Abstract
Each year, millions of rats undergo surgery for research purposes and receive analgesics to alleviate pain. We sought to evaluate the efficacy of common analgesics in tests of hot-plate nociception and postsurgical pain by using the Rat Grimace Scale. Rats received a single dose of one of several drug–dose combinations and were tested by using the hot-plate test (acute pain) or after laparotomy (with either prophylactic or intraoperative analgesic). The efficacy of analgesics for hot-plate pain was generally not predictive of efficacy for surgical pain. Carprofen and ketoprofen were rarely effective in any of the conditions tested. With the exception of the opioid buprenorphine, several of the drugs we tested required higher-than-recommended doses to alleviate pain. Taken together, our data suggest that current analgesic use frequently is insufficient, and many rats may experience significant postsurgical pain even when analgesics are used in commonly recommended doses.
Abbreviation: RGS, Rat Grimace Scale
Studies using animal models allow an understanding of the underlying basis of human conditions at a cellular and molecular level, validation of future human research, and identification of specific targets for pharmaceutical interventions.12 Given that millions of rats are used in research,20 practices that minimize both pain and distress to the greatest degree possible are crucial.5 However, in one survey,15 only 20% of researchers reported administering analgesics to rodents after surgery, suggesting that many animals potentially experience pain.
A lack of pain indicators is often the reason for insufficient analgesia,15 possibly due to a lack of consensus regarding a determination method. To assess pain, researchers have used telemetry,4 extensive videorecording,16,17 and ultrasonic vocalizations,7,13,22 but such methods can be time-consuming, overly technical, or expensive to implement, resulting in their limited use by the research community. In addition, each method can be confounded by the fact that rodents actively conceal pain behaviors,1 especially when they sense a predator.18
The treatment of pain itself varies as well. Drug and dosage recommendations vary from institution to institution. Buprenorphine, carprofen, and ketoprofen in addition to other opioids frequently are suggested for moderate to severe pain, but few relevant supporting data are available for all recommended drugs. Assessing the effectiveness of these drugs has traditionally been limited to acute pain testing, which assesses a type of pain vastly different from postsurgical pain, making the acute tests poor models.12
The prophylactic versus intraoperative timing of the analgesic administration can also influence efficacy. Prophylactic treatment may prevent sensitization that leads to postsurgical hypersensitivity; for example, ketoprofen, a NSAID, is more effective when given prophylactically than intraoperatively.14 However, the opioids buprenorphine and morphine are most effective when given before and during surgery.2,4
In the present study, we used the Rat Grimace Scale (as described in reference 19) to determine effective drug dose and schedule combinations for minimizing postsurgical pain in laboratory rats. Previous work has shown that commonly recommended drugs and doses often are insufficient for postsurgical pain in mice,11 but whether these findings similarly apply to rats has been unknown. We also assessed various drugs for their potential to reduce acute pain, to determine whether acute analgesia is predictive of postsurgical efficacy.
Materials and Methods
All experiments complied with animal care and use guidelines and were approved by the University of Alabama IACUC. Rats of both sexes (n = 246, 123 male and 123 female) were included in all testing, but because no sex-associated differences emerged, all data were combined for reporting. All drugs and vehicle injections were injected at a volume of 1 mL/kg. Buprenorphine and carprofen were diluted in saline; acetaminophen, ibuprofen, and ketoprofen were diluted in 30% polyethylene glycol.
Animals.
To extend previous work that focused on mice,19 all subjects were Wistar rats (weight, 250 to 300 g) obtained from Charles River Laboratories (Hartford, CT). Rats were housed in groups of 4, under a 12:12-h light:dark cycle (lights on, 2130) and provided with food (7017 NIH31, Harlan Laboratories, Indianapolis, IN) and sterile water (Hydropac, Seaford, DE) free choice. Animals were housed in acrylic cages with aspen woodchip bedding in a pathogen-free room with food and water checked daily, and cages were changed twice a week. Cotton squares were added for enrichment purposes. Air handlers controlled and filtered the air in the housing room separately from the rest of the building. Cohorts of rats were tested in the hot-plate test, given a week for drug washout, and then were tested in the laparotomy assay. To avoid sensitization or tolerance effects, no rat received the same drug class twice. All drugs were administered at a volume of 1 ml/kg. All studies were approved by the University of Alabama at Birmingham IACUC. The University of Alabama at Birmingham is AAALAC-accredited.
Hot-plate assay—acute pain.
The hot plate (IITC Life Science, Woodland Hills, CA) was set to 53 °C. All subjects were tested at baseline and at 30 and 60 min after injection. To prevent tissue damage, a cutoff of 60 s was used. The analgesics used were buprenorphine (0.01 to 0.05 mg/kg SC; Sigma, St Louis, MO; n = 4 to 7 per group), carprofen (5 to 25 mg/kg SC; Pfizer, ; n = 4 to 6), saline (Hospira, SC, n = 7), acetaminophen (25 to 100 mg/kg SC; MP Biomedicals, ; n = 3 to 7), ibuprofen (15 to 30 mg/kg SC; MP Biomedicals; n = 5 or 6), ketoprofen (10 to 25 mg/kg SC; MP Biomedicals; n = 4 to 7), and 30% polyethylene glycol (SC; EMD Millipore, Billerica, MA; n = 7). For this assay, common doses were chosen to assess the utility to predict efficacy; we typically used additional doses in other tests to determine the threshold for analgesia.
Laparotomy—spontaneous pain.
A laparotomy, used as a representative surgery that produces spontaneous pain, was performed under isoflurane–oxygen (2%) anesthesia. After shaving and disinfection (alternating povidone–iodine and alcohol, repeated 3 times), a 2-cm incision was made through the skin, fascia, and muscle by using a scalpel. Wounds (muscle wall and skin) were closed by using surgical glue (Vetbond, 3M, Maplewood, MN).
Prophylactic analgesia.
For all prophylactic treatments with analgesics, rats were injected 15 min prior to laparotomy. Analgesics used were buprenorphine (0.01 to 0.025 mg/kg SC; n = 4 or 5 per group), carprofen (5 to 25 mg/kg SC; n = 5 to 7), saline ( SC; n = 7), acetaminophen (50 to 100 mg/kg SC, n = 4 to 8/group), ibuprofen (5 to 30 mg/kg SC, n = 3 or 4/group), ketoprofen (10 to 25 mg/kg SC, n = 2 to 5/group), and 30% polyethylene glycol (SC; n = 10).
Intraoperative analgesia.
Intraoperative analgesics were administered while the surgery was being performed and the rat was anesthetized. Analgesics used were buprenorphine (0.01 to 0.05 mg/kg SC, n = 6 per group), carprofen (5 to 25 mg/kg SC, n = 5 to 12), saline ( SC, n = 13), acetaminophen (25 to 100 mg/kg SC, n = 6 to 8), ibuprofen (5 to 30 mg/kg SC, n = 6 to 8), ketoprofen (5 to 25 mg/kg SC, n = 4 to 9), and 30% polyethylene glycol ( SC, n = 10).
Digital videorecording.
Rats (4 at a time) were placed on a tabletop and allocated between 4 isolated cubicles. The cubicles (21.25 × 8.75 × 10.00 cm) were made of clear acrylic with an opaque, stainless steel, separating wall and perforated, stainless steel floor. Digital high-definition video cameras (HMX-QF20 Full HD, Samsung) were placed on both sides of the cubicles to maximize the opportunity to record clear images of rats’ heads. Rats were recorded in 30-min sessions in a closed room, with no experimenters present.18 Baseline recordings were collected on a day prior to the day of surgery. On the test day, recording began 45 min after surgery.
Analysis of videos.
To determine pain scores for each rat, representative images were taken from each 3-min time bin and randomized into a PowerPoint (Microsoft, Redmond, WA) presentation. Each image was scored independently by 2 raters who were blinded to the experimental condition. For both scoring experiments, intraclass correlations were computed for the baseline (prophylactic, 0.68; intraoperative, 0.67) and test (prophylactic, 0.73; intraoperative, 0.77) scores. Each rater was responsible for rating each image on all 4 action units (orbit, nose–cheek, whiskers, and ears). For each of the 2 raters, a mean was calculated for each action unit within a single test session (baseline or after surgery) and entered into SPSS (IBM, Armonk, NY). Mean scores were calculated for the baseline and postsurgery conditions (for each rater and then as a combined mean), and a difference score was calculated. Additional details regarding scoring pain by using the Rat Grimace Scale are found in reference 19.
Statistical analysis.
All statistical analyses were performed by using SPSS version 22 (IBM). Data from each assay were analyzed by using univariate ANOVA, followed by the Dunnett posthoc test to compare each drug with its vehicle. An α level of 0.05 was applied to designate significance.
Results
All treatments showed a significant (P < 0.05) effect of drug (Table 1). For all statistical analyses, data were separated according to the vehicle used and assay—that is, all drugs with the same vehicle (saline or 30% polyethylene glycol) were analyzed together in each of the 3 assays (Table 1). Significant effects of drug are as compared with their vehicle. Figures for laparotomy data represent the change in scores from baseline to postsurgery. All data for the hot-plate assay represent the maximal percentage change in response. We define an effective drug or dose as one that significantly (P < 0.05) reduces pain (that is, increases latency in the hot-plate assay, decreases the behavior score after laparotomy) when compared with the control condition.
Table 1.
Main effects of drug treatment for all ANOVA from all assays, separated according to drug vehicle
| Vehicle | Test | F | df | P |
| Saline | Hot plate | 4.393 | 6, 33 | 0.002 |
| 30% polyethylene glycol | Hot plate | 2.740 | 8, 40 | 0.016 |
| Saline | Intraoperative | 3.314 | 7, 53 | 0.005 |
| 30% polyethylene glycol | Intraoperative | 2.606 | 10, 66 | 0.010 |
| Saline | Prophylactic | 2.587 | 5, 27 | 0.049 |
| 30% polyethylene glycol | Prophylactic | 5.227 | 8, 34 | 0.001 |
Drugs diluted in saline.
Buprenorphine.
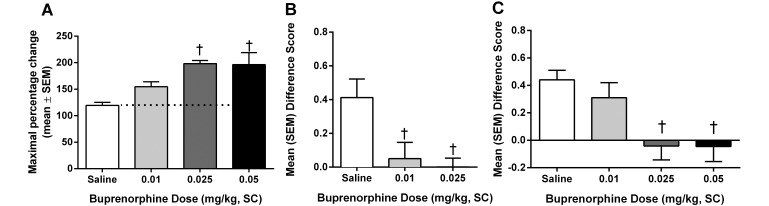
Three doses of buprenorphine were used: 0.01, 0.025, and 0.05 mg/kg. Of these, only doses of 0.025 and 0.05 mg/kg significantly (P < 0.01) increased the threshold of the animals in the hot-plate assay (Figure 1 A). When used as an analgesic for postsurgical pain, buprenorphine was effective at 0.01 and 0.025 mg/kg (P < 0.05) when injected prophylactically (Figure 1 B) and at 0.025 and 0.05 mg/kg (P < 0.01) when administered intraoperatively (Figure 1 C).
Figure 1.
Analgesic effects of buprenorphine on (A) acute pain when tested by using the hot plate at 53 °C, (B) spontaneous pain when administered prophylactically (15 min prior to surgery), and (C) spontaneous pain when administered intraoperatively (during surgery). †, Value is significantly (P < 0.01) different from that for saline.
Carprofen.
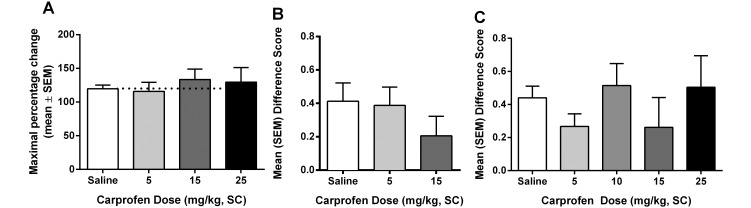
Carprofen was ineffective as an analgesic in all tests (Figure 2).
Figure 2.
Analgesic effects of carprofen on (A) acute pain when tested by using the hot plate at 53 °C, (B) spontaneous pain when administered prophylactically (15 min prior to surgery), and (C) spontaneous pain when administered intraoperatively (during surgery). All differences were nonsignificant.
Drugs diluted in 30% polyethylene glycol.
Acetaminophen.
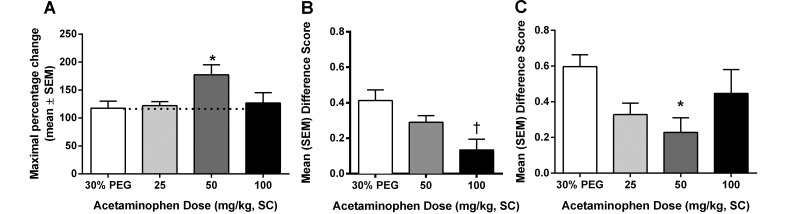
Three doses of acetaminophen were used in these assays: 25, 50, and 100 mg/kg. In the hot-plate assay (Figure 3 A), acetaminophen was effective (P < 0.05) at the 50-mg/kg dose only. When used as an analgesic for postsurgical pain, the drug was significantly effective at 50 mg/kg (P < 0.05) when administered intraoperatively (Figure 3 C) and at 100 mg/kg (P < 0.01) prophylactically (Figure 3 B).
Figure 3.
Analgesic effect of acetaminophen on (A) acute pain when tested by using the hot plate at 53 °C, (B) spontaneous pain when administered prophylactically (15 min prior to surgery), and (C) spontaneous pain when administered intraoperatively (during surgery). Value is significantly (*, P < 0.05; †, P < 0.01) different from that for 30% PEG.
Ibuprofen.
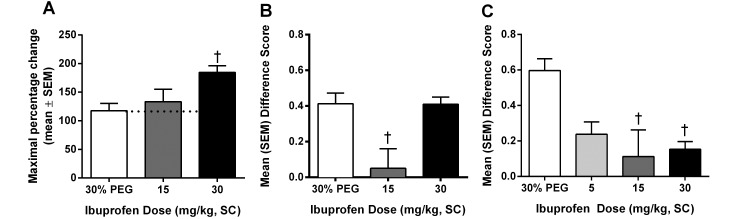
Three doses of ibuprofen were used in these assays: 5, 15, and 30 mg/kg. In the hot-plate assay (Figure 4 A), ibuprofen significantly (P < 0.01) increased the percentage change at the 30-mg/kg dose. When administered intraoperatively (Figure 4 C), all doses were effective (P < 0.05) in reducing postsurgical pain. However when ibuprofen was administered prophylactically (Figure 4 B), only the 15-mg/kg dose was effective (P < 0.01).
Figure 4.
Analgesic effect of ibuprofen on (A) acute pain when tested by using the hot plate at 53 °C, (B) spontaneous pain when administered prophylactically (15 min prior to surgery), and (C) spontaneous pain when administered intraoperatively (during surgery). †, Value is significantly (P < 0.01) different from that for 30% PEG.
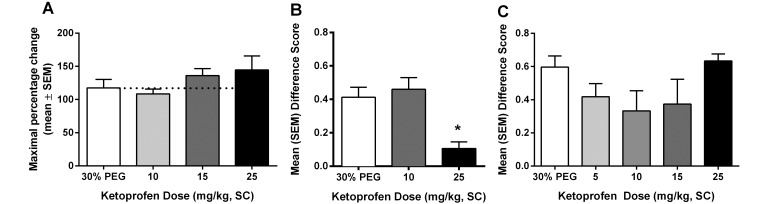
Ketoprofen.
Four doses of ketoprofen were used in these assays: 5, 10, 15, and 25 mg/kg. Ketoprofen had no effect in the hot-plate assay (Figure 5 A) at any dose. Similarly, all doses of ketoprofen were ineffective when the drug was administered intraoperatively (Figure 5 C). For postsurgical pain, ketoprofen was effective when given prophylactically (Figure 5 B) at the 15-mg/kg (P < 0.01) and 25-mg/kg (P < 0.05) doses.
Figure 5.
Analgesic effect of ketoprofen on (A) acute pain when tested by using the hot plate at 53 °C, (B) spontaneous pain when administered prophylactically (15 min prior to surgery), and (C) spontaneous pain when administered intraoperatively (during surgery). *, Value is significantly (P < 0.05) different from that for 30% PEG.
Discussion
To determine the efficacy of common analgesics for reducing pain after laparotomy, we administered common analgesic drugs intraoperatively or prophylactically. Our data indicate that the timing of injections relative to surgery can alter whether a drug significantly reduces postsurgical pain and that many common analgesics are ineffective altogether or at various points within their recommended dose ranges. The data also suggest that the effectiveness of analgesics in acute pain is not necessarily indicative of their efficacy regarding postsurgical pain.
In addition to humane concerns, pain must be minimized to avoid confounding experimental outcomes. Spontaneous pain is the most common complication after surgery and may seriously affect experimental results in animals when not treated sufficiently. Currently available means to assess spontaneous pain in animals include complex behavioral assessment,16,17 ultrasonic vocalizations,7,13,22 and surgically implanted telemetry,4 but all of these methods have drawbacks.
One study16 evaluated the use of behavioral indicators in an approach that was intended to be less subjective than other methods for measuring pain. Although the authors assert that their measures are more effective and less subjective than most, rats were filmed for 8 h, behavioral analysis software was used for data collection, and the researchers reviewed 150 behaviors before establishing a list of the most representative. By using the pared list of behaviors, rats were filmed to determine the frequency and duration of specific behaviors associated with pain.17 Software was used to collect data, and all footage was reviewed by a single treatment-blinded observer, thus preventing measures of interrater reliability and allowing for objectivity. Despite its reported validity, this method is complicated, time-consuming, and impractical for common use. Our review of these behavioral assessments suggests that at least 22 different behaviors appear in these reports,9,16,17 with only 5 being consistently reported (arch, fall, stagger, twitch, and writhe). In addition, a recent report9 suggested that the 5 common behaviors occur “so infrequently” that they should be combined into a composite score. These comments lend credence to our claim that the method, although seemingly valid and comparable to facial expression scoring,9 is more complicated.
The measurement of ultrasonic vocalizations has been used as another method to assess both evoked and chronic pain, with mixed results. After using both partial sciatic nerve ligations and formalin to induce a chronic pain state, one study22 recorded no vocalizations. In contrast, others have found that ultrasonic vocalizations correlate with testing of mechanical hypersensitivity.7 Furthermore, ultrasonic vocalizations have been recorded during the formalin test and can be suppressed by administration of morphine.13 Unfortunately, recording ultrasonic vocalizations requires special chambers, recording equipment, and software to collect and analyze the data, making this method an unlikely means to rapidly and routinely assess pain in laboratory animals.
Armed with the knowledge that rodents often suppress their pain in the presence of an experimenter,1,18 animal care staff and veterinarians must be able to assess pain rapidly (within seconds). With the publication of the grimace scales for assessing spontaneous pain in mice8 and rats,19 pain can be assessed reliably, quickly, and after minimal training. Additional analysis can be done by using recorded videos. These methods have led to the determination that common NSAID are ineffective at their recommended doses for postsurgical pain in mice.11 Here, we show similar findings for carprofen and ketoprofen, which respectively were completely and moderately ineffective for postsurgical pain in rats. We further found that buprenorphine was effective in rats, as it is in mice.11 Acetaminophen, although ineffective in mice,11 was effective in rats, whereas ibuprofen was effective in rats but has not been tested in mice.
In terms of studies examining postoperative analgesia in rats after laparotomy, our results are consistent with previous explorations in only one case: buprenorphine is an effective analgesic. Early seminal work examining body weight change, food and water intake, and locomotor activity showed that buprenorphine (0.05 mg/kg) was more effective than saline as an analgesic.10 This effect was replicated (for the most part) several years later by the same group.6 Whereas we found no analgesic effect for carprofen and moderate analgesia for ketoprofen, others have found these drugs to be significantly better than saline in treating post-laparotomy pain.16 Subcutaneous carprofen and ketoprofen (at identical doses to those we used [0.5 mg/kg]) were effective in reducing weight loss after surgery.3 With the publication of pain-related behaviors as outcome measures, buprenorphine, carprofen, and ketoprofen have been shown to be efficacious in reducing several of these behaviors.16,17 Another group examined locomotor activity and food and water intake and found carprofen to be effective in reducing pain.23 Why we were unable to document analgesia due to carprofen is unknown, but the reason may reflect the specificity of the Rat Grimace Scale for pain expression. Whereas pain clearly affects locomotor activity and food intake, several other factors, such as time of day, relative hunger, and the presence of other conspecifics can affect these measures as well. Some of these factors also affect facial expressions of pain in rats,18 but we controlled for these effects in the current study.
Our current data support the reevaluation of commonly recommended analgesics for postsurgical pain in laboratory rats. Regarding each of the tested drugs, the opioid buprenorphine was effective in all conditions, but higher doses were needed for acute pain and intraoperative injection than for preemptive injection. Unfortunately single exposures to opioid drugs can have long-lasting effects21 and, as controlled substances, have limited usefulness. From the NSAID class of drugs, acetaminophen and ibuprofen performed similarly, with moderate to high doses showing analgesia. In contrast, the most commonly recommended NSAID, carprofen and ketoprofen, were never or only rarely, respectively, effective as analgesics. Ketoprofen was more effective when given prophylactically, suggesting that its onset of action may be slower than other utilized drugs or that using ketoprofen before surgery to reduce the inflammatory process has some benefits for subsequent pain. Our data indicate that the recommended drugs and doses of NSAID should be reconsidered.
The guidelines set forth in the United States and United Kingdom for addressing pain and distress in laboratory animals are intended for general use for various surgical treatments, but research suggests they are falling short of full adoption.15 This discrepancy may be the result of conflicting or variable recommendations between institutions or due to limited empirical research on which to base recommendations. Until recently, measures of spontaneous pain in laboratory rats have been highly subjective, required expensive equipment, or were inconsistent and unreliable. The publication of the Rat Grimace Scale19 as an alternative to more complicated and expensive measures provides a simpler and reliable method for assessing spontaneous pain in laboratory rats that is available to all researchers and animal care staff. We assert that institutions should reevaluate their recommended drugs and doses with reference to the appropriate surgical model, as illustrated in the current study.
Acknowledgments
The authors declare no conflict of interest.
References
- 1.Adamson TW, Kendall LV, Goss S, Grayson K, Touma C, Palme R, Chen JQ, Borowsky AD. 2010. Assessment of carprofen and buprenorphine on recovery of mice after surgical removal of the mammary fat pad. J Am Assoc Lab Anim Sci 49:610–616. [PMC free article] [PubMed] [Google Scholar]
- 2.Brennan TJ, Umali EF, Zahn PK. 1997. Comparison of pre- versus postincision administration of intrathecal bupivacaine and intrathecal morphine in a rat model of postoperative pain. Anesthesiology 87:1517–1528. [DOI] [PubMed] [Google Scholar]
- 3.Flecknell PA, Orr HE, Roughan JV, Stewart R. 1999. Comparison of the effects of oral or subcutaneous carprofen or ketoprofen in rats undergoing laparotomy. Vet Rec 144:65–67. [DOI] [PubMed] [Google Scholar]
- 4.Goecke JC, Awad H, Lawson JC, Boivin GP. 2005. Evaluating postoperative analgesics in mice using telemetry. Comp Med 55:37–44. [PubMed] [Google Scholar]
- 5.Hawkins P. 2002. Recognizing and assessing pain, suffering and distress in laboratory animals: a survey of current practice in the UK with recommendations. Lab Anim 36:378–395. [DOI] [PubMed] [Google Scholar]
- 6.Hayes JH, Flecknell PA. 1999. A comparison of pre- and postsurgical administration of bupivacaine or buprenorphine following laparotomy in the rat. Lab Anim 33:16–23. [DOI] [PubMed] [Google Scholar]
- 7.Kurejova M, Nattenmuller U, Hildebrandt U, Selvaraj D, Stosser S, Kuner R. 2010. An improved behavioural assay demonstrates that ultrasound vocalizations constitute a reliable indicator of chronic cancer pain and neuropathic pain. Mol Pain 6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. 2010. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 7:447–449. [DOI] [PubMed] [Google Scholar]
- 9.Leach MC, Klaus K, Miller AL, Scotto di Perrotolo M, Sotocinal SG, Flecknell PA. 2012. The assessment of postvasectomy pain in mice using behaviour and the Mouse Grimace Scale. PLoS ONE 7:e35656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liles JH, Flecknell PA. 1994. A comparison of the effects of buprenorphine, carprofen, and flunixin following laparotomy in rats. J Vet Pharmacol Ther 17:284–290. [DOI] [PubMed] [Google Scholar]
- 11.Matsumiya LC, Sorge RE, Sotocinal SG, Tabaka JM, Wieskopf JS, Zaloum A, King OD, Mogil JS. 2012. Using the Mouse Grimace Scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J Am Assoc Lab Anim Sci 51:42–49. [PMC free article] [PubMed] [Google Scholar]
- 12.Mogil JS, Davis KD, Derbyshire SW. 2010. The necessity of animal models in pain research. Pain 151:12–17. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira AR, Barros HM. 2006. Ultrasonic rat vocalizations during the formalin test: a measure of the affective dimension of pain? Anesth Analg 102:832–839. [DOI] [PubMed] [Google Scholar]
- 14.Prado WA, Pontes RM. 2002. Presurgical ketoprofen, but not morphine, dipyrone, diclofenac or tenoxicam, preempts postincisional mechanical allodynia in rats. Braz J Med Biol Res 35:111–119. [DOI] [PubMed] [Google Scholar]
- 15.Richardson CA, Flecknell PA. 2005. Anaesthesia and postoperative analgesia following experimental surgery in laboratory rodents: are we making progress? AlternLab Anim 33:119–127. [DOI] [PubMed] [Google Scholar]
- 16.Roughan JV, Flecknell PA. 2001. Behavioural effects of laparotomy and analgesic effects of ketoprofen and carprofen in rats. Pain 90:65–74. [DOI] [PubMed] [Google Scholar]
- 17.Roughan JV, Flecknell PA. 2003. Evaluation of a short duration behaviour-based postoperative pain scoring system in rats. Eur J Pain 7:397–406. [DOI] [PubMed] [Google Scholar]
- 18.Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JC, McPhail M, Delaney A, Wigerblad G, Schumann AP, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS. 2014. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods 11:629–632. [DOI] [PubMed] [Google Scholar]
- 19.Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, Mapplebeck JC, Wei P, Zhan S, Zhang S, McDougall JJ, King OD, Mogil JS. 2011. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain 7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trull FL, Rich BA. 1999. More regulation of rodents. Science 284:1463. [DOI] [PubMed] [Google Scholar]
- 21.Vanderschuren LJ, De Vries TJ, Wardeh G, Hogenboom FA, Schoffelmeer AN. 2001. A single exposure to morphine induces long-lasting behavioural and neurochemical sensitization in rats. Eur J Neurosci 14:1533–1538. [DOI] [PubMed] [Google Scholar]
- 22.Wallace VC, Norbury TA, Rice AS. 2005. Ultrasound vocalisation by rodents does not correlate with behavioural measures of persistent pain. Eur J Pain 9:445–452. [DOI] [PubMed] [Google Scholar]
- 23.Zegre Cannon C, Kissling GE, Goulding DR, King-Herbert AP, Blankenship-Paris T. 2011. Analgesic effects of tramadol, carprofen or multimodal analgesia in rats undergoing ventral laparotomy. Lab Anim (NY) 40:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]