Abstract
Tramadol is a centrally acting weak μ opioid agonist that has few of the adverse side effects common to other opioids. Little work has been done to establish an effective analgesic dose of tramadol specific for surgical laparotomy and visceral manipulation in mice. We used general appearance parameters to score positive indicators of pain including posture, coat condition, activity, breathing, and interactions with other mice, activity events (that is, the number of times each mouse stretched up in a 3-min period) used as an indicator of decreased pain, von Frey fibers, and plasma levels of corticosterone to determine whether tramadol at 20, 40, or 80 mg/kg prevented postoperative pain in male and female C57BL/6 mice. A ventral midline laparotomy with typhlectomy was used as a model of postoperative pain. In male mice, none of the markers differed between groups that received tramadol (regardless of dose) and the saline-treated controls. However, general appearance scores and plasma corticosterone levels were lower in female mice that received 80 mg/kg tramadol compared with saline. In summary, for severe postoperative pain after laparotomy and aseptic typhlectomy, tramadol was ineffective in male C57BL/6 mice at all doses tested. Although 80 mg/kg ameliorated postoperative pain in female C57BL/6 mice, this dose is very close to the threshold reported to cause toxic side effects, such as tremors and seizures. Therefore, we do not recommend the use of tramadol as a sole analgesic in this mouse model of postoperative pain.
Abbreviations: GAP, general appearance parameter; g(f), grams–force; SU, stretching up
The use of animals in biomedical research comes with an obligation to protect the wellbeing of the species used. Part of our obligation to protect the wellbeing of research animals is to prevent unnecessary pain and distress by providing effective analgesia. The 8th edition of the Guide describes the correct use of analgesics in research animals as “…an ethical and scientific imperative” (p 120).21 The advancement of biomethodologies has increased the effectiveness of rodents as models of human disease and, in turn, has created a need for broader options regarding effective analgesics in the smaller species. Although a standard and efficient analgesic option in rodents, NSAID have immunomodulatory effects, making these analgesics inappropriate for various studies involving the immune system. Opioids are another common type of analgesic, but some opioids, including codeine, remifentanil, morphine, and fentanyl, like NSAID, cause immune system derangements.1 Buprenorphine, a partial µ receptor agonist, is the most common opioid used for rodent analgesia.44 However, buprenorphine has several dose-dependent side effects, including respiratory depression, increased activity, decreased food intake, and pica.27,42 Therefore, more choices are needed to treat severe pain in mice and rats.
Tramadol is a centrally acting analgesic that is structurally similar to codeine and morphine. Both enantiomers of tramadol enhance the inhibition of pain transmission through the modulation of neurotransmitters: the (+)-enantiomer inhibits serotonin reuptake, whereas the (–)-enantiomer inhibits norepinephrine reuptake.18 In addition, the (+)-enantiomer and the primary metabolite of tramadol, (+)-O-desmethyl-tramadol, both act as μ-opioid receptor agonists.18 The different properties of the tramadol enantiomers create synergistic analgesic effects, which improves tolerability by decreasing various side effects common to other opioids, such as respiratory depression, constipation, and abuse potential.18,43 As a result, tramadol was not classified as a scheduled substance under the Controlled Substances Act until recently.16
Tramadol has been used successfully as a sole analgesic for moderate to severe perioperative pain in several species, including humans,43 dogs,33 cats,8,53 and rats.52 It also has been documented as effective in rat and mouse models of neuropathic, orthopedic, and visceral pain at doses ranging from 1.25 to 100 mg/kg.2,28,35 Tramadol's documented effectiveness for other pain models in rodents make it an ideal candidate for a wide variety of rodent research models. In addition, we were very interested in the suitability of tramadol for studies of inflammation, because our previous studies showed little adverse effect of low-dose tramadol on select immune parameters in a cecal ligation and puncture model of sepsis.20 However, we did not assess analgesia in those studies, and there are no published studies to date regarding the efficacy of tramadol for the postoperative pain associated with abdominal surgery in mice. Therefore, we assessed tramadol in an abdominal surgery model similar to the model we used previously21 but lacking the sepsis component. We hypothesized that tramadol would provide effective postoperative analgesia in a mouse model of aseptic laparotomy. Specifically, we tested this hypothesis by using a series of scoring parameters to compare the behaviors, appearances, and mechanical sensitivity of male and female mice that received various doses of tramadol after a typhlectomy procedure. In addition, in separate experiments, we measured plasma corticosterone levels and body temperature fluctuations in mice after surgery to compare stress levels and thermoregulatory abilities after tramadol administration.
Materials and Methods
Animals.
Male (n = 133) and female (n = 110) C57BL/6 mice (age, 10 to 12 wk) were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed at 3 to 5 mice of the same sex in ventilated microisolation cages. Each cage was supplied with a compressed cotton nesting pad for enrichment on arrival into the facility. All mice had free-choice access to water and commercial chow (Laboratory Rodent Diet 5001, PMI Lab Diet, St Louis, MO). The rodent housing rooms were maintained at 72 ± 2 °F (22 ± 1 °C) on a 12:12-h light:dark cycle. All animals were acclimated for at least 5 d prior to surgical manipulation. An SPF status was maintained for mouse hepatitis virus, minute virus of mice, mouse parvovirus, enzootic diarrhea of infant mice virus, ectromelia virus, Sendai virus, pneumonia virus of mice, Theiler murine encephalomyelitis virus, reovirus, lymphocytic choriomeningitis virus, mouse adenovirus, and polyomavirus. All experiments were approved by The University of Michigan's Committee on the Use and Care of Animals.
Surgical manipulation.
All mice underwent a ventral midline laparotomy with aseptic typhlectomy to model postoperative pain from visceral manipulation. On the morning of surgery, a vaginal cytology was performed on all female mice by using standard methods to stage estrous.7 Anesthesia was induced by using an isoflurane induction chamber, and mice were maintained via face mask on 2.0% isoflurane mixed with oxygen at 2.5 L/min. Mice received 1.0 mL of 37 °C saline subcutaneously in the left flank and either tramadol or saline according to their treatment group in the right flank prior to the initiation of surgery. Surgeries were performed on a warming surgery table to provide supplemental heat during the anesthetic period. The abdomen was shaved and prepped by swabbing with 3 alternating scrubs of chlorhexidine and saline. An incision approximately 0.5 cm was made on midline through the skin and linea alba. The cecum was exteriorized to the ileocecocolic junction. A single monofilament nylon 4-0 suture was placed 3/4 of the distance from the apex of the cecum (1/4 of the distance from the ileocecocolic junction). The cecum was excised distal to the suture, and any remaining fecal material was removed from the remaining cecal tissue with iodine-soaked sterile gauze. The cecum and ileocecocolic junction were washed with 0.1 mL of sterile 0.9% NaCl and replaced in the abdomen. The linea alba and associated abdominal musculature was closed with 4-0 silk suture in a continuous pattern, and the overlying skin was closed with tissue glue. All mice were placed in an isolated recovery area and provided supplemental heat support until fully ambulatory, at which point they were placed into a clean cage containing 76A Diet Gel (Clear H2O, Portland, ME) and a new cotton nesting pad.
Analgesic administration.
Tramadol powder (a generous gift from Midas Pharmaceuticals, Parsippany, NJ) was compounded in sterile 0.9% NaCl and filtered through a 0.22-μM syringe filter into a sterile glass vial and stored in a dark, cool cabinet. Tramadol has been shown to be stable in solution for 15 d, and all solutions for this study were used within 10 d of preparation.4 The range of tramadol doses selected was based on previously published literature for various pain models, including cancer and neurologic pain models.5,24,35,48 Mice were randomly assigned to receive either saline or tramadol at 20, 40, or 80 mg/kg SC (corresponding to concentrations of 0, 4, 8, and 16 mg/mL, respectively) immediately prior to surgery, followed by 2 additional doses 12 h apart. All analgesic and control volumes were between 0.13 and 0.15 mL, depending on weight of the mice, to reduce variability of fluid amounts administered preoperatively. Saline was used as the control for these studies rather than another analgesic because of our interest in using tramadol in studies of inflammation, which might be affected by analgesic treatment, and in light of the lack of historical data with this particular model.
Pain assessment.
To determine tramadol's efficacy at curbing postoperative pain, a group of male (n = 45) and female (n = 44) mice were scored for 3 separate assessments, which included general appearance parameters (GAP), stretching-up (SU) events, and response to von Frey filaments.
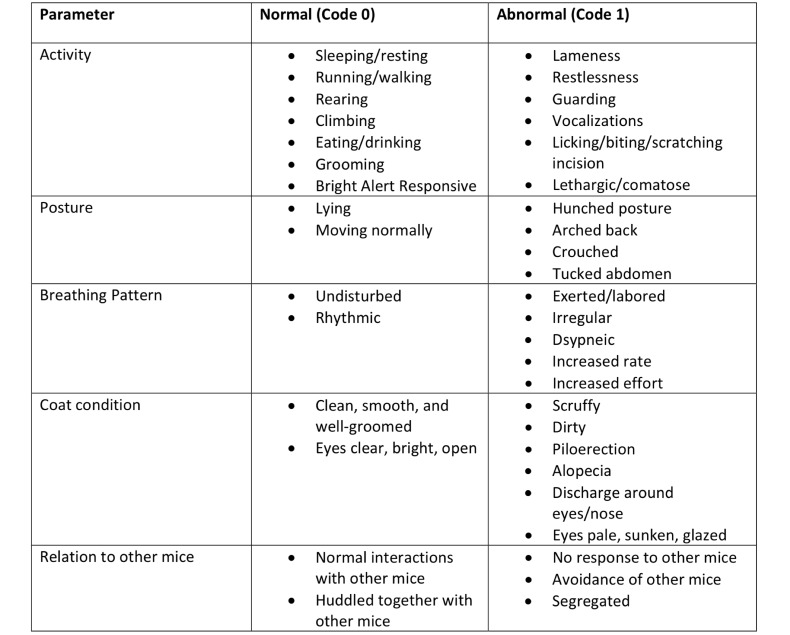
GAP scores were calculated on the basis of previously published parameters of activity, posture, breathing pattern, coat condition, and interactions with other mice.34 A blinded observer assessed each mouse at cage side on the morning after surgery. Mice were assigned a score of 0 (normal) or 1 (abnormal) for each parameter. The highest score achievable was 5, which indicated abnormality in all categories. Figure 1 represents the ethogram for predefined normal and abnormal behaviors in each parameter.
Figure 1.
Ethogram of normal and abnormal behaviors for GAP assessments. Mice were assigned a score of 0 or 1 for the categories of activity, posture, breathing pattern, coat condition, and interaction with other mice.
To assess SU events, on the morning after surgery, cages of mice were placed in a changing station, with tops and food hoppers removed to encourage activity and exploratory behavior. A blinded observer in the room counted the SU events that occurred during a 3-min period. A positive SU event was defined as vertical raising of the body onto the hindlimbs, resulting in full extension of the hocks and abdomen. Horizontal stretches against the cage floor or sides were excluded. We inferred that an increase in the number of SU events indicated decreased postoperative pain given that the surgery was performed through the abdominal musculature.
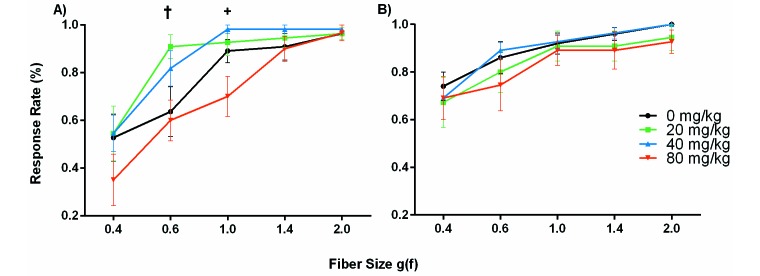
Mechanical testing with von Frey filaments was performed in the afternoon of the day after surgery and used 5 mechanical von Frey filaments of 0.4, 0.6, 1.0, 1.4, and 2.0 grams–force (Anesthesio Von Frey Hairs, Varese, Italy). The manual von Frey system requires application of different fiber sizes to produce a range of pressures. Mice were placed in rigid transparent plastic boxes measuring 15.24 cm high and 146.41 cm2 on a raised fiberglass mesh screen with 18 × 16 openings for every 2.54 cm2. All animals were acclimated to the platform the day before surgery as well as 10 min prior to von Frey assessment. Starting with the smallest fiber, each size of filament was applied around the incision 5 times, each time in a different location on the abdomen. A washout period of 2 min was used between fiber sizes. A blinded observer monitored for a response with each application. A positive response was predefined as jumping, licking or scratching of the incision, or abdominal retraction. Data were recorded as the percentage of the 5 applications that yielded a positive response for a given fiber size.
Corticosterone.
A second experiment was performed in male (n = 70) and female (n = 66) C57BL/6 mice to determine corticosterone levels as a means to measure postoperative levels of systemic stress. Plasma collection for all time points were done between 0930 and 1030. Mice were deeply anesthetized with isoflurane, blood was collected through the retroorbital sinus into EDTA microtubes, and cervical dislocation was performed. The plasma was diluted 1:5 and processed according to the manufacturer's instructions (Corticosterone Enzyme Immunoassay Kit, Cayman Chemical, Ann Arbor, MI).
Body temperature.
Previous work in our laboratory demonstrated a significant increase in mortality in ICR mice that received high doses of tramadol (80 mg/kg) after cecal ligation and puncture when compared with mice that received buprenorphine at 0.1 mg/kg.20 We wanted to determine whether tramadol caused significant changes in body temperatures, which might have contributed to the increased mortality in the CLP mice that received high doses of tramadol. Implantable programmable temperature transponders (model IPTT-300, Biomedic Data Systems, Seaford, DE) were placed in a separate group of male mice (n = 18) prior to surgery. The transponders were injected subcutaneously over the thoracic vertebrae at the same time as administration of the first analgesics. Mice that received temperature transponders underwent the same typhlectomy procedure and postoperative analgesic schedule as mice in the pain assessment cohorts.
Study design.
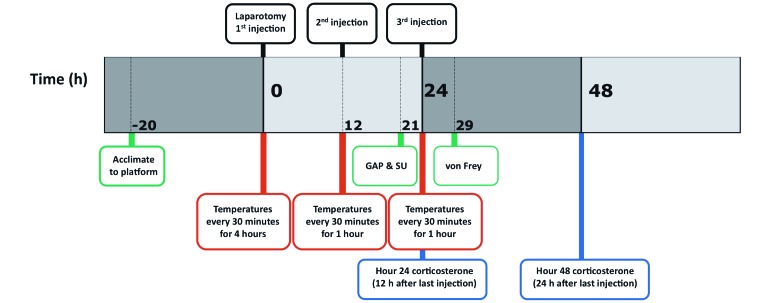
Figure 2 represents a time line for the 3 experiments. For the pain assessments and plasma corticosterone measurements, male and female C57BL/6 mice were assigned randomly to control or treatment groups (n = 10 to 12 per group); treatment groups received tramadol at 20, 40, or 80 mg/kg SC, whereas the control group received an equivalent volume of saline. The GAP and SU scoring took place 9 h after the second tramadol or saline injection; manual von Frey testing occurred 5 h after the third tramadol or saline injection. Blood for the corticosterone experiments was collected at 24 or 48 h postoperatively, which corresponded to 12 or 24 h after the second and third analgesic injections (n = 7 to 10 per group). Thermoregulatory experiments were conducted with male mice randomly assigned to receive either saline or tramadol at 80 mg/kg (n = 8 to 10 per group). Temperatures were recorded cage side every 30 min for 6 h after the administration of preoperative analgesics and every 30 min for 1 h after each subsequent injection of analgesic or saline.
Figure 2.
Timetable for all experiments. Mice underwent laparotomy with typhlectomy and received either tramadol (20, 40, or 80 mg/kg SC) or saline as indicated at the 1st, 2nd, and 3rd injection time points. Pain (green), corticosterone (blue), and temperature (red) were assessed postoperatively.
Statistical analysis.
All data analyses were performed by using Prism (6th edition, GraphPad Software, La Jolla, CA). For GAP and SU, statistical analysis was performed on total scores. Von Frey scores were determined by calculating the percentage response rate elicited for each fiber. The GAP, SU, and corticosterone results were tested for normality by using the D'Agostino and Pearson omnibus normality test. GAP and corticosterone results were compared by using one-way ANOVA, with correction for multiple comparisons by the Bonferroni test. SU data were compared by using the Kruskal–Wallis nonparametric test and Dunn test for correcting multiple comparisons. To compare response rates to the von Frey fibers, 2-way ANOVA with the Bonferroni multiple-comparison correction was used. Body temperature results were analyzed by using the Holm–Sidak method.
Results
Stage of estrous cycle.
Vaginal cytologies of female mice were performed on the morning before surgery. The percentage of mice in each stage was 1.5%, 0.0%, 43.9%, and 54.5% for proestrus, estrus, metestrus, and diestrus, respectively, such that 98.5% of female mice were in low-estrogen and low-progesterone stages of the estrous cycle at the time of the initial analgesic administration and surgery. All remaining results for female mice were combined into a single group irrespective of estrous stage.
Pain assessments.
GAP scores.
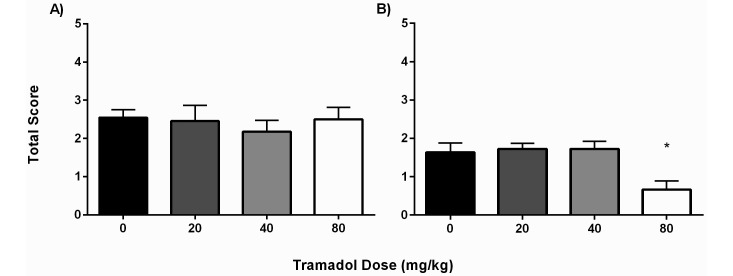
A representative group of both male (n = 8) and female (n = 7) mice were scored the day before surgery to provide baseline behaviors. All mice received a total score of 0.0 with the exception of one female mouse, which received a score of 1.0 due to a scruffy coat. For male mice, there were no differences in GAP scores between the saline group (2.55 ± 0.69) and mice that received 20, 40, or 80 mg/kg (2.45 ± 1.37, 2.18 ± 0.98, and 2.50 ± 1.09, respectively; Figure 3 A). However, among female mice, the GAP score was significantly (P < 0.05) lower in the group that received 80 mg/kg (0.67 ± 0.78) when compared with those that received saline (1.64 ± 0.81), 20 mg/kg tramadol (1.73 ± 0.47), or 40 mg/kg tramadol (1.73 ± 0.65; Figure 3 B). Comparing the sexes, the difference in GAP score was significant (P < 0.0001) only between male and female mice that received 80 mg/kg tramadol.
Figure 3.
GAP total scores (mean ± SEM) for (A) male and (B) female C57BL/6 mice according to treatment group (n = 10–12 mice per group). A blinded observer assessed the animals at 24 h postoperatively, which corresponded to 9 h after the 2nd tramadol or saline injection. The GAP score of female mice that received 80 mg/kg was significantly lower than those of all other groups. *, P < 0.05 compared with all other groups.
SU scores.
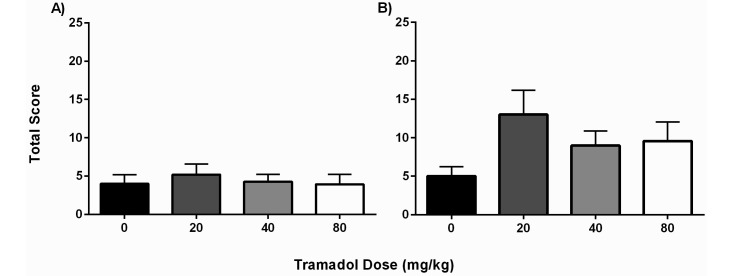
The ‘stretching up’ behavior was used as an indicator of decreased abdominal pain after laparotomy. Baseline scores were obtained from a representative group of both male (n = 8) and female (n = 7) mice on the day prior to surgery. The mean SU scores for male mice before any manipulation was 23.5 ± 5.8, and their SU values decreased to 5.9 ± 5.0 events 21 h after surgery; these animals were included as part of the saline group. The baseline for female mice started at 20.0 ± 6.5 preoperatively and fell to 6.4 ± 3.4 after surgery. The dramatic drop in the number of SU events suggests a surgery-associated effect in both groups. Overall, the average SU scores of male mice did not differ between the control group (4.0 ± 3.95) and the 20- (5.2 ± 4.56), 40- (4.3 ± 3.20), and 80- (3.9 ± 4.58) mg/kg groups, suggesting that tramadol did not significantly improve the willingness to stretch the abdominal area (Figure 4 A). Female mice did not have any significant differences in SU scores between treatment groups, with average scores of 5.00 ± 3.92 for the saline group and 13.00 ± 10.53, 9.00 ± 6.28, and 9.58 ± 8.53 for the 20-, 40-, and 80-mg/kg tramadol groups, respectively.
Figure 4.
SU total scores (mean ± SEM) for (A) male and (B) female C57BL/6 mice according to treatment group (n = 10–12 mice per group). A blinded observer assessed the animals at 24 h postoperatively, which corresponded to 9 h after the 2nd tramadol or saline injection. For both male and female mice, none of the treatment groups differed significantly from the saline group. The female mice that received tramadol at 20 mg/kg yielded 2 scores that were outliers; these were retained in the data set for full representation of the findings.
Comparing the sexes, male mice again demonstrated an increased propensity toward postoperative pain behaviors, with all male treatment groups having lower average SU scores than those of corresponding female mice. The SU scores were significantly (P = 0.0168) lower in the male mice when compared with the female mice only in the 20-mg/kg group. The higher score in the female mice at this treatment dose is most likely due to the 2 female mice that had SU scores over 30.
Von Frey testing.
In all groups, the average percentage response rate to the 5 applications of each fiber increased toward 100% as the fiber size increased, indicating that the smallest fiber (0.4 g[f]) was the most sensitive at indicating differences between the groups (Figure 5). For the male mice, average percentage response rates to the 0.4-g(f) fiber were 52.7% ± 32.6%, 54.6% ± 38.0%, 54.6% ± 25.4%, and 35.0% ± 37.3% for the 0-, 20-, 40-, and 80-mg/kg groups, respectively. Although a dose-dependent trend may be inferred, the response rate to the 0.4-g(f) fiber was not significantly different between the saline group and any of the other treatment groups (Figure 5 A). Interestingly, the 80-mg/kg group was the only group that consistently demonstrated a low response rate to each fiber and, therefore less sensitivity than all other groups. In fact, response rates of the 20- and 40-mg/kg treatment groups to the 0.6- and 1.0-g(f) fibers were significantly (P < 0.05) higher than those of the 80-mg/kg mice. Von Frey assessments for the female mice showed no significant differences between treatment groups (Figure 5 B). The 0-, 20-, 40-, and 80-mg/kg groups had response rates of 74.0% ± 19.0%, 67.3% ± 35.0%, 69.1% ± 30.2%, and 69.1% ± 30.2%, respectively, with the 0.4g(f) fiber, suggesting no effect of tramadol treatment on hyperalgesia due to the indicated fiber sizes. Comparing the sexes, male mice had lower average responses (P = 0.0475) than did female mice in the 80-mg/kg group, suggesting that the female C57BL/6 mice were more sensitive to mechanical stimulation.
Figure 5.
Percentage response rate (mean ± SEM) to von Frey fibers of increasing size for (A) male and (B) female C57BL/6 mice according to treatment group (n = 10–12 mice per group). A blinded observer monitored response to mechanical fiber stimulation at 29 h postoperatively, which corresponded to 5 h after the 3rd tramadol or saline injection. No significant differences were seen between saline and treatment groups at any fiber size. The average response was significantly (†, P = 0.01; +, P = 0.02) lower in male mice that received tramadol at 80 mg/kg compared with 20 mg/kg at the 0.6 g(f) fiber and with those that received 40 mg/kg at the 1.0 g(f) fiber.
Corticosterone.
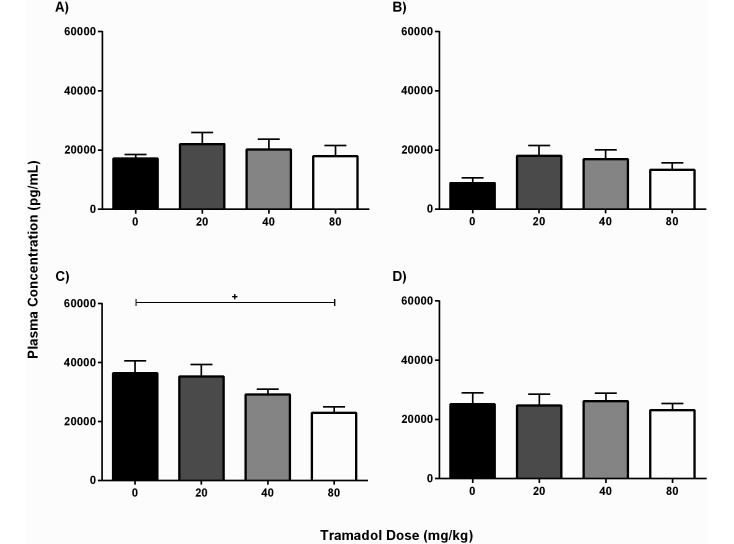
Male mice that received only saline had an average plasma corticosterone level of 1.72 ± 4.13 × 104 pg/mL at 24 h after surgery, and this value significantly (P = 0.02) declined to 8.84 ± 4.24 × 103 pg/mL at 48 h. All corticosterone levels in male mice treated with tramadol were comparable to their control values at both time points (Figure 6 A and B), suggesting that tramadol did not affect corticosterone levels in the male mice. In contrast, the plasma corticosterone levels at 24 h postoperatively of female mice in the 80-mg/kg group (2.29 ± 0.69 × 104 pg/mL) were significantly (P < 0.05) lower than those of female mice given saline (3.64 ± 1.32 × 104 pg/mL; Figure 6 C). The corticosterone levels in the control female mice decreased to 2.51 ± 0.95 × 104 pg/mL by 48 h after surgery, when there were no significant differences between groups (Figure 6 D). Comparing the sexes, female mice that received saline had significantly higher average plasma corticosterone concentrations than did male mice at both 24 h (P = 0.0006) and 48 h (P = 0.003) after surgery. This trend was also evident in comparisons of the 40- and 80-mg/kg groups.
Figure 6.
Plasma corticosterone concentration (pg/mL; mean ± SEM) according to treatment group (n = 7–10 mice per group per time point). In male mice, no groups differed significantly at either (A) 24 h or (B) 48 h after laparotomy. In female mice, plasma corticosterone was significantly (+, P = 0.02) lower at (C) 24 h postoperatively in those that received tramadol at 80 mg/kg compared with saline; (D) no groups differed significantly at 48 h postoperatively.
Body temperature.
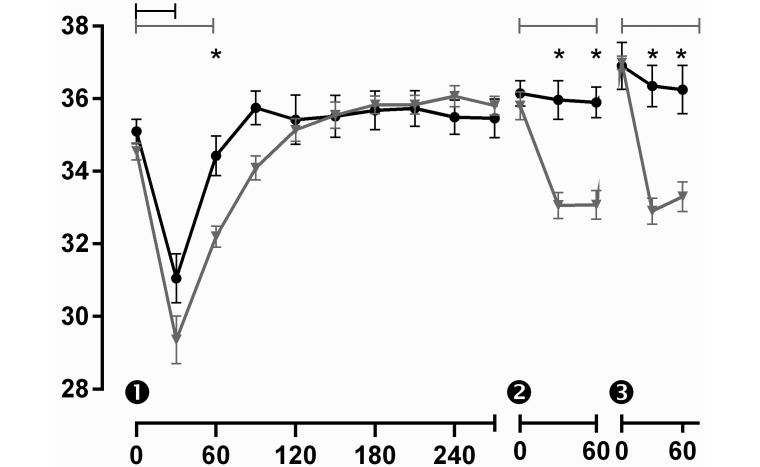
Despite the warmed subcutaneous fluids and supplemental heat sources provided during and after surgery, all mice had a significant drop in temperature 30 min after anesthetic induction (Figure 7). By 60 min after induction, the temperature seemed to be recovering to baseline values, but the mice in the 80-mg/kg group had a significantly (P < 0.05) lower average body temperature (32.2 ± 0.9 °C) than did mice in the saline group (34.4 ± 1.8 °C). The 80-mg/kg group consistently had average body temperatures that were 1 to 2 °C lower than those of the control group for 2 h after anesthetic induction, demonstrating a slower return to baseline body temperatures in the tramadol-treated mice. Although the initial drop in body temperature was probably a result of surgery, the trend continued after subsequent tramadol administrations. For the second and third injections, mice received tramadol or saline without adjunct anesthesia. At 30 min after each of these injections, the 80-mg/kg group once again had significantly lower body temperatures when compared with control mice (33.2 ± 0.9 °C compared with 36.8 ± 0.3 °C; P < 0.05). These lower body temperatures for the 80-mg/kg group persisted at least 60 min after the administration of tramadol, although an eventual return to normal body temperature was always evident prior to the next injection.
Figure 7.
Body temperature (°C; mean ± SEM) for male C57BL/6 mice that received 3 injections (indicated as 1, 2, and 3) of either saline or tramadol at 80 mg/kg (n = 8–10 mice per group). Mice were anesthetized (time 0) for implantation of temperature transponders, laparotomy, and typhlectomy. Temperatures were measured every 30 min by using a handheld wand that read implanted subcutaneous microchips. Whereas body temperature was decreased for 30 min after surgery in mice that received saline (black symbols), mice that received tramadol (gray symbols) had significant drops in temperature that persisted as long as 60 min after each tramadol injection. *, Significant (P < 0.05) difference between treatment groups at a given time point; brackets indicate significant differences (P < 0.05) between time points within a treatment group.
Discussion
Pain assessments including GAP scores, SU events, and response to von Frey filaments were used in this study to ascertain any differences in analgesic effects between mice that received saline or 20, 40, or 80 mg/kg tramadol. Overall, none of the tramadol doses produced effective postoperative analgesia in male C57BL/6 mice, whereas GAP and SU scores differed according to tramadol dose in female mice. Female mice in the 80-mg/kg group returned to normal activity and appearance more quickly than did female mice in the lower dose groups as indicated by the significantly lower GAP score. Although none of the treatment groups differed significantly for the SU scores, the female 20-mg/kg group exhibited higher average scores with a wider standard deviation than did all other groups. This group had 2 animals with exceptionally high SU scores (greater than 30), whereas all other mice of the same group averaged only 9 SU events. These 2 mice had surgery on the same day and were cohoused. Although these 2 mice appear to be outliers, we retained the data for analysis to accurately and fully represent all of the findings. Comparisons of pre- and postsurgical scores in mice showed clear changes in both GAP and SU tests, suggesting they are reliable indicators of postoperative pain. The results of mechanical testing with von Frey fibers is more difficult to interpret for several reasons. First, we acclimated the animals to the von Frey stand and environment but did not perform a preoperative assessment to reduce sensitization to the actual testing. However, this feature inhibits our ability to discern inter-individual differences in skin sensitivity that could have introduced variability in our data. Second, the von Frey measurements were performed only 5 h after the preceding tramadol or saline administration. Sedative effects from the higher doses of tramadol could have been present and resulted in diminished response to the fibers. The male mice exhibited differences between those that received 80 mg/kg tramadol when compared with mice that received doses of 20 and 40 mg/kg. This result could have been due to a sedative effect in the 80-mg/kg group, although the GAP and SU scores did not show similar dose-associated sedative effects. Alternatively, the 20- and 40-mg/kg groups might have demonstrated a hyperalgesic effect. Paradoxical opioid induced hyperalgesia has been documented as a postoperative side effect and has specifically been tied to sub-analgesic doses of opioids.19,49 However, we are unable to differentiate opioid-induced hyperalgesia secondary to subeffective dosing of 20 and 40 mg/kg tramadol from other causes of hyperalgesia, such as acute opioid tolerance. Therefore, we cannot assume that the mechanical differences between low and high doses of tramadol seen in the male mice are an indicator of competent postoperative analgesia by the 80-mg/kg dose.
Plasma corticosterone levels were measured 24 and 48 h after surgery to coincide with time points of 12 and 24 h after tramadol or saline treatments. Plasma corticosterone levels have been used to reliably quantify postoperative stress and may correlate with efficacy of analgesic treatment.46 Although the timing and method of sample collection can greatly influence plasma corticosterone concentrations,10,46,51 our results overall suggest tramadol administration did not alter corticosterone levels in the male mice but, at high doses, did lower corticosterone in female mice. This finding suggests that the female mice experienced less stress when given tramadol at the highest dose and is in keeping with the GAP and SU results. Interestingly, none of the male or female mice that received tramadol demonstrated a significant increase in corticosterone. Both morphine and fentanyl are known to cause strong immune system modulation and have been shown to induce dramatic spikes in corticosterone due to activation of the hypothalamic–pituitary–adrenal axis.17 Our results support the theory that tramadol has less immunomodulatory effects than other opioids.
One important consideration that has emerged from our research is the dramatic sex-dependent differences seen in the results of the pain assessments. Although our experiments in male and female mice were not run simultaneously, comparison of the study parameters indicates differences. The GAP and SU scores suggest that laparotomy affected the male mice more adversely than the female mice, which is in contradiction of common findings in human postoperative studies.47 Although the GAP scores displayed significant differences between the 80-mg/kg groups only, the scores were persistently higher across all treatment groups for the male mice, including the saline group, suggesting a higher incidence of pain behavior in male mice postoperatively. However, very little has been done to compare sex-associated differences in postlaparotomy pain in rodents. In contrast, our female mice showed an increase in hypersensitivity to the von Frey fibers. This finding correlates with previous studies that showed exaggerated allodynia in female rats relative to male rats throughout diestrus and proestrus.22,32,36,40,50 It is important to note 98.5% of the female mice were in diestrus or metestrus at the time of surgery. This distribution is most likely due to the cohousing of female mice, which can prolong the diestrus and metestrus stages,32,50 which are the low-estrogen phases of estrous. Pain sensitivity peaks during the high-estradiol and -progesterone stages of the estrous cycle.22,40 Therefore, the female mice in the current study might have shown greater sensitivity, with GAP and SU scores more similar to those of the male mice, if they had been in either proestrus or estrus.
Although there were differences between males and females in overall pain responses, there also appeared to be differences in tramadol's efficacy. The highest dose of tramadol appeared to be analgesic in female but not male mice, as determined by GAP and SU scores as well as corticosterone levels. This finding is in contrast to previous work that demonstrated a lower ED50 value for tramadol in male Swiss mice than their female counterparts.14 The results of the previous study14 might be explained by hormonal effects such as estradiol's negative effect on µ-opioid receptor binding and luteinizing hormone's desensitization of opioid receptors to µ agonists. However, sexual dimorphism with opioid analgesics cannot be broadly characterized, given that several variables can affect the expression of sex-associated differences, including the strain, stock, or genotype of the rodents; potency, dose, and route of the opioid; and even the age of the animals.6,13,23 One possible reason for the increased sensitivity in our female mice is the unique dual mechanism of action of tramadol. The synergistic action of the μ-opioid agonist with the serotonin reuptake inhibition may result in an increased sensitivity of female rodents, which previously have been shown to be more sensitive than male rodents to selective serotonin reuptake inhibitors.25,30 Another possible mechanism is the κ receptor action of tramadol. Women consistently show greater analgesia sensitivity to combined μ- and κ-opioid analgesics than do men.6 Rodent studies have had more variable differences in analgesic efficacy of κ-opioid agonists, with sexual dimorphisms dependent on species, strain, and nociceptive assay.38
Finally, we evaluated temperature regulation after peri- and postoperative administration of 80 mg/kg tramadol because previous research led us to suspect a hypothermic effect.20 Opioid agonists have varying effects on the thermoregulatory system of rats and mice, causing either or both hypothermia and hyperthermia.3,41 In humans, tramadol can cause a decrease in both the sweating and shivering thresholds as well as a decrease in vasoconstriction, which is only partially reversible with naloxone.15 However, more recently, the homeostatic relationship between μ and κ agonists on body temperature has been detailed, with μ agonists causing hyperthermia and κ agonists balancing this effect with hypothermia.11,39 The significant hypothermia caused by tramadol with and without surgery supports other literature demonstrating a κ-agonist component to tramadol's mechanism of action.29,45 Our results revealed dramatic drops in body temperature after tramadol administration with and without concurrent anesthesia. This effect should be considered carefully when using tramadol in research models in which changes in temperature might skew survival curves and other results.
Taking into account all of our pain assessments and corticosterone data, we conclude that tramadol at 20, 40, or 80 mg/kg subcutaneously would not provide effective analgesia for severe postoperative pain in male mice. Interestingly, unlike other opioid analgesics, female C57BL/6 mice potentially have greater sensitivity to tramadol than do male mice; we hypothesize that this effect is due to the dual action of tramadol as an opioid agonist and a serotonin reuptake inhibitor. The dose of 80 mg/kg likely is effective for the management of postoperative pain due to visceral manipulation in female mice, but this dose is potentially toxic. Previous studies have estimated a tramadol dose of 86.5 mg/kg to induce seizures in 50% of the population.37 Although we saw no detrimental side effects with the 80-mg/kg dose, other investigators at our facility described at least one occurrence of seizure activity in a mouse that received 80-mg/kg tramadol. Due to the potential for seizures along with the extreme, albeit transient, temperature changes seen after administration, we are hesitant to recommend using a tramadol dose of 80 mg/kg. Although it offered insufficient efficacy for our invasive surgical model, tramadol as a sole analgesic remains a viable choice for mild to moderate pain in some cases.26 In addition, tramadol in combination with other analgesics has proven to have synergistic effects.9,31,52 Finally, with the new NIH policy to balance male and female representation in animal studies, the significant differences in tramadol's efficacy between male and female rodents should be considered carefully.12
References
- 1.Al-Hashimi M, Scott SW, Thompson JP, Lambert DG. 2013. Opioids and immune modulation: more questions than answers. Br J Anaesth 111:80–88. [DOI] [PubMed] [Google Scholar]
- 2.Apaydin S, Uyar M, Karabay NU, Erhan E, Yegul I, Tuglular I. 2000. The antinociceptive effect of tramadol on a model of neuropathic pain in rats. Life Sci 66:1627–1637. [DOI] [PubMed] [Google Scholar]
- 3.Baker AK, Meert TF. 2002. Functional effects of systemically administered agonists and antagonists of μ, Δ, and κ opioid receptor subtypes on body temperature in mice. J Pharmacol Exp Ther 302:1253–1264. [DOI] [PubMed] [Google Scholar]
- 4.Barcia E, Martin A, Azuara ML, Sanchez Y, Negro S. 2007. Tramadol and hyoscine N-butyl bromide combined in infusion solutions: compatibility and stability. Supportive Care Cancer 15:57–62. [DOI] [PubMed] [Google Scholar]
- 5.Berrocoso E, De Benito MD, Mico JA. 2007. Role of serotonin 5-HT1A and opioid receptors in the antiallodynic effect of tramadol in the chronic constriction injury model of neuropathic pain in rats. Psychopharmacology (Berl) 193:97–105. [DOI] [PubMed] [Google Scholar]
- 6.Bodnar RJ, Kest B. 2010. Sex differences in opioid analgesia, hyperalgesia, tolerance and withdrawal: central mechanisms of action and roles of gonadal hormones. Horm Behav 58:72–81. [DOI] [PubMed] [Google Scholar]
- 7.Byers SL, Wiles MV, Dunn SL, Taft RA. 2012. Mouse estrous cycle identification tool and images. PLoS ONE 7:e35538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cagnardi P, Villa R, Zonca A, Gallo M, Beccaglia M, Luvoni GC, Vettorato E, Carli S, Fonda D, Ravasio G. 2011. Pharmacokinetics, intraoperative effect, and postoperative analgesia of tramadol in cats. Res Vet Sci 90:503–509. [DOI] [PubMed] [Google Scholar]
- 9.Chavarria-Bolanos D, Perez-Urizar J, Grandfils C, Pozos-Guillen A. 2014. Peripheral synergism between tramadol and ibuprofen in the formalin test. Drug Dev Res 75:224–230. [DOI] [PubMed] [Google Scholar]
- 10.Chelini MO, Souza NL, Cortopassi SR, Felippe EC, Oliveira CA. 2006. Assessment of the physiologic stress response by quantification of fecal corticosteroids. J Am Assoc Lab Anim Sci 45:8–11. [PubMed] [Google Scholar]
- 11.Chen X, McClatchy DB, Geller EB, Tallarida RJ, Adler MW. 2005. The dynamic relationship between μ and κ opioid receptors in body temperature regulation. Life Sci 78:329–333. [DOI] [PubMed] [Google Scholar]
- 12.Clayton JA, Collins FS. 2014. Policy: NIH to balance sex in cell and animal studies. Nature 509:282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahan A, Kest B, Waxman AR, Sarton E. 2008. Sex-specific responses to opiates: animal and human studies. Anesth Analg 107:83–95. [DOI] [PubMed] [Google Scholar]
- 14.Dai X, Brunson CD, Rockhold RW, Loh HH, Ho IK, Ma T. 2008. Gender differences in the antinociceptive effect of tramadol, alone or in combination with gabapentin, in mice. J Biomed Sci 15:645–651. [DOI] [PubMed] [Google Scholar]
- 15.De Witte JL, Kim JS, Sessler DI, Bastanmehr H, Bjorksten AR. 1998. Tramadol reduces the sweating, vasoconstriction, and shivering thresholds. Anesth Analg 87:173–179. [DOI] [PubMed] [Google Scholar]
- 16.Drug Enforcement Administration 2014. Schedule of controlled substances: placement of tramadol into schedule IV. Final rule. Fed Regist 79:37623–37630. [PubMed] [Google Scholar]
- 17.Franchi S, Panerai AE, Sacerdote P. 2007. Buprenorphine ameliorates the effect of surgery on hypothalamus–pituitary–adrenal axis, natural killer cell activity, and metastatic colonization in rats in comparison with morphine or fentanyl treatment. Brain Behav Immun 21:767–774. [DOI] [PubMed] [Google Scholar]
- 18.Grond S, Sablotzki A. 2004. Clinical pharmacology of tramadol. Clin Pharmacokinet 43:879–923. [DOI] [PubMed] [Google Scholar]
- 19.Holtman JR, Jr, Wala EP. 2005. Characterization of morphine-induced hyperalgesia in male and female rats. Pain 114:62–70. [DOI] [PubMed] [Google Scholar]
- 20.Hugunin KM, Fry C, Shuster K, Nemzek JA. 2010. Effects of tramadol and buprenorphine on select immunologic factors in a cecal ligation and puncture model. Shock 34:250–260. [DOI] [PubMed] [Google Scholar]
- 21.Institute for Laboratory Animal Research 2011Guide for the care and use of laboratory animals. Washington (DC): National Academies Press. [Google Scholar]
- 22.Kayser V, Berkley KJ, Keita H, Gautron M, Guilbaud G. 1996. Estrous and sex variations in vocalization thresholds to hindpaw and tail pressure stimulation in the rat. Brain Res 742:352–354. [DOI] [PubMed] [Google Scholar]
- 23.Kest B, Wilson SG, Mogil JS. 1999. Sex differences in supraspinal morphine analgesia are dependent on genotype. J Pharmacol Exp Ther 289:1370–1375. [PubMed] [Google Scholar]
- 24.Keyhanfar F, Shamsi Meymandi M, Sepehri G, Rastegaryanzadeh R, Heravi G. 2013. Evaluation of antinociceptive effect of preGABAlin in mice and its combination with tramadol using tail flick test. Iran J Pharm Res 12:483–493. [PMC free article] [PubMed] [Google Scholar]
- 25.Lebron-Milad K, Tsareva A, Ahmed N, Milad MR. 2013. Sex differences and estrous cycle in female rats interact with the effects of fluoxetine treatment on fear extinction. Behav Brain Res 253:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leppert W. 2009. Tramadol as an analgesic for mild to moderate cancer pain. Pharmacol Rep 61:978–992. [DOI] [PubMed] [Google Scholar]
- 27.Liles JH, Flecknell PA. 1992. The effects of buprenorphine, nalbuphine, and butorphanol alone or following halothane anaesthesia on food and water consumption and locomotor movement in rats. Lab Anim 26:180–189. [DOI] [PubMed] [Google Scholar]
- 28.Lopopolo M, Affaitati G, Fabrizio A, Massimini F, Lapenna D, Giamberardino MA, Costantini R. 2014. Effects of tramadol on viscero-visceral hyperalgesia in a rat model of endometriosis plus ureteral calculosis. Fundam Clin Pharmacol 28:331–341. [DOI] [PubMed] [Google Scholar]
- 29.Manocha A, Sharma KK, Mediratta PK. 2005. On the mechanism of anticonvulsant effect of tramadol in mice. Pharmacol Biochem Behav 82:74–81. [DOI] [PubMed] [Google Scholar]
- 30.McEuen JG, Semsar KA, Lim MA, Bale TL. 2009. Influence of sex and corticotropin-releasing factor pathways as determinants in serotonin sensitivity. Endocrinology 150:3709–3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKeon GP, Pacharinsak C, Long CT, Howard AM, Jampachaisri K, Yeomans DC, Felt SA. 2011. Analgesic effects of tramadol, tramadol–gabapentin, and buprenorphine in an incisional model of pain in rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 50:192–197. [PMC free article] [PubMed] [Google Scholar]
- 32.McLean AC, Valenzuela N, Fai S, Bennett SA. 2012. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp 67:e4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgaz J, Navarrete R, Munoz-Rascon P, Dominguez JM, Fernandez-Sarmiento JA, Gomez-Villamandos RJ, Granados MM. 2013. Postoperative analgesic effects of dexketoprofen, buprenorphine, and tramadol in dogs undergoing ovariohysterectomy. Res Vet Sci 95:278–282. [DOI] [PubMed] [Google Scholar]
- 34.Morton DB, Griffiths PH. 1985. Guidelines on the recognition of pain, distress, and discomfort in experimental animals and an hypothesis for assessment. Vet Rec 116:431–436. [DOI] [PubMed] [Google Scholar]
- 35.Mouedden ME, Meert TF. 2007. Pharmacological evaluation of opioid and nonopioid analgesics in a murine bone cancer model of pain. Pharmacol Biochem Behav 86:458–467. [DOI] [PubMed] [Google Scholar]
- 36.Nicotra L, Tuke J, Grace PM, Rolan PE, Hutchinson MR. 2014. Sex differences in mechanical allodynia: how can it be preclinically quantified and analyzed? Front Behav Neurosci 8:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raffa RB, Stone DJ., Jr 2008. Unexceptional seizure potential of tramadol or its enantiomers or metabolites in mice. J Pharmacol Exp Ther 325:500–506. [DOI] [PubMed] [Google Scholar]
- 38.Rasakham K, Liu-Chen LY. 2011. Sex differences in κ-opioid pharmacology. Life Sci 88:2–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rawls SM, Benamar K. 2011. Opioid, cannabinoid, and transient receptor potential (TRP) systems: effects on body temperature. Front Biosc (Schol Ed)i 3:822–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sapsed-Byrne S, Ma D, Ridout D, Holdcroft A. 1996. Estrous cycle phase variations in visceromotor and cardiovascular responses to colonic distension in the anesthetized rat. Brain Res 742:10–16. [DOI] [PubMed] [Google Scholar]
- 41.Savic Vujovic KR, Vuckovic S, Srebro D, Ivanovic M, Dosen-Micovic L, Vucetic C, Dzoljic E, Prostran M. 2013. A comparison of the antinociceptive and temperature responses to morphine and fentanyl derivatives in rats. Arch Pharm Res 36:501–508. [DOI] [PubMed] [Google Scholar]
- 42.Schaap MW, Uilenreef JJ, Mitsogiannis MD, van ‘t Klooster JG, Arndt SS, Hellebrekers LJ. 2012. Optimizing the dosing interval of buprenorphine in a multimodal postoperative analgesic strategy in the rat: minimizing side effects without affecting weight gain and food intake. Lab Anim 46:287–292. [DOI] [PubMed] [Google Scholar]
- 43.Scott LJ, Perry CM. 2000. Tramadol: a review of its use in perioperative pain. Drugs 60:139–176. [DOI] [PubMed] [Google Scholar]
- 44.Stokes EL, Flecknell PA, Richardson CA. 2009. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab Anim 43:149–154. [DOI] [PubMed] [Google Scholar]
- 45.Sun HL, Zheng JW, Wang K, Liu RK, Liang JH. 2003. Tramadol reduces the 5HTP-induced head-twitch response in mice via the activation of μ- and κ-opioid receptors. Life Sci 72:1221–1230. [DOI] [PubMed] [Google Scholar]
- 46.Sundbom R, Jacobsen KR, Kalliokoski O, Hau J, Abelson KS. 2011. Postoperative corticosterone levels in plasma and feces of mice subjected to permanent catheterization and automated blood sampling. In Vivo 25:335–342. [PubMed] [Google Scholar]
- 47.Tighe PJ, Riley JL, 3rd, Fillingim RB. 2014. Sex differences in the incidence of severe pain events following surgery: a review of 333,000 pain scores. Pain Med 15:1390–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai YC, Won SJ. 2001. Effects of tramadol on T lymphocyte proliferation and natural killer cell activity in rats with sciatic constriction injury. Pain 92:63–69. [DOI] [PubMed] [Google Scholar]
- 49.Wala EP, Holtman JR., Jr 2011. Buprenorphine-induced hyperalgesia in the rat. Eur J Pharmacol 651:89–95. [DOI] [PubMed] [Google Scholar]
- 50.Whitten WK. 1959. Occurrence of anoestrus in mice caged in groups. J Endocrinol 18:102–107. [DOI] [PubMed] [Google Scholar]
- 51.Wright-Williams S, Flecknell PA, Roughan JV. 2013. Comparative effects of vasectomy surgery and buprenorphine treatment on faecal corticosterone concentrations and behaviour assessed by manual and automated analysis methods in C57 and C3H mice. PLoS ONE 8:e75948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zegre Cannon C, Kissling GE, Goulding DR, King-Herbert AP, Blankenship-Paris T. 2011. Analgesic effects of tramadol, carprofen or multimodal analgesia in rats undergoing ventral laparotomy. Lab Anim (NY) 40:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeiler GE, Dzikiti BT, Fosgate GT, Stegmann FG, Venter FJ, Rioja E. 2014. Anaesthetic, analgesic, and cardiorespiratory effects of intramuscular medetomidine–ketamine combination alone or with morphine or tramadol for orchiectomy in cats. Vet Anaesth Analg 41:411–420. [DOI] [PubMed] [Google Scholar]