Abstract
Alopecia has been reported to occur in several species of captive NHP. Much of this research has focused on macaque monkeys; whether other primate species such as baboons are affected similarly is unknown. Because alopecia can be a focus of inspectors and a possible marker of wellbeing, the purpose of the current study was to survey the occurrence of alopecia in 2 baboon populations and to identify potential risk factors. Subjects were 262 group-housed and 279 corral-housed baboons. Alopecia was assessed cage-side (group-housed) and on sedated animals (corral-housed). Although alopecia was mild in both populations, there were significant effects of season and sex. Alopecia was greater in the winter (group-housed) and the fall (corral-housed) and in female baboons. In addition, the group-housed baboons showed a significant negative effect of age and a lesser effect of group size on alopecia. These results demonstrate that variables other than those associated with animal management practices can affect hair loss in baboons.
Alopecia, or hair loss, is a multifaceted condition that occurs in several species of captive NHP.23 The extent of hair loss can vary, ranging from diffuse hair thinning and small, focal bare spots to large areas of missing hair,10,11,15,30 but most reported cases are mild.2,30 Alopecia is not an uncommon condition; as many as 34% to 86.5% of rhesus monkeys maintained in laboratory environments exhibit some form of hair loss.16,20,31 However, the etiology of this condition is poorly understood, given the numerous and diverse potential factors contributing to hair loss, including sex, age, season, housing condition, pregnancy, social rank, behavior, hormonal changes, disease, and stress.2,16,20,22,23,30,32 Because alopecia is often readily visible and because it can be a potential indicator of impaired health or wellbeing, it is often a focus of inspectors and facility managers. Therefore, it is important to assess the extent and etiology of alopecia in captive primate populations to better understand hair loss as an indicator of animal wellbeing.
Intrinsic characteristics of animals, including species, sex, and age, are known to play a role in hair loss. Within the genus Macaca, species vary in terms of overall amounts of alopecia. In several studies, pigtailed macaques (M. nemestrina) showed greater amounts of alopecia than did either rhesus (M. mulatta) or cynomolgus (M. fascicularis) macaques,5,17 and rhesus macaques showed greater amounts of alopecia than did cynomolgus macaques.16,17 In addition, hair loss appears to affect female more than male NHP,3,16,17,20,30 which may be due in part to pregnancy.2,7 However, this finding is not universal; in one study, no sex-associated difference was observed,5 whereas in another study, male macaques were affected more than were female.18 In addition, age can affect the severity of alopecia; younger animals tend to have a thicker hair coat than do older animals,2,5,16,17,30 but in some cases, young adult animals (for example, 4 to 10 y old) had significantly higher alopecia scores than did older adults (for example, older than 10 y).17
Hair growth or hair loss can also be influenced by environmental factors such as season or housing condition. In rhesus macaques, hair loss or molting can be seasonal,32 with the poorest coats occurring during the spring or winter months and better coats occurring during the autumn months.2,30 Similarly, seasonal hair loss has been reported to occur in other species of macaques, including pigtail, cynomolgus, and Japanese macaques, with hair loss occurring primarily in the fall or winter,17,38 and in wild vervet monkeys (Cercopithecus aethiops), with hair loss peaking during November through January.13 In addition to seasonal effects, captive housing conditions can affect alopecia. For example, NHP housed outdoors tended to have a better coat condition than did those housed indoors,30 and animals that originated from an outdoor colony before being moved indoors tended to have a lower incidence of alopecia than did those born and raised indoors and currently housed under the same conditions.16 Similarly, rhesus macaques housed in enclosures with gravel substrates had significantly more alopecia than did those in grass-covered enclosures. This poor coat condition in animals on gravel substrates may be due to decreased time spent foraging and increased time spent grooming.1,2
Social factors, such as dominance rank and group size, can influence hair loss. For example, low- or middle-ranking rhesus monkeys tend to have worse coats than do their high-ranking counterparts.2,13 Some of this hair loss may be due to hair-pulling by self or others.20,27 Despite the rank-related effects, social housing is associated overall with increased coat quality. Pair-housed NHP were noted to have a better hair coat than did those that were singly housed,17 and moving animals into pairs or social groups helped to reduce alopecia.9 However, coat condition was reported to worsen with a decrease in available space per animal,30 and increasing animal density intensified the influence of pregnancy on hair loss.2 These effects may result from increased stress30 or increased grooming in response to increased cage density.14
Whereas the extent of and risk factors for alopecia have primarily been assessed in macaque monkeys,1,2,16-18,20,22,31 less is known about the occurrence of alopecia in baboon populations. Therefore, the purpose of our current study was to assess the extent of alopecia in captive baboons and to determine whether housing conditions and factors other than animal management practices, such as season, sex, and age, contribute to alopecia in this population.
Materials and Methods
All animals in both studies were housed at the Southwest National Primate Research Center, which is AAALAC-accredited, and maintained in accordance with the Guide for the Care and Use of Laboratory Animals.12 All work was approved by the IACUC of the Southwest National Primate Research Center.
Study 1: Group-housed baboons.
Subjects.
The subjects were 317 olive and olive hybrid baboons (Papio hamadryas spp; male, 156; female, 161; age, 2 to 29 y [mean, 8 y]). They were housed in 25 social groups (13 same-sex groups, 1 juvenile group, 11 harem groups) that ranged in size from 5 to 21 baboons (mean, 14) in outdoor cages that measured approximately 45 m2 and contained indoor access. Of the 317 subjects, 55 (24 male, 31 female) were unavailable for scoring in the winter. Therefore, data analyses were conducted on the remaining 262 baboons (male, 132; female, 130; age, 2 to 25 y [mean, 7.5 y]).
Procedures.
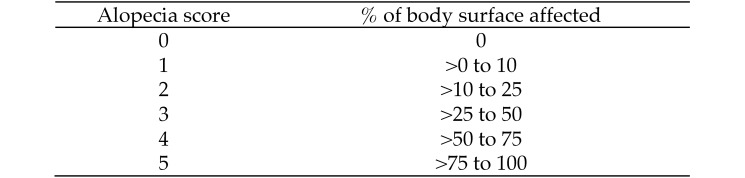
Two observers assessed and achieved consensus regarding each animal's coat condition by using the ‘rule of nines’ and the alopecia scoring system developed by the National Primate Research Center Behavioral Management Consortium.4 The baboons were awake and in their social groups during scoring, and the observers viewed the animals from outside of the cage. Alopecia scores were based on the percentage of body surface affected (Figure 1). Scoring took place during Summer 2009 (June and July) and in the winter (December 2009 and January 2010) to assess the effect of season on alopecia.
Figure 1.
Alopecia score according to percentage of body surface affected.
Study 2: Corral-housed baboons.
Subjects.
The subjects were 334 olive and olive hybrid baboons (Papio hamadryas spp.; male, 118; female, 216; age, 2.4 to 17.9 y [mean, 7.4 y]). They were housed outdoors in two 6-acre, same-sex corrals. In addition, 2 adult vasectomized male baboons were housed in the female corral, but these 2 male baboons were excluded from this study.
Procedures.
Alopecia scoring took place while the baboons were sedated for twice-annual physical examinations, which occurred between 2009 and 2011 (2 in April and 2 in October). Two observers closely inspected each animal while it was recovering from sedation and scored alopecia by using the previously described scoring system.4 The observers achieved consensus for each alopecia score. Only animals that had at least one score each for April and October were included in the study. This criterion reduced the sample size by 55, resulting in a sample size of 279 baboons (male, 86; female, 193; age, 2.4 to 17.9 y [mean, 7.5 y]). For subjects that were scored for a given month during both years, only the first score for that month was used.
Data analyses.
The data were analyzed by using the R language environment for statistical computing26 within the RStudio29 development environment, including the dplyr,34 ggplot237, knitr,33 lubridate,8 moments,19 repolr,25 RODBC,28 stringr,35 testthat,36 XLConnect,21 and xtable6 packages. The package repolr, which uses a modified version of the generalized estimating equation method for model fitting, was used to fit a proportional-odds logistic-regression model to the ordinal alopecia score data. We chose this model because it accommodates ordered categorical dependent variables and both numeric and categorical independent variables. Independent predictive variables used are defined below within the description of each study.
Study 1: Group-housed baboons.
Season, sex, and pregnancy status were each treated as categorical predictor variables having 2 states. Group size was treated as a numeric variable and defined as the average number of cage mates each animal had for the 2 mo of June and July for the summer group sizes and the 2 mo of December and January for the winter group sizes. Age was coded as a numeric variable and measured to the nearest day.
There were not enough pregnant animals to include pregnancy within the regression analysis. In addition, the number of pregnant baboons during the summer (n = 3) was too small for statistical analysis. However, 11 baboons were pregnant during the winter assessments. To test the effect of pregnancy on hair coat during the winter, a 2-sample t test was conducted on these female alopecia scores, with the scores being treated as numeric values.
Study 2: Corral-housed baboons.
Season and sex were each treated as categorical predictor variables, and age was coded as a numeric variable, as in study 1. Pregnancy and group size could not be used, because no animals were at risk of being pregnant and because the baboons were housed in 2 social groups only.
Results
Study 1: Group-housed baboons.
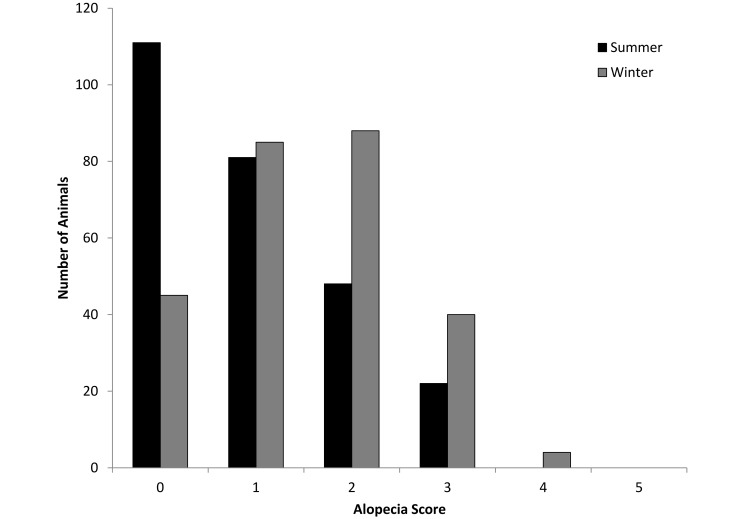
The extent of alopecia in the group-housed baboons was mild. On a scale of 0 to 5, the average alopecia score was 0.9 (range, 0 to 3) for the summer and 1.5 (range, 0 to 4) for the winter (Figure 2). In the winter, pregnant female baboons (n = 11) had significantly (t128 = –2.21, P < 0.05) higher alopecia scores than did nonpregnant female baboons.
Figure 2.
Alopecia scores in the group-housed animals.
Our intent was to select the model with the lowest Quasi Akaike Information Criterion.24 However, we found that the coefficients were unstable during the initial fits of a 3-level proportional-odds model using season, age, sex, and group size with interactions among all independent variables. This instability was likely caused by having too few observations for some of the combinations of variables. We therefore reduced the model to a 2-level proportional-odds model and tested all possible 2-way interactions. The model with the lowest Quasi Akaike Information Criterion was that in which there was a sex × age interaction with a coefficient (log odds, 0.05; odds, 1.05), indicating that young female baboons were more likely to have a higher alopecia score than that explained by a model without interactions. However, although the coefficient estimates were very similar to the estimates provided by the 1-level proportional-odds model, all but the coefficient for seasonality failed to be significantly different from 0. We therefore chose to describe in detail the results of the 1-level proportional-odds model, which included season, sex, age, and group size (Table 1).
Table 1.
Regression coefficients and robust standard errors for group-housed baboons
| Log (odds) | Odds | Robust SE | P | |
| Season | −1.14 | 0.32 | 0.119 | < 0.001 |
| Sex | 0.64 | 1.89 | 0.204 | 0.002 |
| Age | 0.05 | 1.05 | 0.020 | 0.011 |
| Group size | 0.04 | 1.04 | 0.022 | 0.092 |
The estimated seasonal effect was −1.14. However, this result is on the log-odds scale. Interpretation is easier when the exponential function is used on the estimate to obtain the effects in terms of odds. This adjustment yields a treatment effect of 0.32 (P < 0.001), indicating that baboons during winter were 3.1 times more likely to have alopecia than were animals in the summer.
Similarly the sex-effect estimate was 0.64 on the log-odds scale and 1.89 (P < 0.005) on the odds scale, indicating that female baboons were 1.9 times more likely to have alopecia than were male. The age effect estimate was 0.05 on the log-odds scale and 1.05 (P < 0.05) on the odds scale, indicating that younger baboons were, on average, more likely to experience hair loss than were older animals. The group-size effect estimate was 0.04 on the log-odds scale and 1.04 (P < 0.10) on the odds scale, indicating that animals within smaller compared with larger groups tended to experience more hair loss.
Study 2: Corral-housed baboons.
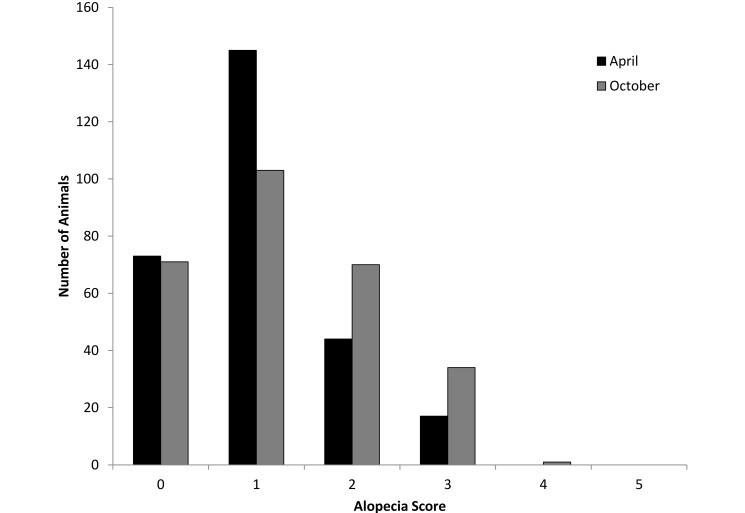
Like that in group-housed animals, the extent of alopecia in the corralled baboons was mild. On a scale of 0 to 5, the average alopecia score was 1.0 (range, 0 to 3) in April and 1.2 (range, 0 to 4) in October (Figure 3).
Figure 3.
Alopecia scores in the corral animals.
For these data, we were able to select the model with the lowest Quasi Akaike Information Criterion. The independent variables available for modeling alopecia scores were seasonality, sex, and age, with age modeled as a continuous variable. The best-fitting model included only season and sex (Table 2). The corralled baboons were housed in only 2 groups, preventing any estimate of group-size effect, and it is likely that the limited age distribution among both sexes, and particularly within the male baboons, reduced our ability to detect age-associated effects within the corral-housed animals. The maximal age for male baboons in study 2 was below the third quartile for all other groups and below the mean for female baboons in both studies (Table 3).
Table 2.
Regression coefficients for best-fitting model for corral-housed animals.
| Log (odds) | Odds | Robust SE | P | |
| season | −0.44 | 0.64 | 0.121 | < 0.001 |
| sex | 0.35 | 1.42 | 0.209 | 0.097 |
Table 3.
Age distribution (y) according to sex
| Study | Sex | Minimum | First quartile | Median | Mean | Third quartile | Maximum |
| 1 | Female | 1.7 | 3.7 | 8.2 | 8.8 | 11.7 | 24.6 |
| 1 | Male | 1.7 | 3.4 | 3.8 | 6.3 | 8.4 | 20.7 |
| 2 | Female | 2.6 | 5.1 | 6.5 | 8.5 | 12.6 | 17.9 |
| 2 | Male | 2.4 | 4.8 | 5.2 | 5.4 | 5.8 | 7.9a |
Note that the maximal age for male baboons in study 2 is below the third quartile of all other groups and below the mean age of female baboons in both studies.
The estimated seasonal effect was −0.44 on the log-odds scale and 0.64 on the odds scale (P < 0.001). This result indicates that the average baboon is 1.56 times more likely to have a higher alopecia score in the winter than in the summer. The sex-effect estimate was 0.35 on the log-odds scale and 1.42 on the odds scale (P < 0.10), indicating a trend that female baboons were 1.4 times more likely to have hair loss than were male baboons.
Discussion
This study showed that mild alopecia was present in 2 populations of baboons, with a median score of 1 on a scale of 0 to 5, and that both sex and season affected hair coats in both populations. These results were consistent across the 2 studies even though the baboons in the 2 studies had different housing conditions (medium-sized caged groups compared with large corralled groups) and different degrees of animal contact (viewed from outside of the cage compared with physical contact with sedated animals).
Our study found that female baboons exhibited greater hair loss than did male baboons. This finding is consistent with many studies that report greater amounts of alopecia in female macaques.3,16,20,30 Greater hair loss in female NHP may in part be due to pregnancy, given that a poor hair coat often is pronounced in the final months of pregnancy and during the month after parturition.2,7 This explanation may have been the case in our group-housed baboons, because some of the female baboons were pregnant at the time of observation, and those that were pregnant in the winter had higher alopecia scores than did those that were not pregnant. Baboons in this colony give birth year-round, although births increase in the spring. However, due to the small percentage of pregnant female baboons in the group-housed population, especially during the summer, there are insufficient data to detect a significant effect of pregnancy on overall alopecia scores in female baboons. In addition, the corral-housed female baboons were housed with 2 vasectomized male baboons in an otherwise all-female group and were not pregnant at the time of observation; however, these corralled female baboons, like those group-housed, exhibited a trend toward greater hair loss than that observed in male baboons. Therefore the factors that underlie the sex-associated differences in alopecia in the 2 populations are unclear. Other factors, such as social conditions, may have played a role.
In addition to sex, season affected hair loss in the baboons, with levels of alopecia being the highest in the fall (corral-housed) and winter (group-housed). Seasonality in hair loss has been reported to occur in other primate species, and the seasons with the greatest hair loss tend to be fall, winter, and spring.2,13,17,30,32,38 For example, poor coats were reported to occur in the winter for vervet monkeys and Japanese macaques,13,38 in the winter and spring for rhesus monkeys,2,30 and in the fall and winter for cynomolgus monkeys.17 Differences in seasonality may be due in part to species-specific features or to differences in climate. Possible mechanisms for seasonal changes in alopecia include temperature, humidity, and photoperiod,2,17,30 all of which can affect outdoor animals.
In the group-housed baboons, younger animals had more hair loss than did older animals. This finding contradicts reports of rhesus monkeys, in which older animals frequently were noted to have poorer hair coats than those of younger animals.2,5,16,23,30 However, this age-associated effect is inconsistent; in one study, older adult macaques had significantly lower alopecia scores than did younger adults.17 Because our observers assessed the hair coats of group-housed animals from outside of the cage and at a short distance, mild age-related hair thinning may have gone unnoticed in the present study. Alternatively, baboons may not consistently experience the age-related hair thinning or hair loss that is often reported in macaques. In addition, our ability to detect and measure age-associated effects was reduced by the age distributions in both of our studies. Specifically, our baboons were young predominantly, with a median age of 5.7 y, and only 12% of baboons were 14 y of age or older. The effect of age on alopecia in baboons therefore needs further investigation.
In the group-housed baboons, group size, and therefore cage density, did not play a significant role in alopecia. However, there was a trend toward increased alopecia in smaller groups. This finding contrasts with reports in rhesus monkeys, in which coat condition worsened as the amount of space per animal decreased.30 In our current study, the larger groups in the group-housed population tended to consist of younger, and therefore smaller, baboons, a factor may have lessened the influence of group size on available space. In addition, the enclosures for group-housed baboons were relatively large, at approximately 45 m2. Perhaps the baboons in this population were not sufficiently constricted for animal density or social stress to affect alopecia. In addition, the positive benefits of outdoor housing30 may have helped to mitigate the effect of group size on alopecia.
As in rhesus monkeys, hair loss in baboons is complex and can be affected by many different variables. However, some variables, such as sex and season, may play a greater role in baboon alopecia than do other variables, such as age or cage density. Although alopecia may be a focus of some inspectors or facility managers, all hair loss does not necessarily reflect animal management practices. Our study highlights the importance of identifying which variables do influence alopecia in captive primate populations, to help direct appropriate care.
Acknowledgments
We thank Felicia Ponce, Laura Condel, Sarah McKinney, Heath Nevill, Diana Mejido, Cheryl Jones, and Brittany Peterson for their assistance with alopecia scoring. We also thank Priscilla Williams for her assistance with the data records and data analysis. This research was supported by grant nos. R24OD01180-15 to Melinda Novak (University of Massachusetts) and P51OD011133 to Texas Biomedical Research Institute (SNPRC).
References
- 1.Beisner BA, Isbell LA. 2008. Ground substrate affects activity budgets and hair loss in outdoor captive groups of rhesus macaques (Macaca mulatta). Am J Primatol 70:1160–1168. [DOI] [PubMed] [Google Scholar]
- 2.Beisner BA, Isbell LA. 2009. Factors influencing hair loss among female captive rhesus macaques (Macaca mulatta). Appl Anim Behav Sci 119:91–100. [Google Scholar]
- 3.Conti G, Hansman C, Heckman JJ, Novak MFX, Ruggiero A, Suomi SJ. 2012. Primate evidence on the late health effects of early-life adversity. Proc Natl Acad Sci USA 109:8866–8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crockett CM, Baker KC, Lutz CK, Coleman K, Fahey MA, Bloomsmith MA, McCowan B, Sullivan J, Weed JL. 2009. Developing a reliable laboratory primate alopecia scoring system for interfacility collaboration and online training.Am J Primatol 71:73. [Google Scholar]
- 5.Crockett CM, Bentson KL, Bellanca RU. 2007. Alopecia and overgrooming in laboratory monkeys vary by species but not sex, suggesting a different etiology than self-biting. Am J Primatol 69:87–88. [Google Scholar]
- 6.Dahl DB. [Internet] 2014. xtable: export tables to LaTeX or HTML. R package version 1.7-4. [Cited 30 October 2014] Available at: http://CRAN.R-project.org/package=xtable.
- 7.Davis EB, Suomi SJ. 2006. Hair loss and replacement cycles in socially housed, pregnant, rhesus macaques. Am J Primatol 68:58. [DOI] [PubMed] [Google Scholar]
- 8.Grolemund G, Wickham H. 2011. Dates and times made easy with lubridate. J Stat Softw 40:1–25. [Google Scholar]
- 9.Harding K. 2013. Behavior treatment of alopecia in Macaca fascicularis: comparison of outcomes. Am J Primatol 75:51. [Google Scholar]
- 10.Honess P, Gimpel JL, Wolfensohn SE, Mason GJ. 2005. Alopecia scoring: the quantitative assessment of hair loss in captive macaques. Altern Lab Anim 33:193–206. [DOI] [PubMed] [Google Scholar]
- 11.Horenstein VD-P, Williams LE, Brady AR, Abee CR, Horenstein MG. 2005. Age-related diffuse chronic telogen effluvium-type alopecia in female squirrel monkeys (Saimiri boliviensis boliviensis). Comp Med 55:169–174. [PubMed] [Google Scholar]
- 12.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 13.Isbell LA. 1995. Seasonal and social correlates of changes in hair, skin, and scrotal condition in vervet monkeys (Cercopithecus aethiops) of Amboseli National Park, Kenya. Am J Primatol 36:61–70. [DOI] [PubMed] [Google Scholar]
- 14.Judge PG, de Waal FBM. 1997. Rhesus monkey behaviour under diverse population densities: coping with long-term crowding. Anim Behav 54:643–662. [DOI] [PubMed] [Google Scholar]
- 15.Kimura T. 2008. Systemic alopecia resulting from hyperadrenocorticism in a Japanese monkey. Lab Primate Newsl 47:5–9. [Google Scholar]
- 16.Kramer J, Fahey M, Santos R, Carville A, Wachtman L, Mansfield K. 2010. Alopecia in rhesus macaques correlates with immunophenotypic alterations in dermal inflammatory infiltrates consistent with hypersensitivity etiology. J Med Primatol 39:112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroeker R, Bellanca RU, Lee GH, Thom JP, Worlein JM. 2014. Alopecia in 3 macaque species housed in a laboratory environment. Am J Primatol 76:325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luchins KR, Baker KC, Gilbert MH, Blanchard JL, Liu DX, Myers L, Bohm RP. 2011. Application of the diagnostic evaluation for alopecia in traditional veterinary species to laboratory rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 50:926–938. [PMC free article] [PubMed] [Google Scholar]
- 19.Komsta L, Novomestky F. [Internet] 2012. moments: moments, cumulants, skewness, kurtosis, and related tests. R package version 0.13. [Cited 30 October 2014] Available at: http://CRAN.R-project.org/package=moments
- 20.Lutz CK, Coleman K, Worlein J, Novak MA. 2013. Hair loss and hair-pulling in rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 52:454–457. [PMC free article] [PubMed] [Google Scholar]
- 21.Mirai Solutions. [Internet] 2014. XLConnect: Excel connector for R. R package version 0.2-9. [Cited 30 October 2014] Available at: http://CRAN.R-project.org/package=XLConnect.
- 22.Novak MA, Hamel AF, Coleman K, Lutz CK, Worlein J, Menard M, Ryan A, Rosenberg K, Meyer JS. 2014. Hair loss and hypothalamic–pituitary–adrenocortical axis activity in captive rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 53:261–266. [PMC free article] [PubMed] [Google Scholar]
- 23.Novak MA, Meyer JS. 2009. Alopecia: possible causes and treatments, particularly in captive nonhuman primates. Comp Med 59:18–26. [PMC free article] [PubMed] [Google Scholar]
- 24.Pan W. 2001. Akaikes information criterion in generalized estimating equations. Biometrics 57:120–125. [DOI] [PubMed] [Google Scholar]
- 25.Parsons N. [Internet] 2013. repolr: repeated-measures proportional odds logistic regression. R package version 2.0. [Cited 30 October 2014] Available at: http://CRAN.R-project.org/package=repolr.
- 26.R Core Team. [Internet] 2014. R: a language and environment for statistical computing. [Cited 30 October 2014] Available at: http://www.R-project.org/.
- 27.Reinhardt V, Reinhardt A, Houser D. 1986. Hair pulling and eating in captive rhesus monkey troops. Folia Primatol (Basel) 47:158–164. [DOI] [PubMed] [Google Scholar]
- 28.Ripley B, Lapsley M. [Internet] 2013. RODBC: ODBC database access. R package version 1.3-10, [Cited 30 October 2014] Available at: http://CRAN.R-project.org/package=RODBC.
- 29.R Studio. [Internet] 2014. RStudio: integrated development environment for R (version 0.98.1087) [Cited 30 October 2014] Available at: http://www.rstudio.org/download/daily/desktop/mac/.
- 30.Steinmetz HW, Kaumanns W, Dix I, Heistermann M, Fox M, Kaup FJ. 2006. Coat condition, housing condition and measurement of faecal cortisol metabolites- a non-invasive study about alopecia in captive rhesus macaques (Macaca mulatta). J Med Primatol 35:3–11. [DOI] [PubMed] [Google Scholar]
- 31.Steinmetz HW, Kaumanns W, Dix I, Neimeier KA, Kaup FJ. 2005. Dermatologic investigation of alopecia in rhesus macaques (Macaca mulatta). J Zoo Wildl Med 36:229–238. [DOI] [PubMed] [Google Scholar]
- 32.Vessey SH, Morrison JA. 1970. Molt in free-ranging rhesus monkeys, Macaca mulatta. J Mammal 51:89–93. [Google Scholar]
- 33.Xie Y. [Internet] 2014. knitr: a general-purpose package for dynamic report generation in R. R package version 1.7. [Cited 30 October 2014] Available at: http://yihui.name/knitr/.
- 34.Wickham H, Francois R. [Internet] 2014. dplyr: a grammar of data manipulation. R package version 0.3.0.2. [Cited 30 October 2014] Available at: http://CRAN.R-project.org/package=dplyr.
- 35.Wickham H. [Internet] 2012. stringr: make it easier to work with strings. R package version 0.6.2. [Cited 30 October 2014] Available at: http://CRAN.R-project.org/package=stringr.
- 36.Wickham H. [Internet] 2011. testthat: get started with testing. [Cited 30 October 2014] Available at: http://journal.r-project.org/archive/2011-1/RJournal_2011-1_Wickham.pdf.
- 37.Wickham H. [Internet] 2009. ggplot2: elegant graphics for data analysis. [Cited 30 October 2014] Available at: http://had.co.nz/ggplot2/book.
- 38.Zhang P. 2011. A noninvasive study of alopecia in Japanese macaques Macaca fuscata. Curr Zool 57:26–35. [Google Scholar]