Abstract
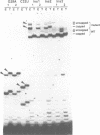
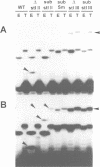
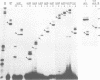
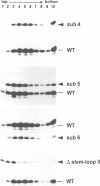
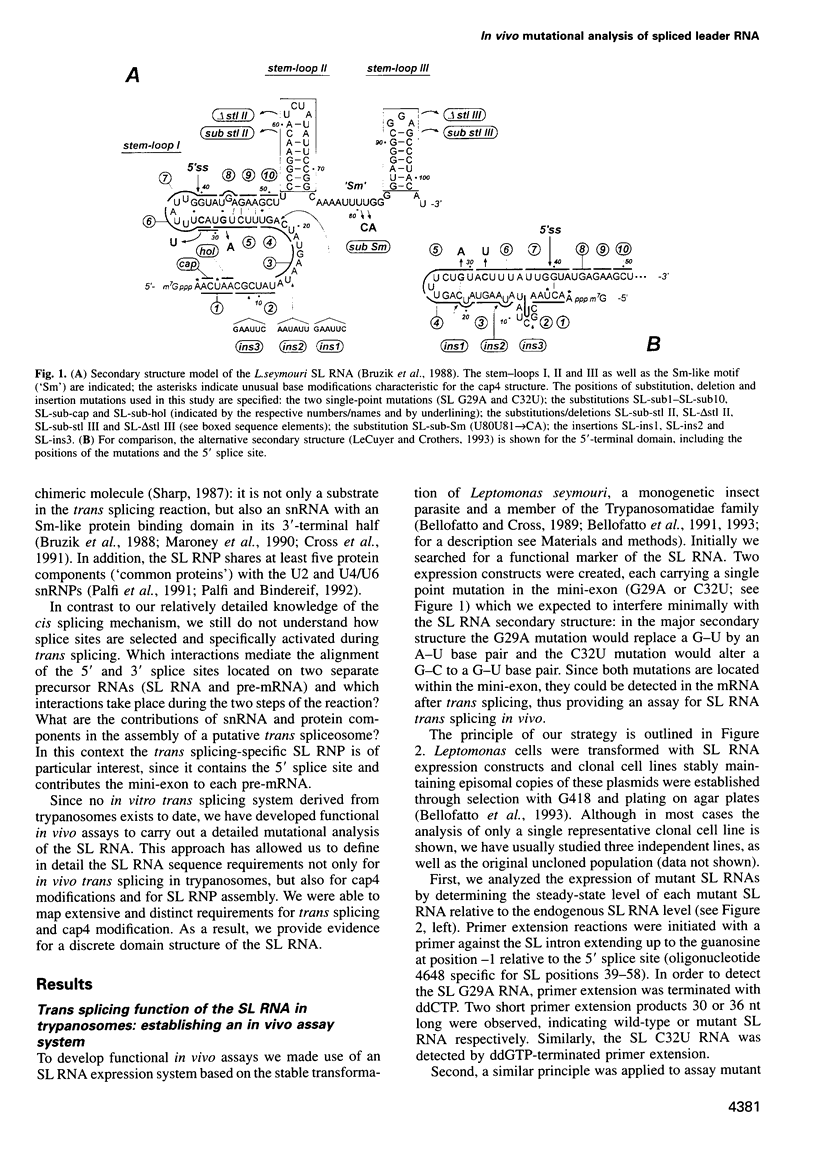
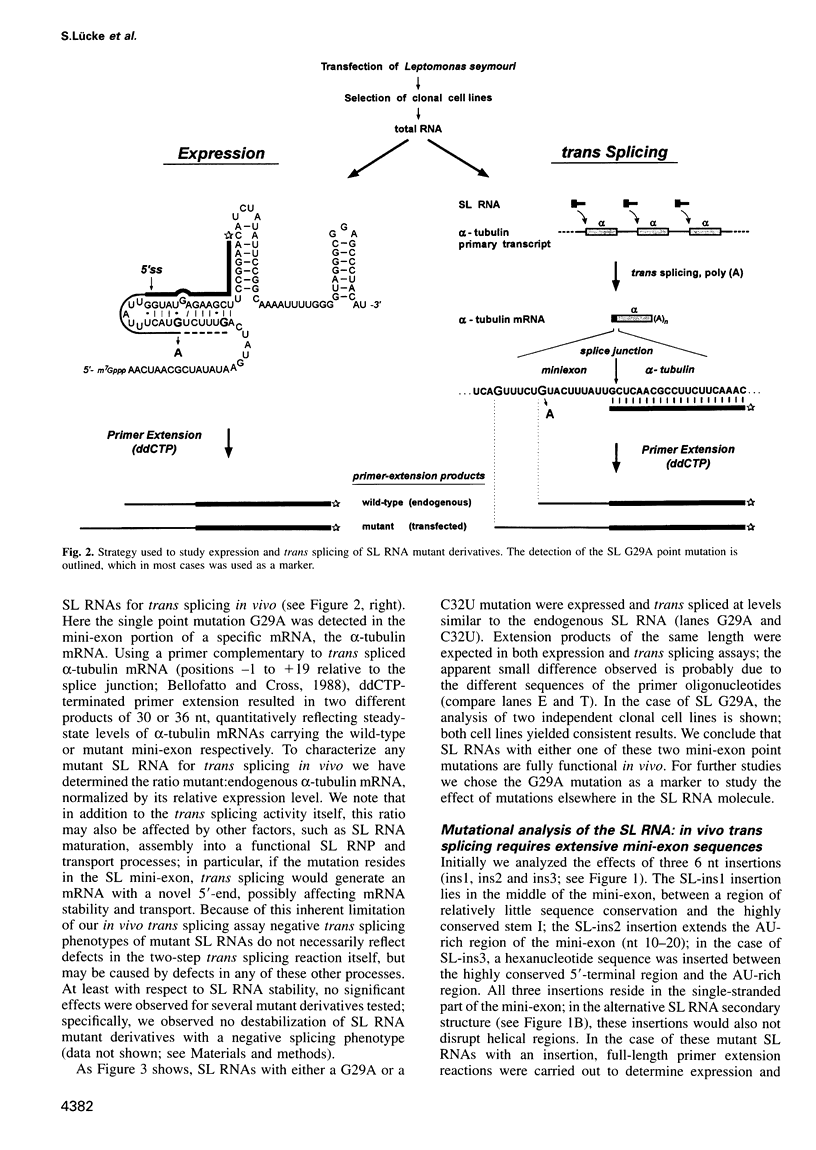
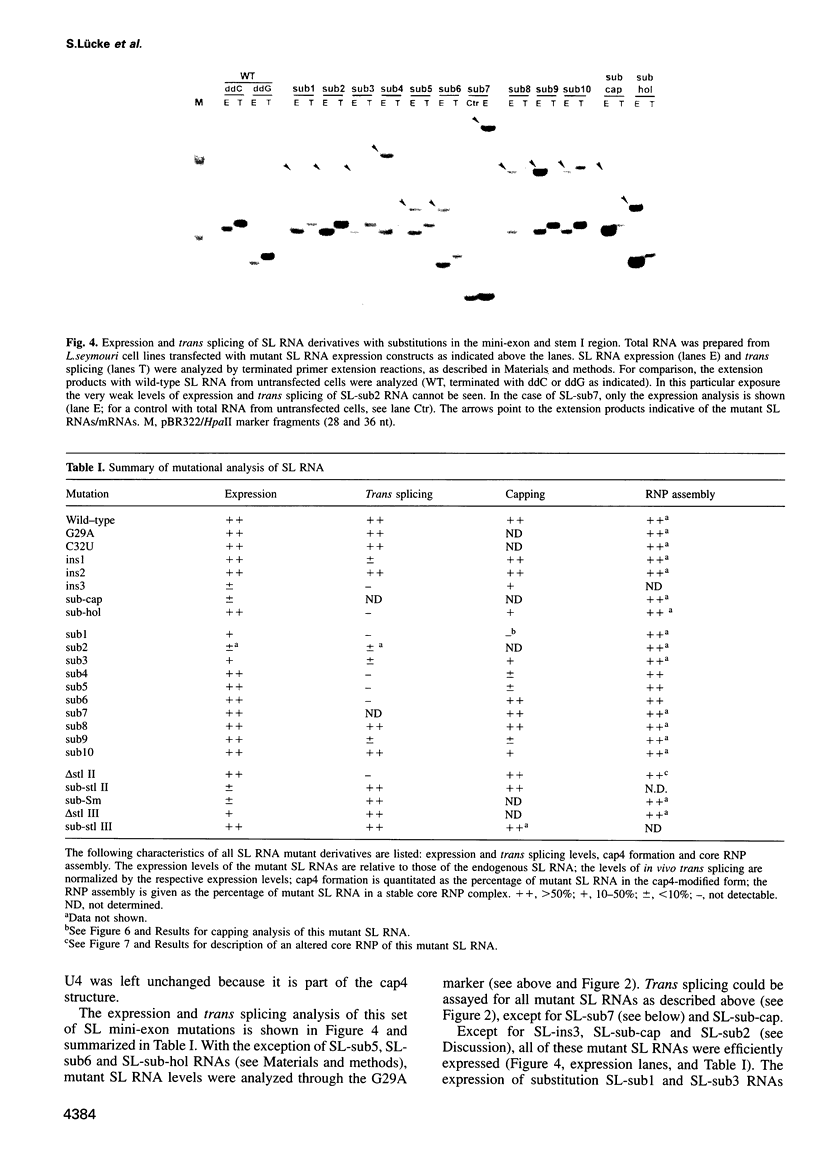
In trypanosomes mRNAs are generated through trans splicing. The spliced leader (SL) RNA, which donates the 5'-terminal mini-exon to each of the protein coding exons, plays a central role in the trans splicing process. We have established in vivo assays to study in detail trans splicing, cap4 modification, and RNP assembly of the SL RNA in the trypanosomatid species Leptomonas seymouri. First, we found that extensive sequences within the mini-exon are required for SL RNA function in vivo, although a conserved length of 39 nt is not essential. In contrast, the intron sequence appears to be surprisingly tolerant to mutation; only the stem-loop II structure is indispensable. The asymmetry of the sequence requirements in the stem I region suggests that this domain may exist in different functional conformations. Second, distinct mini-exon sequences outside the modification site are important for efficient cap4 formation. Third, all SL RNA mutations tested allowed core RNP assembly, suggesting flexible requirements for core protein binding. In sum, the results of our mutational analysis provide evidence for a discrete domain structure of the SL RNA and help to explain the strong phylogenetic conservation of the mini-exon sequence and of the overall SL RNA secondary structure; they also suggest that there may be certain differences between trans splicing in nematodes and trypanosomes. This approach provides a basis for studying RNA-RNA interactions in the trans spliceosome.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian N. Trans splicing of nuclear pre-mRNAs. Cell. 1990 Jun 29;61(7):1157–1160. doi: 10.1016/0092-8674(90)90674-4. [DOI] [PubMed] [Google Scholar]
- Agami R., Aly R., Halman S., Shapira M. Functional analysis of cis-acting DNA elements required for expression of the SL RNA gene in the parasitic protozoan Leishmania amazonensis. Nucleic Acids Res. 1994 Jun 11;22(11):1959–1965. doi: 10.1093/nar/22.11.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONE G. J., STEINERT M. Isotopes incorporated in the nucleic acids of Trypanosoma mega. Nature. 1956 Aug 11;178(4528):308–309. doi: 10.1038/178308a0. [DOI] [PubMed] [Google Scholar]
- Bangs J. D., Crain P. F., Hashizume T., McCloskey J. A., Boothroyd J. C. Mass spectrometry of mRNA cap 4 from trypanosomatids reveals two novel nucleosides. J Biol Chem. 1992 May 15;267(14):9805–9815. [PubMed] [Google Scholar]
- Bellofatto V., Cooper R., Cross G. A. Discontinuous transcription in Leptomonas seymouri: presence of intact and interrupted mini-exon gene families. Nucleic Acids Res. 1988 Aug 11;16(15):7437–7456. doi: 10.1093/nar/16.15.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellofatto V., Cross G. A. Characterization of RNA transcripts from the alpha tubulin gene cluster of Leptomonas seymouri. Nucleic Acids Res. 1988 Apr 25;16(8):3455–3469. doi: 10.1093/nar/16.8.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellofatto V., Cross G. A. Expression of a bacterial gene in a trypanosomatid protozoan. Science. 1989 Jun 9;244(4909):1167–1169. doi: 10.1126/science.2499047. [DOI] [PubMed] [Google Scholar]
- Bellofatto V., Hartree D. E., Torres-Munoz J. Leptomonas seymouri as a model system for the analysis of gene expression in trypanosomatids. J Parasitol. 1993 Oct;79(5):637–644. [PubMed] [Google Scholar]
- Bellofatto V., Torres-Muñoz J. E., Cross G. A. Stable transformation of Leptomonas seymouri by circular extrachromosomal elements. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6711–6715. doi: 10.1073/pnas.88.15.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzik J. P., Steitz J. A. Spliced leader RNA sequences can substitute for the essential 5' end of U1 RNA during splicing in a mammalian in vitro system. Cell. 1990 Sep 7;62(5):889–899. doi: 10.1016/0092-8674(90)90264-f. [DOI] [PubMed] [Google Scholar]
- Bruzik J. P., Van Doren K., Hirsh D., Steitz J. A. Trans splicing involves a novel form of small nuclear ribonucleoprotein particles. Nature. 1988 Oct 6;335(6190):559–562. doi: 10.1038/335559a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cross M., Günzl A., Palfi Z., Bindereif A. Analysis of small nuclear ribonucleoproteins (RNPs) in Trypanosoma brucei: structural organization and protein components of the spliced leader RNP. Mol Cell Biol. 1991 Nov;11(11):5516–5526. doi: 10.1128/mcb.11.11.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freistadt M. S., Cross G. A., Branch A. D., Robertson H. D. Direct analysis of the mini-exon donor RNA of Trypanosoma brucei: detection of a novel cap structure also present in messenger RNA. Nucleic Acids Res. 1987 Dec 10;15(23):9861–9879. doi: 10.1093/nar/15.23.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freistadt M. S., Cross G. A., Robertson H. D. Discontinuously synthesized mRNA from Trypanosoma brucei contains the highly methylated 5' cap structure, m7GpppA*A*C(2'-O)mU*A. J Biol Chem. 1988 Oct 15;263(29):15071–15075. [PubMed] [Google Scholar]
- Günzl A., Cross M., Bindereif A. Domain structure of U2 and U4/U6 small nuclear ribonucleoprotein particles from Trypanosoma brucei: identification of trans-spliceosomal specific RNA-protein interactions. Mol Cell Biol. 1992 Feb;12(2):468–479. doi: 10.1128/mcb.12.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon G. J., Maroney P. A., Ayers D. G., Shambaugh J. D., Nilsen T. W. Transcription of a nematode trans-spliced leader RNA requires internal elements for both initiation and 3' end-formation. EMBO J. 1990 Jun;9(6):1915–1921. doi: 10.1002/j.1460-2075.1990.tb08318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon G. J., Maroney P. A., Denker J. A., Nilsen T. W. Trans splicing of nematode pre-messenger RNA in vitro. Cell. 1990 Jun 29;61(7):1247–1255. doi: 10.1016/0092-8674(90)90689-c. [DOI] [PubMed] [Google Scholar]
- Hannon G. J., Maroney P. A., Yu Y. T., Hannon G. E., Nilsen T. W. Interaction of U6 snRNA with a sequence required for function of the nematode SL RNA in trans-splicing. Science. 1992 Dec 11;258(5089):1775–1780. doi: 10.1126/science.1465612. [DOI] [PubMed] [Google Scholar]
- Harris K. A., Jr, Crothers D. M., Ullu E. In vivo structural analysis of spliced leader RNAs in Trypanosoma brucei and Leptomonas collosoma: a flexible structure that is independent of cap4 methylations. RNA. 1995 Jun;1(4):351–362. [PMC free article] [PubMed] [Google Scholar]
- Hartree D., Bellofatto V. Essential components of the mini-exon gene promoter in the trypanosomatid Leptomonas seymouri. Mol Biochem Parasitol. 1995 Apr;71(1):27–39. doi: 10.1016/0166-6851(95)00034-x. [DOI] [PubMed] [Google Scholar]
- Horowitz D. S., Krainer A. R. Mechanisms for selecting 5' splice sites in mammalian pre-mRNA splicing. Trends Genet. 1994 Mar;10(3):100–106. doi: 10.1016/0168-9525(94)90233-x. [DOI] [PubMed] [Google Scholar]
- Jones M. H., Guthrie C. Unexpected flexibility in an evolutionarily conserved protein-RNA interaction: genetic analysis of the Sm binding site. EMBO J. 1990 Aug;9(8):2555–2561. doi: 10.1002/j.1460-2075.1990.tb07436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandels-Lewis S., Séraphin B. Involvement of U6 snRNA in 5' splice site selection. Science. 1993 Dec 24;262(5142):2035–2039. doi: 10.1126/science.8266100. [DOI] [PubMed] [Google Scholar]
- Laird P. W., Zomerdijk J. C., de Korte D., Borst P. In vivo labelling of intermediates in the discontinuous synthesis of mRNAs in Trypanosoma brucei. EMBO J. 1987 Apr;6(4):1055–1062. doi: 10.1002/j.1460-2075.1987.tb04858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird P. W., ten Asbroek A. L., Borst P. Controlled turnover and 3' trimming of the trans splicing precursor of Trypanosoma brucei. Nucleic Acids Res. 1987 Dec 23;15(24):10087–10103. doi: 10.1093/nar/15.24.10087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCuyer K. A., Crothers D. M. The Leptomonas collosoma spliced leader RNA can switch between two alternate structural forms. Biochemistry. 1993 May 25;32(20):5301–5311. doi: 10.1021/bi00071a004. [DOI] [PubMed] [Google Scholar]
- Lesser C. F., Guthrie C. Mutations in U6 snRNA that alter splice site specificity: implications for the active site. Science. 1993 Dec 24;262(5142):1982–1988. doi: 10.1126/science.8266093. [DOI] [PubMed] [Google Scholar]
- Maroney P. A., Hannon G. J., Denker J. A., Nilsen T. W. The nematode spliced leader RNA participates in trans-splicing as an Sm snRNP. EMBO J. 1990 Nov;9(11):3667–3673. doi: 10.1002/j.1460-2075.1990.tb07578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroney P. A., Hannon G. J., Shambaugh J. D., Nilsen T. W. Intramolecular base pairing between the nematode spliced leader and its 5' splice site is not essential for trans-splicing in vitro. EMBO J. 1991 Dec;10(12):3869–3875. doi: 10.1002/j.1460-2075.1991.tb04956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy-Burke C., Taylor Z. A., Buck G. A. Characterization of the spliced leader genes and transcripts in Trypanosoma cruzi. Gene. 1989 Oct 15;82(1):177–189. doi: 10.1016/0378-1119(89)90043-7. [DOI] [PubMed] [Google Scholar]
- McNally K. P., Agabian N. Trypanosoma brucei spliced-leader RNA methylations are required for trans splicing in vivo. Mol Cell Biol. 1992 Nov;12(11):4844–4851. doi: 10.1128/mcb.12.11.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaeli S., Roberts T. G., Watkins K. P., Agabian N. Isolation of distinct small ribonucleoprotein particles containing the spliced leader and U2 RNAs of Trypanosoma brucei. J Biol Chem. 1990 Jun 25;265(18):10582–10588. [PubMed] [Google Scholar]
- Mottram J., Perry K. L., Lizardi P. M., Lührmann R., Agabian N., Nelson R. G. Isolation and sequence of four small nuclear U RNA genes of Trypanosoma brucei subsp. brucei: identification of the U2, U4, and U6 RNA analogs. Mol Cell Biol. 1989 Mar;9(3):1212–1223. doi: 10.1128/mcb.9.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W. J., Watkins K. P., Agabian N. Identification of a novel Y branch structure as an intermediate in trypanosome mRNA processing: evidence for trans splicing. Cell. 1986 Nov 21;47(4):517–525. doi: 10.1016/0092-8674(86)90616-1. [DOI] [PubMed] [Google Scholar]
- Nilsen T. W. Trans-splicing of nematode premessenger RNA. Annu Rev Microbiol. 1993;47:413–440. doi: 10.1146/annurev.mi.47.100193.002213. [DOI] [PubMed] [Google Scholar]
- Palfi Z., Bindereif A. Immunological characterization and intracellular localization of trans-spliceosomal small nuclear ribonucleoproteins in Trypanosoma brucei. J Biol Chem. 1992 Oct 5;267(28):20159–20163. [PubMed] [Google Scholar]
- Palfi Z., Günzl A., Cross M., Bindereif A. Affinity purification of Trypanosoma brucei small nuclear ribonucleoproteins reveals common and specific protein components. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9097–9101. doi: 10.1073/pnas.88.20.9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfi Z., Xu G. L., Bindereif A. Spliced leader-associated RNA of trypanosomes. Sequence conservation and association with protein components common to trans-spliceosomal ribonucleoproteins. J Biol Chem. 1994 Dec 2;269(48):30620–30625. [PubMed] [Google Scholar]
- Patnaik P. K., Bellofatto V., Hartree D., Cross G. A. An episome of Trypanosoma brucei can exist as an extrachromosomal element in a broad range of trypanosomatids but shows different requirements for stable replication. Mol Biochem Parasitol. 1994 Jul;66(1):153–156. doi: 10.1016/0166-6851(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Patnaik P. K., Kulkarni S. K., Cross G. A. Autonomously replicating single-copy episomes in Trypanosoma brucei show unusual stability. EMBO J. 1993 Jun;12(6):2529–2538. doi: 10.1002/j.1460-2075.1993.tb05908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry K. L., Watkins K. P., Agabian N. Trypanosome mRNAs have unusual "cap 4" structures acquired by addition of a spliced leader. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8190–8194. doi: 10.1073/pnas.84.23.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby S. W., Abelson J. An early hierarchic role of U1 small nuclear ribonucleoprotein in spliceosome assembly. Science. 1988 Nov 18;242(4881):1028–1035. doi: 10.1126/science.2973660. [DOI] [PubMed] [Google Scholar]
- Saito R. M., Elgort M. G., Campbell D. A. A conserved upstream element is essential for transcription of the Leishmania tarentolae mini-exon gene. EMBO J. 1994 Nov 15;13(22):5460–5469. doi: 10.1002/j.1460-2075.1994.tb06881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. Trans splicing: variation on a familiar theme? Cell. 1987 Jul 17;50(2):147–148. doi: 10.1016/0092-8674(87)90207-8. [DOI] [PubMed] [Google Scholar]
- Sontheimer E. J., Steitz J. A. Three novel functional variants of human U5 small nuclear RNA. Mol Cell Biol. 1992 Feb;12(2):734–746. doi: 10.1128/mcb.12.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A. Splicing takes a holliday. Science. 1992 Aug 14;257(5072):888–889. doi: 10.1126/science.1386941. [DOI] [PubMed] [Google Scholar]
- Sutton R. E., Boothroyd J. C. Evidence for trans splicing in trypanosomes. Cell. 1986 Nov 21;47(4):527–535. doi: 10.1016/0092-8674(86)90617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschudi C., Ullu E. Destruction of U2, U4, or U6 small nuclear RNA blocks trans splicing in trypanosome cells. Cell. 1990 May 4;61(3):459–466. doi: 10.1016/0092-8674(90)90527-l. [DOI] [PubMed] [Google Scholar]
- Ullu E., Matthews K. R., Tschudi C. Temporal order of RNA-processing reactions in trypanosomes: rapid trans splicing precedes polyadenylation of newly synthesized tubulin transcripts. Mol Cell Biol. 1993 Jan;13(1):720–725. doi: 10.1128/mcb.13.1.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullu E., Tschudi C. Trans splicing in trypanosomes requires methylation of the 5' end of the spliced leader RNA. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10074–10078. doi: 10.1073/pnas.88.22.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G. L., Wieland B., Bindereif A. trans-spliceosomal U6 RNAs of Crithidia fasciculata and Leptomonas seymouri: deviation from the conserved ACAGAG sequence and potential base pairing with spliced leader RNA. Mol Cell Biol. 1994 Jul;14(7):4565–4570. doi: 10.1128/mcb.14.7.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]