL.
The interassay coefficient of variation was 8.75% when a pool of human saliva (undiluted; Bioreclamation, Westbury, NY) was assayed repeatedly (n = 5; 0.86 ± 0.1 ng/mL). The interassay coefficient of variation was 12.97% when a pool of human saliva (diluted 2-fold) was assayed repeatedly (n = 5; 0.43 ± 0.1ng/mL). The interassay coefficient of variation was 11.23% when a pool of human saliva (diluted 4-fold) was assayed repeatedly (n = 5; 0.20 ± 0 ng/mL).
Statistical analysis.
The effects of housing (treatment) and day of euthanasia on cortisol levels were assessed by using fixed-effects 2-way ANOVA. An initial exploratory analysis indicated that groups with higher average cortisol levels also had greater variation in this response variable. To make the variation more uniform, we used a logarithmic transform of each fish's cortisol per unit weight as the dependent variable in our analyses. This action made the assumptions of normality and homoscedasticity (standard deviations were equal) of our analyses reasonable. Indicator covariates were used for the 3 treatments in this study (individually housed, paired without vegetation, and paired with vegetation). Interaction terms between treatment and day were included in the model. Comparisons of morbidity and mortality rates between pairs housed with and without vegetation were assessed by using the log-rank test. Two-sided P values less than 0.05 were considered significant. Analyses were run with Stata version 13 (StataCorp, College Station).
Our ANOVA of the log-cortisol values assumed that the residuals were normally distributed and have constant within-group standard deviations. As a crosscheck of these assumptions, we also ran supplemental nonparametric Kruskal–Wallis analyses to compare the 3 housing environments on each day of euthanasia. When the results were significant, Wilcoxon rank-sum tests were used to evaluate differences between pairs of treatments. We used a Fisher protected least-squares approach to reduce the risk of spurious findings due to multiple comparisons.12
The results from 3 fish were not within the reliable limits of the assay and were eliminated from this analysis, as were those from the 5 pairs in which one member died.
Results
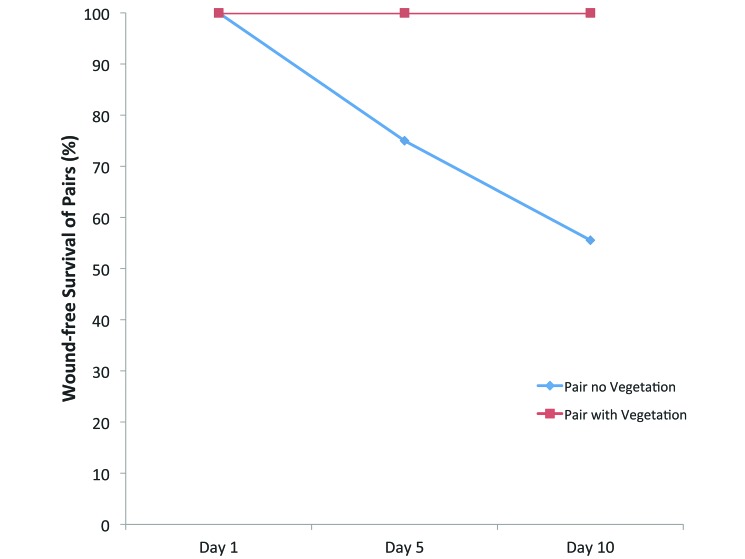
All fish survived the first time point (1 d after housing set-up). However, by 5 d, animals in the NVeg pairs began to die due to fighting. During the first run, 1 of 3 NVeg pairs was eliminated because the female fish was found dead on day 5, and 2 of 3 NVeg pairs were eliminated on day 10 for the same reason. For the second run, additional pairs were established to accommodate the losses during the first run. During the second run, the male fish of one pair in the NVeg treatment group was alive but wounded on day 5, and 2 pairs were eliminated on day 10 because the male fish had died. No lesions were found on any of the fish in the individually housed or Veg groups, and all of them survived to their endpoints (Figure 1). Therefore, a total of 6 of 24 (25%) NVeg pairs suffered morbidity or mortality due to aggression, whereas none of the 21 Veg pairs suffered such losses (P = 0.02).
Figure 1.
Wound-free survival of pairs (%). No morbidity or mortality was observed on day 1 for either group. The Pair no Vegetation (NVeg) group had one death and one injury among 8 pairs by day 5 and 4 deaths among 9 pairs by day 10. No deaths or injury were seen in the Pair with Vegetation (Veg) group.
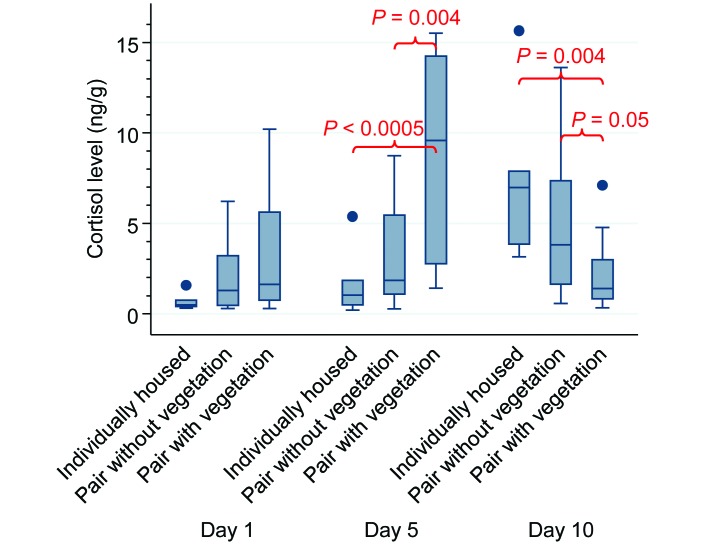
Treatment and day had highly significant effects on cortisol levels (F = 5.4; df = 8 and 85; P< 0.00005; Table 1 and Figure 2). There was no significant difference in cortisol levels between treatments on day 1, but on days 5 and 10 cortisol levels differed significantly between treatments (day 5, P = 0.0005; day 10, P = 0.009). On day 5, cortisol levels were higher in Veg pairs than in either NVeg pairs (P = 0.004) and individually housed fish (P < 0.0005). By day 10, however, cortisol levels were lower in Veg pairs than in NVeg pairs (P = 0.05) or individually housed fish (P = 0.004). The results of our nonparametric analyses were consistent with and supported our parametric findings. In addition, cortisol levels in individually housed male zebrafish increased over time; the day-10 cortisol level was higher (P < 0.0005) than that on day 1. Five pairs of fish, all in the NVeg group, had to be eliminated from the study because one of the mates was found dead. Values from one fish in the NVeg group and 2 fish in the Veg group were not included in the analysis because their results were below the reliable limits of the cortisol assay. Cortisol levels did not differ between runs, sexes, or time points for any group.
Table 1.
Estimated geometric mean cortisol levels by group and day of the experiment.
| Day | Group | Cortisol (ng/g fish) |
Pa | |
| Geometric mean | 95% confidence interval | |||
| 1 | Ind | 0.57 | 0.25−1.31 | not applicable |
| NVeg | 2.25 | 0.83−6.06 | 0.11 | |
| Veg | 3.08 | 1.14−8.30 | 0.03 | |
| 5 | Ind | 1.70 | 0.52−5.49 | 0.37 |
| NVeg | 3.39 | 1.23−9.38 | 0.02 | |
| Veg | 11.56 | 4.18−32.00 | <0.0005 | |
| 10 | Ind | 11.29 | 3.49−36.50 | <0.0005 |
| NVeg | 5.97 | 2.09−17.10 | 0.001 | |
| Veg | 2.54 | 0.94−6.86 | 0.07 | |
Ind, individually housed; NVeg, pair-housed without vegetation; Veg, pair-housed with vegetation
Compared with value for individually housed group on day 1
Figure 2.
Cortisol levels of experimental fish (nanograms per gram of fish). No significant difference was seen between treatment groups on day 1. On day 5, the Pair with Vegetation (Veg) group had significantly higher cortisol values than did the Pair no Vegetation (NVeg) group (P = 0.004) and the individually housed group (P< 0.0005). On day 10, the Veg group had significantly lower cortisol values than did the NVeg group (P = 0.05) and the individually housed group (P = 0.004).
Discussion
Studying nonstressed subjects is important both for the wellbeing of the animals and the validity of the data obtained.15 Husbandry practices are a potential source of stress in animals, and we are now beginning to learn which practices are, indeed, stressful. Because zebrafish have a known preference for habitat complexity in the laboratory,19 evaluating the effects of this feature during potentially stressful situations, such as pair housing, was of interest. Evaluating the suitability of housing and husbandry conditions for zebrafish through measurement of cortisol levels can be a useful approach to determining how best to care for these animals in the laboratory setting. Our study demonstrated that although cortisol can be a reliable marker of stress in zebrafish,1,2,6,24,27,30 it should not be the sole method used to evaluate husbandry.
The goal of the current study was to test the hypothesis that adding habitat complexity to the tank of pair housed zebrafish improves welfare and reduces stress levels. Cortisol levels did not differ between Veg and NVeg pairs on day 1. Vegetation was associated with higher cortisol among pairs on day 5 but lower levels by day 10. The experimental fish had never been exposed to vegetation or any other habitat complexity prior to the experiment. This result suggests that the novel element in the environment induces stress and that fish may require as much as 10 d to adapt to vegetation and use it to avoid aggression by their mates. This finding supports other authors who have recommended a prolonged acclimation period of 10 d when using zebrafish to test stress responses.8 The high levels of aggression, injury, and even death observed in the NVeg pairs is consistent with the high cortisol levels observed by day 10. Aggression without death or obvious injury was observed in the Veg pairs. There are several possible explanations for our results.
One possible explanation is that adding vegetation does not decrease stress levels in single-paired zebrafish during the acclimation phase. A study in juvenile zebrafish found no difference in cortisol levels between small groups with or without habitat complexity.39 This lack of difference in cortisol may have been due to undeveloped endocrine systems of fish at this age,37 but our study in adult zebrafish supports the finding that adding complexity did not reduce stress, at least according to cortisol levels during the first 5 d. The same study also found that aggression remained high until the fifth day in the barren tanks, whereas the tanks with complexity didn't see a drop in aggression until day 10; the authors proposed that the addition of habitat complexity prolongs the establishment of a dominant–subordinate relationship.39 Our study saw a similar trend (Figure 2). Perhaps the presence of vegetation early during the acclimation period does not alter stress but instead merely provides zebrafish a refuge from injury and death. Regardless of cortisol levels, the death and injury observed in the NVeg pairs poses an animal welfare issue that should be evaluated further.
Another possible interpretation is that NVeg pairs have spuriously low cortisol values; the argument for this explanation lies in the loss of pairs due to injury in this group. The lower-than-expected values in the NVeg pairs on day 5 might reflect pairs that were compatible and were therefore not particularly stressed. Cortisol levels in fish may not rise significantly unless there is a life-threatening stressor.37 The pairs that were removed from our study had lost a mate due to fighting, and we did not measure the cortisol levels in these pairs. Perhaps if these fish had survived, their cortisol concentrations might have raised the NVeg group mean. The cortisol values from the Veg pairs were higher than those of the NVeg group on day 5, supporting the previous proposal that the dominant–subordinate relationship in these fish is delayed.39 In addition, in other fish species, high cortisol levels can actually inhibit aggression;11 this effect must be considered as an alternative cause of the decreased aggression in the Veg group.
Two additional factors to consider are the use of a dark background to surround the zebrafish tanks and the housing of tanks on the recirculating water system. We wanted to prevent the effects of other stressors, such as the visualization of other fish outside the home tank2,27 from confounding our results and to keep our experimental tanks on the facility's recirculating water system to avoid the stress associated with manual water changes.27 Zebrafish prefer dark backgrounds,5,22 and housing zebrafish with dark backgrounds lowers cortisol levels compared with those in zebrafish housed with light backgrounds.28 To eliminate the background as a source of stress, we chose a dark color for our dividers. Perhaps using a dark background, which is not a common practice, and continual water changes on the recirculating system helped keep cortisol levels in our study fish lower than what is typical in a single mating-pair housing set-up. However, these features did not prevent death and injury in the NVeg group.
Although cortisol values differed significantly between groups, they may indicate only mild increases in stress. In addition, our results may reflect, in part, characteristics of the strain we used. Zebrafish strains are known to differ in their behavior and propensity for stress and anxiety.7,25
A particularly interesting finding in our study was that cortisol levels rose in individually housed male zebrafish as their time in isolation increased. Zebrafish are a social species,4 and social isolation for an extended period of time may be stressful to these animals. The study we selected as a reference for using individually housed zebrafish as controls reported low cortisol levels after housing for 2 weeks.27 Cortisol levels were not measured throughout those 2 weeks in that study,27 so it is possible that the fish might have experienced stress early on which then decreased after 2 wk. Additional studies are needed to evaluate the potential stress of individually housing zebrafish, of both sexes, given that fish may need to be isolated for long periods during particular types of experiments.
Limitations to our study included its small sample size. In the future, this experiment should be repeated with larger sample sizes and additional time points. In addition, we were unable to determine the dominant fish in all pairs, and an attempt to videotape and qualify the behavior between mates was unsuccessful. Whereas the identity of the dominant fish was obvious in some tanks (that is, it was observed to chase and bite), not all pairs could be evaluated due to either a lack of interaction during video recording or because interaction occurred beyond the field of view of the camera. For most tanks that were scored, the subordinate fish had the higher cortisol value. We were not confident in this analysis, however, as manual quantification of zebrafish behavior has been criticized for its potential for error.7 Measuring behavior in zebrafish can be challenging, because which behaviors are manifestations of an anxious state of being is unclear.5 However, some studies have shown that cortisol concentration is elevated in fish that have demonstrated behavior that has been classified as indicative of anxiety or stress.7,13 Many studies are starting to use computer analysis of zebrafish behavior as a generally more accepted method to qualify behavior and stress.7 Computer program analysis eliminates human error and the possible confounding effect of a human presence at the tank side on stress and anxiety. Such equipment was unavailable for this study but likely would have provided interesting additional information.
Another limitation is how little we know about the normal range of values for cortisol in zebrafish. Other studies report a wide range of values, which also differ between the 2 assays used commonly, radioimmunoassay and ELISA.32 Our reported range of cortisol levels is consistent with previous findings using cortisol radioimmunoassay and, other than a few outliers, our range of cortisol levels did not exceed the ranges reported in other studies26,30 (Table 1). Regarding the evaluation of trends, other studies have reported whole-body cortisol concentrations near 1 ng/g in ‘nonstressed’ control fish.30 Our individually housed zebrafish had similar levels on day 1, indicating that these fish were not stressed at that point. In addition, our finding of similar cortisol levels between male and female zebrafish supports previous findings of no difference between the sexes in terms of behavior and aggression.25,36
Although our study did not show a reduction in cortisol levels with the addition of vegetation to pair-housed zebrafish until day 10, it did demonstrate a potential welfare-associated situation when housing pairs for an extended period of time. After 1 d together, all pairs survived, but by day 5, members of pairs in the NVeg treatment group began to die. In light of this observation and to prevent potential injury and death of fish, we recommend the addition of habitat complexity, such as plastic vegetation, that provides infrastructure within the water column when single pairs of zebrafish must be maintained for more than 24 h.
In conclusion, 2 important implications arise from our findings. First, and most importantly, housing pairs of zebrafish for an extended time poses a serious animal welfare concern. Our results very clearly showed that zebrafish pairs housed for 5 d or more had a significantly higher likelihood of experiencing injury or death, Adding plastic vegetation prevented these serious outcomes. Second, relying on cortisol data alone to evaluate the suitability of husbandry conditions may be insufficient to support well informed management decisions. The cortisol levels that we measured in the present study did not reflect the magnitude of the welfare issue that we observed. We hope our current study sheds new light on potential welfare issues surrounding common husbandry practices for zebrafish.
Acknowledgments
We thank Owen McGuinness for his assistance in developing our cortisol assay methodology, Joshua T Gamse and Qiang Guan for providing their zebrafish expertise and support during the course of our study, Carla Harris for her technical assistance with cortisol extraction, and Dale Plummer for his assistance with our statistical analyses.
References
- 1.Aluru N, Vijayan MM. 2009. Stress transcriptomics in fish: a role for genomic cortisol signaling. Gen Comp Endocrinol 164:142–150. [DOI] [PubMed] [Google Scholar]
- 2.Barcellos LJG, Ritter F, Kreutz LC, Quevedo RM, Bolognesi da Silva L, Bedin AC, Finco J, Cericato L. 2007. Whole-body cortisol increases after direct and visual contact with a predator in zebrafish, Danio rerio. Aquaculture 272:774–778. [Google Scholar]
- 3.Basquill SP, Grant JWA. 1998. An increase in habitat complexity reduces aggression and monopolization of food by zebrafish (Danio rerio). Can J Zool 76:770–772. [Google Scholar]
- 4.Blaser R, Gerlai R. 2006. Behavioral phenotyping in zebrafish: comparison of 3 behavioral quantification methods. Behav Res Methods 38:456–469. [DOI] [PubMed] [Google Scholar]
- 5.Blaser RE, Chadwick L, McGinnis GC. 2010. Behavioral measures of anxiety in zebrafish (Danio rerio). Behav Brain Res 208:56–62. [DOI] [PubMed] [Google Scholar]
- 6.Cachat J, Stewart A, Grossman L, Gaikwad S, Kadri F, Chung KM, Wu N, Wong K, Roy S, Suciu C, Goodspeed J, Elegante M, Bartels B, Elkhayat S, Tien D, Tan J, Denmark A, Gilder T, Kyzar E, Dileo J, Frank K, Chang K, Utterback E, Hart P, Kalueff AV. 2010. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat Protoc 5:1786–1799. [DOI] [PubMed] [Google Scholar]
- 7.Cachat JM, Canavello PR, Elegante MF, Bartels BK, Elkhayat SI, Hart PC, Tien AK, Tien DH, Beeson E, Mohot S, Laffoon AL, Stewart AM, Gaikwad S, Wong K, Haymore W, Kalueff AV. 2011. Modeling stress and anxiety in zebrafish, p 73–88. In: Kalueff AV, Cachat JM. Zebrafish models in neurobehavioral research, vol 52. New York (NY): Springer. [Google Scholar]
- 8.Canavello PR, Cachat JM, Beeson E, Laffoon AL, Grimes C, Haymore W, Elegante MF, Bartels BK, Hart PC, Elkhayat SI, Tien DH, Mohnot S, Amri H, Kalueff AV. 2011. Measuring endocrine (cortisol) responses of zebrafish to stress, p 135–142. In: Kalueff AV, Cachat JM. Zebrafish neurobehavioral protocols, vol 51. New York (NY): Springer. [Google Scholar]
- 9.Carfagnini AG, Rodd FH, Jeffers KB, Bruce AEE. 2009. The effects of habitat complexity on aggression and fecundity in zebrafish (Danio rerio). Environ Biol Fishes 86:403–409. [Google Scholar]
- 10.Clark SM, Sand J, Francis TC, Nagaraju A, Michael KC, Keegan AD, Kusnecov A, Gould TD, Tonelli LH. 2014. Immune status influences fear and anxiety responses in mice after acute stress exposure. Brain Behav Immun 38:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiBattista JD, Anisman H, Whitehead M, Gilmour KM. 2005. The effects of cortisol administration on social status and brain monoaminergic activity in rainbow trout Oncorhynchusmykiss. J Exp Biol 208:2707–2718. [DOI] [PubMed] [Google Scholar]
- 12.Dupont WD. 2009. Statistical modeling for biomedical researchers: a simple introduction to the analysis of complex data, 2nd ed. Cambridge (United Kingdom): Cambridge University Press [Google Scholar]
- 13.Egan RJ, Bergner CL, Harta PC, Cachat JM, Canavello PR, Elegante MF, et al. 2009. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res 205:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engeszer RE, Barbiano LA, Ryan MJ, Parichy DM. 2007. Timing and plasticity of shoaling behaviour in the zebrafish, Danio rerio. Anim Behav 74:1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filby AL, Paull GC, Bartlett EJ, Van Look KJ, Tyler CR. 2010. Physiological and health consequences of social status in zebrafish (Danio rerio). Physiol Behav 101:576–587. [DOI] [PubMed] [Google Scholar]
- 16.Harper C, Lawrence C. 2011. The laboratory zebrafish. Boca Raton (FL): CRC Press. [Google Scholar]
- 17.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 18.Johnson SL, Midson CN, Ballinger EW, Postlethwait JH. 1994. Identification of RAPD primers that reveal extensive polymorphisms between laboratory strains of zebrafish. Genomics 19:152–156. [DOI] [PubMed] [Google Scholar]
- 19.Kistler C, Hegglin D, Würbel H, König B. 2011. Preference for structured environment in zebrafish (Danio rerio) and checker barbs (Puntius oligolepis). Appl AnimBehav Sci 135:318–327. [Google Scholar]
- 20.Larson ET, O'Malley DM, Melloni RH., Jr 2006. Aggression and vasotocin are associated with dominant-subordinate relationships in zebrafish. Behav Brain Res 167:94–102. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence C. 2011. Advances in zebrafish husbandry and management. methods in cell biology, p 429–451. In: Detrich HW, Westerfield M, Zon LI. The zebrafish: genetics, genomics, and informatics, vol104. Waltham (MA): Academic Press. [DOI] [PubMed] [Google Scholar]
- 22.Maximino C, de Brito TM, da Silva Batista AW, Herculano AM, Morato S, Gouveia A., Jr 2010. Measuring anxiety in zebrafish: a critical review. Behav Brain Res 214:157–171. [DOI] [PubMed] [Google Scholar]
- 23.Mazeaud MM, Mazeaud F, Donaldson EM. 1977. Primary and secondary effects of stress in fish. T Am Fish Soc 106:201–212. [Google Scholar]
- 24.Mommsen TP, Vijayan MM, Moon TW. 1999. Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fish 9:211–268. [Google Scholar]
- 25.Moretz JA, Martins EP, Robison BD. 2007. Behavioral syndromes and the evolution of correlated behavior in zebrafish. BehavEcol 18:556–562. [Google Scholar]
- 26.Oliveira TA, Koakoski G, da Motta AC, Piato AL, Barreto RE, Volpato GL, Barcellos LJ. 2014. Death-associated odors induce stress in zebrafish. HormBehav 65:340–344. [DOI] [PubMed] [Google Scholar]
- 27.Parker MO, Millington ME, Combe FJ, Brennan CH. 2012. Housing conditions differentially affect physiological and behavioural stress responses of zebrafish, as well as the response to anxiolytics. PLoSONE 7:e34992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavlidis M, Digka N, Theodoridi A, Campo A, Barsakis K, Skouradakis G, Samaras A, Tsalafouta A. 2013. Husbandry of zebrafish, Danio rerio, and the cortisol stress response. Zebrafish 10:524–531. [DOI] [PubMed] [Google Scholar]
- 29.Pritchard VL, Lawrence J, Butlin RK, Krause J. 2001. Shoal choice in zebrafish, Danio rerio: the influence of shoal size and activity. Anim Behav 62:1085–1088. [DOI] [PubMed] [Google Scholar]
- 30.Ramsay JM, Feist GW, Varga ZM, Westerfield M, Kent ML, Schreck CB. 2006. Whole-body cortisol is an indicator of crowding stress in adult zebrafish, Danio rerio. Aquaculture 258:565–574. [Google Scholar]
- 31.Schroeder P, Jones S, Young IS, Sneddon LU. 2014. What do zebrafish want? Impact of social grouping, dominance and gender on preference for enrichment. Lab Anim 48:328–337. [DOI] [PubMed] [Google Scholar]
- 32.Sink TD, Kumaran S, Lochmann RT. 2007. Development of a whole-body cortisol extraction procedure for determination of stress in golden shiners, Notemigonuscrysoleucas. Fish PhysiolBiochem 33:189–193. [Google Scholar]
- 33.Spence R, Smith C. 2005. Male territoriality mediates density and sex ratio effects on oviposition in the zebrafish, Danio rerio. AnimBehav 69:1317–1323. [Google Scholar]
- 34.Spence R, Fatema MK, Reichard , Huq KA, Wahab MA, Ahmed ZF, Smith C. 2006. The distribution and habitat preferences of the zebrafish in Bangladesh. J Fish Biol 69:1435–1448. [Google Scholar]
- 35.Spence R, Ashton R, Smith C. 2007. Oviposition decisions are mediated by spawning site quality in wild and domesticated zebrafish, Danio rerio. Behaviour 144:953–966. [Google Scholar]
- 36.Spence R, Gerlach G, Lawrence C, Smith C. 2008. The behavior and ecology of the zebrafish, Danio rerio. Biol Rev CambPhilosSoc 83:13–34. [DOI] [PubMed] [Google Scholar]
- 37.Steenbergen PJ, Richardson MK, Champagne DL. 2011. The use of the zebrafish model in stress research. Prog Neuropsychopharmacol Biol Psychiatry 35:1432–1451. [DOI] [PubMed] [Google Scholar]
- 38.Walker C. 1999. Haploid screens and γ-ray mutagenesis. Methods Cell Biol 60:43–70. [DOI] [PubMed] [Google Scholar]
- 39.Wilkes L, Owen SF, Readman GD, Sloman KA, Wilson RW. 2012. Does structural enrichment for toxicology studies improve zebrafish welfare? Appl Anim Behav Sci 139:143–150. [Google Scholar]