Abstract
ABO blood groups have long been associated with cardiovascular disease, thrombosis and acute coronary syndromes. Many studies over the years have shown type O blood group to be associated with lower risk of cardiovascular disease compared to non-type O blood groups. However, the mechanisms underlying this association remain unclear. Although ABO blood group is associated with variations in concentrations of circulating von Willebrand Factor and other endothelial cell adhesion molecules, ABO antigens are also present on several platelet surface glycoproteins and glycosphingolipids. As we highlight in this platelet-centric review, these glycomic modifications may impact platelet function in arterial thrombosis. More broadly, improving our understanding of the role of platelet glycan modifications in acute coronary syndromes may inform future diagnostics and therapeutics for cardiovascular diseases.
Keywords: platelets, thrombosis, glycoprotein, ABO
Introduction
Cardiovascular disease (CVD), including coronary artery disease (CAD), cerebrovascular disease, and peripheral vascular disease, is a leading cause of morbidity and mortality throughout the world and the United States. According to the American Heart Association (AHA), CVD accounted for 31.9% of all mortality within the United States in 2010, with one out of every six deaths attributable to CAD (1). Despite some success in reducing rates of heart disease, these realities demand new innovations to CAD prevention as well as novel therapeutic strategies.
Vascular inflammation, driven by hyperlipidemia and diverse risk factors, promotes leukocyte recruitment and transmigration to the sub-endothelium, mediated in large part by adhesion molecules, chemokines and cytokines expressed on endothelial cells and leukocytes (2). Once atherosclerotic lesions have developed, physical and inflammatory disruption of these plaques incites a platelet-dependent thrombosis and acute coronary syndrome (ACS).
An active area of research is the role of the ABO blood group locus and glycosyltransferases in the pathophysiology of CAD and ACS. A multitude of studies have shown an association between non-O blood groups and both prevalence and incidence of CAD (3,4), as well as increased mortality in patients with ischemic heart disease (5), and size of myocardial infarction (MI) during ACS (6). The mechanism behind the association of ABO blood type and cardiovascular disease has been studied but not yet clearly elucidated. One potential mechanism is that ABO glycosyltransferases modify platelet surface glycoproteins and glycolipids, resulting in terminal modifications that impact the function of platelets. Understanding the ways in which ABO glycans modulate platelet glycoprotein and glycolipid function with respect to platelet activation, aggregation and platelet-driven thrombosis may improve our knowledge of the mechanisms underlying arterial thrombosis and ACS. Thus, this review will examine the existing knowledge of ABO blood group effects on CAD and ACS with a specific focus on the potential impact of ABO on platelet function and arterial thrombosis while also considering more broadly the impact of diverse glycomic modifications on platelet function and biology.
ABO blood group, thrombosis and acute coronary syndromes
ABO blood group phenotype is determined by the expression of specific antigens on red blood cells, endothelial cells, platelets and many other cells and tissues. The presence of A or B antigen results in the expression of A, B or AB phenotype, whereas the lack of both A and B antigen results in the expression of the O phenotype. These ABH antigens comprise of complex terminal carbohydrate molecules on glycoproteins and glycolipids, generated by the addition of N-acetylgalactosamine (A antigen) or D-galactose (B antigen) to existing N-glycan and O-glycan structures through the action of the ABO glycosyltransferases (7). The A and B alleles of the ABO locus encode A and B glycosyltransferases, which in turn catalyzes the transfer of the different carbohydrates onto a common core H antigen, to form an A or B antigen. However, type O individuals only express H antigen, as the O isoform of ABO lacks glycosyltransferase activity (8).
In the past, studies have associated ABO blood groups with CVD, whereby non-type O blood groups are associated with higher risk of CVD, MI and thrombosis than type O blood group (3). Previous genome-wide association studies (GWAS) have also identified associations between ABO blood type and cardiovascular risk factors, such as serum cholesterol and LDL (9), implying a role of ABO glycans in atherogenesis. However, GWAS also have highlighted that the top common genetic polymorphisms associated with MI, in the setting of known angiographic CAD, map to the ABO blood group locus (10). This finding is distinct from other GWAS for more generally defined CAD phenotypes (10,11), which reflect the association with coronary atherosclerosis but not necessarily ACS and MI per se. The same ACS/MI ABO locus variants also associate with venous thrombosis. Thus, these human genetic findings implicate ABO genotype as a potential effector not simply for atherogenesis, but in the pathophysiology of thrombosis as well as ACS and MI in the presence of existing coronary atherosclerosis.
One proposed mechanism to explain the increased risk of coronary thrombosis in non-blood group O individuals, is that ABO A and B glycotransferases modulate levels of circulating von Willebrand factor (VWF), where non-O blood group individuals have higher plasma levels, which is in turn is associated with greater risk of venous and arterial thrombosis (12, 13). VWF modulates thrombosis by promoting subendothelial collagen interactions with platelet glycoprotein (GP) Ia/IIa and is also directly complexed with Factor VIII in circulation. Circulating VWF is known to express ABH antigenicity, where the amount of H antigen expressed influences serum concentrations of VWF (14). Non-blood group O individuals have approximately 25% higher plasma levels of VWF compared to O individuals (15). Much of this variation has been attributed to differences in clearance of VWF, as opposed to synthesis, across different blood groups. Gallinaro et al showed that O blood group individuals had significantly faster clearance of VWF compared to non O individuals, where the elimination half-life of VWF in O versus non O groups was 10.0 ± 0.8 hours vs. 25.5 ± 5.3 hours, respectively; P <0.01 (16). Work by Bowen and colleagues suggest that differences in VWF clearance across blood groups might be secondary to differences in rates of proteolysis of VWF by ADAMTS13, where the O group had significantly higher rates of proteolysis compared to non O groups (17). This difference in susceptibility to proteolysis appears to be related to the presence of A or B antigens and the addition of terminal sugars on VWF glycoproteins, as well as the presence or absence of the H antigen (18). Furthermore, ABO blood groups have also been associated with serum levels of the pro-clotting factor, Factor VIII, with non-O individuals having approximately 25% higher levels than blood group O carriers (15). The Cohorts for Heart and Aging Research in Genome Epidemiology Consortium GWAS did not find any ABO polymorphisms associated with Factor VIII levels independent of blood levels of VWF (19). These results imply that ABO’s association with serum Factor VIII levels are likely attributable to VWF complexing with Factor VIII in circulation.
Although ABO blood type is associated with circulating levels of VWF (single nucleotide polymorphisms that tag the ABO blood groups account for 15.4% of the log variance), there are several other mechanisms that regulate VWF concentration. Studies of healthy European cohorts have shown that VWF level varies depending on age, body mass and common polymorphisms within the VWF gene (20). In addition, murine models have been developed with expression of both increased and decreased levels of VWF (21, 22), despite the fact that mice do not express ABH antigenicity. At least part of this variance in VWF level is thought to be due to differences in the level of VWF siaylation. This hypothesis was investigated by McGrath et al, where desialyated VWF was more prone to cleavage by serine and cysteine proteases, but less susceptible to cleavage by ADAMTS13 (23), supporting the idea that similarly to ABO glycosylation, sialyation of VWF may alter its rate of clearance. In fact, the effect of siaylation may even be understated, as erythrocytes and platelets lose their sialic acid during blood banking via irreversible membrane alterations (24). Although it is unknown if VWF loses sialic acid during sample banking, any tendency for ex vivo desiaylation could make it more difficult to identify the specific targets of siaylation that ultimately impact VWF level.
Beyond associations with levels of circulating VWF, blood group phenotype is also thought to affect endothelial-leukocyte interactions and leukocyte recruitment and transmigration to inflamed endothelium by influencing serum levels of endothelial-derived adhesion molecules, including soluble P-selectin, E-selectin and intercellular adhesion molecule-1 (ICAM-1). A large scale GWAS by Barbalic et al suggested that both soluble P-selectin and soluble ICAM-1 are associated with ABO polymorphisms, with lower levels of sP-selectin and sICAM-1 associated with A1 and A2 blood group alleles (25). Similarly, a GWAS by Patterson et al demonstrated that polymorphisms around the ABO locus are associated with soluble E-selectin levels, accounting for up to 19% of variability, with highest concentrations associated with type O blood group (26). Notably, the direction of ABO blood group association with blood levels of these endothelial-derived adhesion molecules is opposite to that for VWF. Yet, it remains unclear how blood adhesion molecule associations occur – i.e., via glycan modifications that alter secretion, cleavage, turnover or clearance. Despite the association between blood group O and lower rates of cardiovascular events, blood group O carriers have higher levels of soluble P-selectin and sICAM-1. Yet higher concentrations of these adhesion molecules are actually associated with increased risk for cardiovascular events (25). One potential explanation for this confusing observation is that ABO phenotype affects the concentration of adhesions molecules differently during acute pathophysiologies such as ACS/MI compared to at rest. It is also possible that the function of adhesions molecules, in addition to the concentration, differ among ABO groups. Although these associations have been studied with soluble cell adhesion molecules, ABO glycans may also have an impact on levels or function of intracellular or membrane bound adhesion molecules on endothelial cells, which play a direct role in leukocyte-platelet-endothelial interactions.
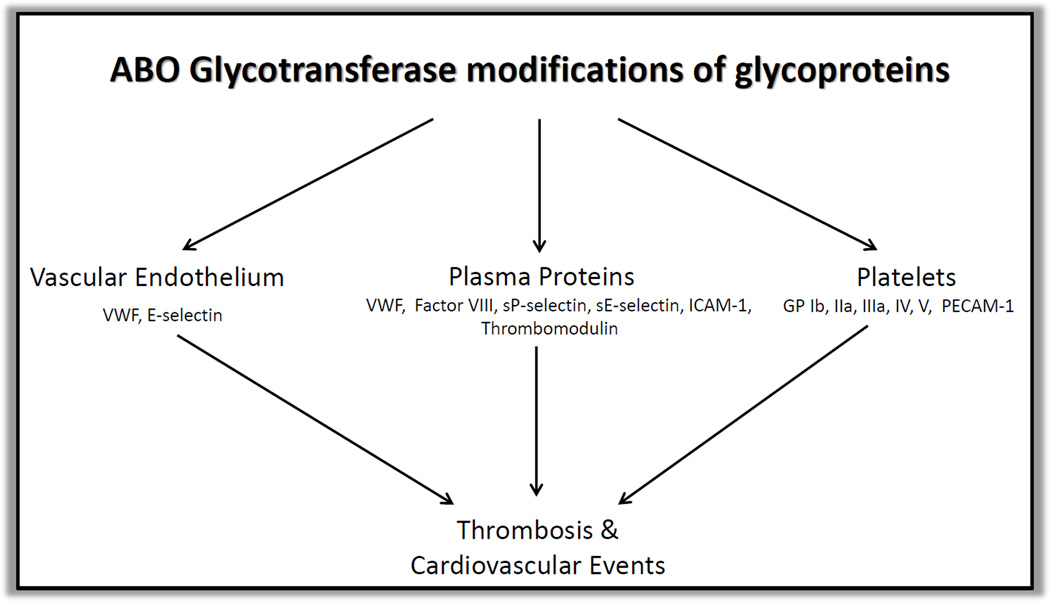
ABO blood group associations may promote ACS/MI in additional ways beyond the effect on serum concentrations of VWF and cell adhesion molecules via distinct modulation of plaque rupture, platelet-dependent thrombosis or both. Although platelet counts do not differ by blood group (3–6), ABH antigens are expressed on both platelet membrane lipids and platelet glycoproteins, and these may influence platelet interactions with fibrinogen and VWF, thereby modulating platelet aggregation and platelet-driven thrombosis.
ABO expression on platelet glycoproteins and glycolipids
Platelet glycoproteins
Blood type A and B carbohydrate antigens are expressed on platelet glycoproteins (GPs), including several GPs that play key roles in thrombotic pathways. Santoso discovered the presence of ABH antigenicity on platelet GPs IIa and IIIa as well as the GP IIb/IIIa complex by utilizing anti A and anti B IgG antibodies and radioimmuno-precipitation (27). The GP IIb/IIIa receptor complex mediates platelet aggregation in ACS and MI via binding of fibrinogen, fibronectin and VWF (28). The role of GP IIb/IIIa in arterial thrombosis has been highlighted by numerous studies showing the benefits of targeting the glycoprotein complex (29). Similarly, GP IIa is part of the GP Ia/IIa complex, which binds collagen and stabilizes platelets to exposed collagen at sites of injured endothelium. It is possible that ABO modification of this GP complex may also impact its function and expression, thereby impacting platelet function in arterial thrombosis. Therefore, knowledge of the extent and exact locations of ABO modification of GPs Ia/IIa and IIb/IIIa may provide additional insight into the mechanistic underpinnings of platelet aggregation and thrombosis in ACS and MI, and also impact future drug therapies. ABH antigens are expressed on several other platelet GPs, including GP Ib (30), GP IV and GP V (31). GPs Ib and V are part of the GP Ib-IX-V complex, which mediates platelet adhesion to injured endothelium via the binding of VWF and thereby initiates events leading to thrombosis (32). Platelet GP Ib-IX-V complex is proposed to play a significant role in arterial thrombosis during shear induced binding of VWF in the setting of abnormal blood flow and shear at sites of atherosclerosis. This binding subsequently induces platelet activation and aggregation via the binding of thrombin and VWF to initiate the formation of a thrombus (33). ABH antigenicity is also expressed on platelet endothelial cell adhesion molecule-1 (PECAM-1) (34). PECAM-1 is a glycoprotein expressed on the surface of platelets and endothelial cells and is thought to play a role in atherogenesis via leukocyte adhesion and transmigration (35). Yet, recent studies by Falati et al used PECAM-1 deficient mice to show that arteriolar thrombi, induced by laser injury, were larger and formed more rapidly (36). Thus, PECAM-1 appears to be a negative regulator of platelet driven thrombosis in vivo. ABO glycomic modifications of PECAM-1 may have a functional impact on platelet driven thrombosis. Understanding the impact of these platelet-related glycan and GP modifications may inform future diagnostics and therapeutics, specifically targeting ABO modified platelet GPs.
One novel mechanism by which glycan modifications of platelet GPs may alter platelet function is via galectin-glycan interactions. Galectins are a family of proteins which specifically bind beta galactosides, glycans which can be bound to proteins via N-linked or O-linked glycosylation (37). Galectins are expressed both extracellularly, in circulation and within extracellular matrices, and intracellularly within the nucleus, cytoplasm and outer plasma membrane. Furthermore, galectins are expressed within a variety of tissue, including endothelial, epithelial and adipose tissue. There have been 15 unique galectins discovered, although only 12 are present in humans, where different galectins can recognize different glycan structures and with different affinities. Galectins Gal-1 and Gal-8 can act as potent platelet agonists, inducing both platelet aggregation via a conformational change of GP IIb/IIIa (38, 39) and platelet adhesion to an extracellular matrix. These galectins are able to mediate their effects on platelets by interacting with specific platelet surface glycans expressed on glycoproteins. Gal-8 binds platelet surface GP Ib and GP IIB, while the exact targets for Gal-1 are still unknown (39). It is possible that ABO glycan modifications could affect how platelet glycoproteins interact with their respective galectins, thereby impacting platelet activation, adhesion and aggregation.
Platelet glycolipids
Platelet glycosphingolipids (GSLs) also play a prominent role in platelet activation, aggregation and thrombosis (40–42) by modulating transmembrane signal transduction. These GSLs affect platelet function by interacting with various cell adhesion molecules such as P-selectin, fibrinogen and VWF. In a study by Merten, sulfatide, a sulfated GSL, was found to affect platelet adhesion by acting as a ligand to P-selectin, where exposure to a sulfatide antagonist decreased the extent of platelet adhesion (41). Guchhait et al further illustrated the role of sulfatide, by exposing platelets to a sulfatide antibody probe (43). By blocking sulfatide, they inhibited platelet adhesion to P-selectin, fibrinogen and VWF. In addition, there was decreased platelet aggregation to agonists collagen, ADP and thrombin, suggesting that sulfatide may also play a role in the formation of stable platelet aggregates. Indeed, in sulfatide deficient mice, there was a longer time to in vitro platelet aggregation with collagen compared to that in wild type mice.
Beyond sulfated GSLs, platelet surfaces express many neutral GSLs and sialylated GSLs, known as gangliosides, with the most abundant being hematoside (GM3), globoside and trihexosylceramide (44). These platelet surface GSLs and gangliosides undergo both quantitative and qualitative changes during platelet activation, thereby impacting platelet adhesion and aggregation. Wang et al demonstrated that exposure of platelets to thrombin reduces by approximately 50% the surface labeling of trihexosylceramide and globoside, while increasing hematoside surface labeling (44). Resting platelets predominantly express the ganglioside GM3 yet platelet activation by ADP, collagen or arachidonic acid increased surface expression of GD3, suggesting that GD3 serves as a messenger in platelet adhesion and activation (40). Activation of platelets by thrombin receptor agonist peptide (TRAP) increases expression of sulfated GSLs on the platelet surface. Further, the addition of micelles containing sulfated GSLs inhibited platelet adhesion to P-selectin (41). In contrast, antagonists of sulfated GSLs reduced ADP and TRAP induced platelet aggregation, while addition of sulfated GSLs increased the extent of platelet aggregation in ex vivo activated platelets (41, 42). Similarly, Santoro demonstrated the inhibitory effect of exogenously added gangliosides on platelet adhesion to fibronectin, fibrinogen and VWF and discovered that the inhibitory effect varied with the number of sialic acid residues expressed by the added gangliosides (45). These results suggest that platelet function may be modulated by gangliosides and their posttranslational modifications. Although further mechanistic and clinical studies are required, overall these data support the concept of a functional role for the dynamic and regulated expression of specific GSLs during platelet activation and in arterial thrombosis.
Like platelet glycoproteins, ABH antigenicity is also expressed on platelet surface GSLs. Cooling et al demonstrated that neutral GSLs, hexosylceramide (A6) and octaosylceramide (A8) express A antigenicity in type A platelets, while no ABH antigenicity was identified on type O platelets (46). Similarly, 5 acidic GSLs were identified that also express A antigenicity on type A platelets. Although the exact structures of the 5 acidic GSLs could not be identified, it is thought that they are likely monosialo, long-chain gangliosides, unlike the complex, branched GSLs that express ABH antigenicity on RBCs. Similarly, GSLs expressing B antigenicity have been identified on type B platelets (47). The role and impact of ABO on GSL functions in platelets, however, remain uncertain particularly as published work suggests that only a small proportion (estimated at less than 1%) of all known platelet GSLs and gangliosides may carry ABH antigens (46). Although limited in quantity, these posttranslational modifications of GSLs may have important effects on platelet function.
Variability in ABO expression on platelets
The degree of ABH antigenic expression on platelet glycoproteins and glycolipids not only varies across ABO blood groups but also within individuals of the same blood group. Hou et al demonstrated that blood group A and B antigens are heavily associated with GP Ib and GP IIb, with GP IIb being the most prominent (48). However, there were differences in antigenic expression, even within a specific blood group phenotype. For example, blood group A is characterized by two specific subgroups, A1 and A2, where the A2 genotype represents a mutation involving single base deletion, which ultimately results in an A2 glycosyltransferase with 30–50 fold less activity than A1 (49). In examining these subgroups, platelets with the A2 polymorphism do not express A antigens on glycoproteins Ib and IIb and expressed only one tenth the amount of antigen on glycolipids as individual with A1 platelets (48).
Several studies have found that a subset of A and B individuals express significantly more A and B antigens on their platelet surfaces than the general population and are termed “high expressers”. Roughly 7% of type A individuals and 4–7% of type B individuals are high expressers for A and B antigen expression, with higher levels of A and B antigens expressed on GP Ib/IX, Ia/IIa, IV, PECAM, IIb/IIIa. While examining type A individuals, Curtis et al used flow cytometry to discover that individuals with an A2 polymorphism have undetectable or barely detectable levels of A antigen, while individuals with an A1 polymorphism express low levels of A antigen (50). Yet, roughly 7% of A1 individuals and 4% of B individuals expressed A and B antigens levels greater than 2 standard deviations above the mean, with higher levels of antigens on various platelet glycoproteins, but especially GP IIb and PECAM. The mechanism behind this differential expression may lie in differences in enzymatic activity as well as in H antigen abundance and modification; high expressers concomitantly show higher levels of A1 and B glycosyltransferase activity and lower levels of H antigen on their red blood cells.
Subsequent studies further examined the determinants of this differential expression of ABH antigenicity within blood groups. Cooling et al studied 131 type A platelet donors (47), where similar to previous studies, A antigen was expressed on platelet membranes, glycoproteins and glycosphingolipids of A1 platelets. ABH antigen expression varied significantly between donors, but did not vary significantly over time within donors. Expression was influenced by specific A subtype, age and sex, with the A1 vs. A2 subtypes being the greatest predictor of platelet ABH antigen expression. Concordance was shown between A1 phenotype and A antigen expression on platelet membranes, GPs and glycosphingolipids. Among the A1 phenotype, there was a linear co-expression of A and H antigens, suggesting that platelet ABH antigen expression is also dependent on the abundance of H antigens, and not solely A and B glycosyltransferase activity. Similarly, blood group B platelets also displayed differences in the expression of B surface antigen. Ogasawara et al demonstrated that 7% of type B individuals were high expressers for B antigen, with increased levels of B glycosyltransferase activity (51). In these high expressers, GP IIb and IIa were shown to express the largest amount of B antigen on the platelet surface. Clinically, high expressers were found to have rapid clearance of ABO mismatched platelet transfusions (52).
While interesting, the molecular basis and clinical significance of high ABH expression, beyond implications in transfusion medicine, is still being investigated. There is however already evidence that the level of platelet ABH expression can vary depending on platelet activation and storage conditions, either through granule translocation or changes in glycosyltransferase activity (53). Therefore, one hypothesis might be that high expression of A or B antigenicity, relative to that of blood group O, influences the risk of coronary thrombosis via the degree of modification of important glycoproteins, such as GP Ib, GP IIa, GP IIb and PECAM. In addition, the degree and impact of posttranslational modification of these platelet glycoproteins may be different at rest and during platelet activation preceding a thrombotic event. Investigating the potential impact of high ABH expression on platelet function at rest and during activation as well as on other cells involved in arterial thrombosis, such as endothelial cells, may inform diagnostics, through biomarker development and risk prediction and by identifying individuals with atherosclerosis who may be at higher risk for thrombosis and therefore, ACS/MI.
Potential for ABO modulation of platelet function and arterial thrombosis
Platelets play a pivotal role in ACS with formation of a platelet aggregate at the site of a ruptured atherosclerotic plaque or locus of endothelial denudation (54). Platelets adhere to sites of injured vessels via the binding of subendothelial collagen to GP Ia/IIa and ultimately forming a platelet rich plug via the binding of VWF or fibrinogen to activated platelet GP IIb/IIIa, the transmembrane receptor complex that serves as the dominant final common pathway in platelet driven arterial thrombosis (55). The importance of platelets in ACS and MI has been established over decades through basic and clinical research, most notably by documentation of acute in vivo platelet activation during ACS and MI (56, 57) and clinical trials that demonstrate the efficacy of oral (58) and parenteral (29) antiplatelet agents in prevention of heart disease and in the treatment of ACS and MI. Thus, ABO modulation of platelet glycolipids, glycoproteins or circulating proteins that interact with platelets (e.g., VWF, which is itself ABO modified) may alter the rate and clinical impact of platelet-driven thrombosis.
Several studies have investigated differences in platelet function and hemostasis markers across blood groups. Moeller et al examined 162 healthy donors with normal in vivo bleeding time endpoints, Factor VIII activity, VWF structure and ristocetin induced platelet aggregation (59). In this study, type O individuals demonstrated a longer bleeding time compared to non-type O individuals (89 ± 14.6 sec vs. 82 ± 13 sec, p < 0.01), indicating an in vitro effect of ABO blood group on primary hemostasis. Favaloro et al examined 452 donors across blood groups and demonstrated an association between type O group and lower levels of plasma VWF, collagen binding activity and Factor VIII coagulant activity (60). Interestingly, type O individuals also had lower levels of ristocetin cofactor activities, suggesting a higher rate of platelet aggregation to ristocetin in non-type O individuals. However, there was no association between blood group status and the ratio of total VWF to functional VWF. The authors concluded that ABO blood group may influence platelet adhesion via the binding of VWF, conferring increased rates of thrombosis to blood in non-blood group O carriers.
Feuring et al demonstrated that although ABO blood group was associated with differences in measures of ex vivo platelet function, ABO blood group status did not influence the effect of a range of GPIIb/IIIa receptor antagonists in blocking the receptor and inhibiting platelet function (61, 62). These studies suggest that ABO affects certain surrogate in vitro measures of platelet function, possibly via glycan modifications of platelet glycoproteins or glycolipids. However, it remains uncertain what effect, if any, platelet ABO glycan modifications have on clinically relevant platelet function and arterial thrombosis in vivo.
Platelet glycobiology beyond ABO modifications
Despite limitations of present knowledge regarding the extent, specific targets, and function of ABO modification of platelet glycoproteins and glycolipids, ABO glycosylation may prove to be an important model for the role of glycocomic pathways in platelet function and ultimately arterial thrombosis. Studies by Wandall et al have shown that platelets contain functional intracellular and surface glycosyltransferases, with the ability to glycosylate exogenously added GP Ib, IIb and VWF (63). Furthermore, although platelets expressed functional glycosyltransferases at rest, they release approximately 50% of their glycosyltransferase activity, as well as sugar nucleotides substrates, upon activation by TRAP or ADP. These results suggest that platelets contain a complete glycosylation apparatus, which upon activation allows platelets to modify surface glycans. In fact, when human platelets were activated in the presence of mouse platelets, sialic acid was noted to be added to mouse platelet surface GP Ib. To test the effect of modification of surface glycoproteins on platelet function, the bleeding times of sialyated platelets were compared against non-sialyated platelets in vitro. Platelets that incorporated sialic acid into their surface glycoproteins were found to be less responsive, with significantly longer bleeding times compared to controls.
Post-translational modification of platelet surface GPs can play an important role in determining platelet specific alloantigenicity. Bak is a platelet specific alloantigen expressed on GP IIb. Take et al. tested the hypothesis that post-translational sialylation of GP IIb affected the expression of Bak epitopes. The authors demonstrated that the binding of four anti-Bak sera to Bak changed depending on whether neuraminidase was administered to desiaylate GP IIb (64). A subsequent study by Goldberger et al, demonstrated changes in electrophoretic mobility and reactivity to antisera when Baka and Bakb platelets were exposed to neuraminidase, thereby desiaylating GP IIb. The authors concluded that in addition to a change in amino acid 843 of GP IIb, from isoleucine to serine, post-translational siaylation of GP IIb was also necessary for the expression of Baka and Bakb epitopes (65).
From a clinical perspective, Davoren et al examined 1162 cases of neonatal alloimmune thrombocytopenia, a disease thought to be mediated by the placental passage of platelet specific alloantibodies, and discovered that alloantibodies to Bak epitopes contributed to 2% of all cases (66). Other polymorphisms in platelet specific antigens have also been associated with various disease states, including coronary thrombosis. A study by Weiss et al showed a higher prevalence of PIA2 (39.4 vs. 19.1%), a polymorphism on another platelet specific antigen, in patients with a history of MI or unstable angina, compared to controls (67). It is therefore possible that post-translational modification, including sialyation, of platelet glycoproteins can impact platelet function via the differential expression of platelet specific alloantigens.
While glycan modification of platelet receptors appears to play a role in platelet function, understanding the exact role of glycan modification of these glycoproteins, including ABO glycosylation, in resting and during activation of platelets may offer new insights into the pathophysiology of thrombosis and provide opportunities for diagnostic and prognostic translations and for novel therapeutics.
Systematic glycomic profiling of platelets
Over the years, many studies have shown that ABO groups are associated with ACS and thrombosis and that ABH antigens are expressed on platelet surface glycoproteins and glycolipids. However, the specific glycans, glycopeptides and glycolipids on platelets, modified by ABO glycans, and the manner in which these molecules influence platelet function is still largely unknown. Importantly, work by Cummings and colleagues have shown that specific glycan modifications do indeed play an important role in platelet function (68). Utilizing mice with targeted deletion of Cosmc, an essential chaperone in O-glycosylation regulation, the authors showed increased incidence of hemorrhage and longer bleeding times as well as decreased GP Ib-IX-V and GP IIb/IIIa function in mouse with deletion of Cosmc. These findings imply that disruption of O-glycosylation leads to impaired platelet adhesion and clotting via changes in platelet glycoprotein function.
Mass spectrometry and glycan binding lectins can provide additional insights into platelet specific glycomic modifications by providing information on changes in structure, content, and configuration of specific platelet glycoproteins and glycolipids (69). A recent study by Mercado et al. utilized mass spectrometry to probe the mechanism in which elevated plasma levels of serotonin accelerate platelet aggregation (70). This study revealed modification of the content of N-glycans on mouse platelet surfaces following infusion of serotonin, with a switch in the N-glycan structures on platelet glycoproteins from predominantly N-acetyl-neuraminic acid to N-glycolyl-neuraminic acid. The authors postulated that these glycoprotein modifications underlie the mechanism by which serotonin enhances platelet aggregation.
In order to further explore the role of ABO groups in modifying platelet function, the complete extent of N-, O-, and GSL species and targets in platelets at rest and during activation will need to be defined in an unbiased manner. Although mass spectrometry has been used to highlight specific proteins in the platelet sheddome (71) and within platelet microparticles (72), fractions that contain key local and systemic signals modulating thrombosis in clinical disease, a complete analysis of all glycoprotein and GSL species is lacking. In the context of ABO, a combination of unbiased proteomics, emerging glycoproteomcis and lectin arrays targeting ABO pathways may be used to uncover which specific peptides carry modifications. Such deep and complete profiling of glycoproteins and GSLs, will allow determination of the presence, abundance and targets of ABO and other glycan modifications in platelets, providing opportunities for clinical translation.
Furthermore, it may be useful to consider ABO as a model for the broader impact of post-translational glycan modification on platelet function. Therefore, deep glycopeptide and glycoproteomic interrogations are required to identify which proteins carry specific modifications and at which residues and domains. Such unbiased profiling will uncover many potentially biologically and clinically important glycoprotein modifications, not just those of ABH antigenicity. These modifications can be studied further, through mutagenesis in vitro and in vivo, for their impact on the function of these proteins, and in vivo for their related platelet activities in thrombosis and vascular disease. Such discovery and follow-up functional analyses will help define the effect of established ABO modifications on glycoproteins, such as GP Ia, GP Ib and GP IIb/IIIa as well as discover novel, and perhaps platelet specific, glycopeptides and -proteins, that modulate platelet function and arterial thrombosis. Although ABO expression on platelet surface glycoproteins and GSLs may be of low relative abundance, these may have important functional consequences, whether via terminal modifications of glycoproteins or glycan modifications of glycolipids that are key signaling molecules in platelet-driven thrombosis at sites of atherosclerotic plaque rupture. Future targeted work will elucidate whether glycomic modifications, including ABH glycans, act on platelet functions via specific proteins and lipids or whether there is a more generalized effect of global carbohydrate type and abundance in altering platelet functions.
Conclusions
A growing body of evidence has demonstrated a strong and reproducible association between ABO blood group status and ACS, whereby non-type O blood group, compared to blood group O carriers, is associated with increased risk of CVD. While the exact mechanism of this effect is largely unknown, several glycoproteins and glycosphingolipids on the surface of platelets are known to express ABH antigenicity. It is hypothesized that ABO modification of these glycans may affect platelet function and thrombosis. Further studies are needed to thoroughly investigate the presence, targets and extent of these ABO modifications and how they modulate platelet function in arterial thrombosis. A better understanding of all types of glycomic modifications and their potential impact on platelet function and thrombosis may impact future diagnostic and therapeutic targets in ACS and CVD.
Schematic of ABO modification of glycoproteins and its impact on arterial thrombosis
Significance.
Several studies have demonstrated a relationship between ABO blood group and acute coronary syndromes, where O blood group has been associated with lower rates of incidence, infarct size and mortality.
Research into this relationship has focused primarily on the impact of ABO blood group on circulating von Willebrand Factor and integrins. However, platelets play a pivotal role in arterial thrombosis. Therefore, our review focuses on ABO blood type from a platelet perspective.
We review the expression of ABH antigens on platelet glycoproteins and glycosphingolipids and highlight studies that examine the effect of ABO blood group on several platelet function assays. We conclude by providing perspective on future studies that can further elucidate the impact of ABO glycan modification on platelet aggregation and function.
Understanding the effect of ABO blood group on platelets highlights the importance of platelet glycoprotein modifications in thrombosis, which may help inform future diagnostic and therapeutic targets.
Acknowledgments
Sources of Funding
Funding sources: This work is supported by U01-HL108636 (to MPR and PA). MPR is also supported by HL111694, DK090505, HL107643 and HL113147. JDC is also supported by HL087115, HL096845, HL114626, and HL115354.
Acronyms and Abbreviations
- CVD
Cardiovascular disease
- CAD
Coronary artery disease
- ACS
Acute coronary syndrome
- MI
Myocardial infarction
- GWAS
Genome wide association study
- VWF
von Willebrand Factor
- GP
Glycoprotein
- ICAM
Intercellular adhesion molecule
- PECAM
Platelet endothelial cell adhesion molecule
- GSL
Glycosphingolipid, Thrombin receptor agonist peptide
Footnotes
Author contributions: Ming Zhong MD drafted the manuscript, and all authors critically revised it for intellectual content and approved the final version.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 3.He M, Wolpin B, Rexrode K, Manson JE, Rimm E, Hu FB, Qi L. ABO blood group and risk of coronary heart disease in two prospective cohort studies. Arterioscler Thromb Vasc Biol. 2012;32:2314–2320. doi: 10.1161/ATVBAHA.112.248757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whincup PH, Cook DG, Phillips AN, Shaper AG. ABO blood group and ischaemic heart disease in British men. BMJ. 1990;300:1679–1682. doi: 10.1136/bmj.300.6741.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpeggiani C, Coceani M, Landi P, Michelassi C, L'abbate A. ABO blood group alleles: A risk factor for coronary artery disease. An angiographic study. Atherosclerosis. 2010;211:461–466. doi: 10.1016/j.atherosclerosis.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Ketch TR, Turner SJ, Sacrinty MT, Lingle KC, Applegate RJ, Kutcher MA, Sane DC. ABO blood types: influence on infarct size, procedural characteristics and prognosis. Thromb Res. 2008;123:200–205. doi: 10.1016/j.thromres.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Storry JR, Olsson ML. The ABO blood group system revisited: a review and update. Immunohematology. 2009;25:48–59. [PubMed] [Google Scholar]
- 8.Jenkins PV, O'Donnell JS. ABO blood group determines plasma von Willebrand factor levels: a biologic function after all? Transfusion. 2006;46:1836–1844. doi: 10.1111/j.1537-2995.2006.00975.x. [DOI] [PubMed] [Google Scholar]
- 9.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reilly MP, Li M, He J, et al. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet. 2011;377:383–392. doi: 10.1016/S0140-6736(10)61996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schunkert H, Konig IR, Kathiresan S, et al. Large-scale assocation analysis identifies 13 new susceptbility loci for coronary artery disease. Nature Genetics. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders YV, Eikenboom J, de Wee EM, van der Bom JG, Cnossen MH, Degenaar-Dujardin MEL, Fijnvandraat K, Kamphuisen PW, Laros-van Gorkom BAP, Meijer K, Mauser-Bunschoten EP, Leebeek FWG. Reduced prevalence of arterial thrombosis in von Willebrand disease. Journal Thrombosis and Haemostasis. 2013;11:845–854. doi: 10.1111/jth.12194. [DOI] [PubMed] [Google Scholar]
- 13.Kraaijenhagen RA, In 'T Anker PS, Koopman MMW, Reitsma PH, Prins MH, van den Ende A, Buller HR. High plasma concentration of factor VIIIc is a major risk factor for venous thromboembolism. Thrombosis and Haemostasis. 2000;83:5–9. [PubMed] [Google Scholar]
- 14.O'Donnell J, Boulton FE, Manning RA, Laffan MA. Amount of H antigen expressed on circulating von Willebrand factor is modified by ABO blood group genotype and is a major determinant of plasma von Willebrand factor antigen levels. Arterioscler Thromb Vasc Biol. 2002;22:335–341. doi: 10.1161/hq0202.103997. [DOI] [PubMed] [Google Scholar]
- 15.O’Donnell J, Laffan MA. The relationship between ABO histo-blood group, factor VIII and von Willebrand factor. Transfusion Medicine. 2001;11:343–351. doi: 10.1046/j.1365-3148.2001.00315.x. [DOI] [PubMed] [Google Scholar]
- 16.Gallinaro L, Cattini MG, Sztukowska M, Padrini R, Sartorello F, Pontara E, Bertomoro A, Daidone V, Pagnan A, Casonato A. A shorter von Willebrand factor survival in O blood group subjects explains how ABO determinants influence plasma von Willebrand factor. Blood. 2008;111:3540–3545. doi: 10.1182/blood-2007-11-122945. [DOI] [PubMed] [Google Scholar]
- 17.Bowen DJ. An influence of ABO blood group on the rate of proteolysis of von Willebrand factor by ADAMTS13. Journal of Thrombosis and Haemostasis. 2003;1:33–40. doi: 10.1046/j.1538-7836.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell JS, McKinnon TA, Crawley JT, Lane DA, Laffan MA. Bombay phenotype is associated with reduced plasma-VWF levels and an increased susceptibility to ADAMTS13 proteolysis. Blood. 2005;106:1988–1991. doi: 10.1182/blood-2005-02-0792. [DOI] [PubMed] [Google Scholar]
- 19.Smith NL, Chen MH, Dehghan A, et al. Novel associations of multiple genetic loci with plasma levels of factor VII, factor VIII, and von Willebrand factor: the CHARGE (Cohorts for Heart and Aging Research in Genome Epidemiology) Consortium. Circulation. 2010;121:1382–1392. doi: 10.1161/CIRCULATIONAHA.109.869156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campos M, Sun W, Yu F, Barbalic M, Tang W, Chambless LE, Wu KK, Ballantyne C, Folsom AR, Boerwinkle E, Dong JF. Genetic determinants of plasma von Willebrand factor antigen levels: a target gene SNP and haplotype analysis of ARIC cohort. Blood. 2011;117:5224–5230. doi: 10.1182/blood-2010-08-300152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellies LG, Ditto D, Levy GG, Wahrenbrock M, Ginsburg D, Varki A, Le DT, Marth JD. Sialyltransferase ST3Gal-IV operates as a dominant modifier of hemostasis by concealing asialoglycoprotein receptor ligands. Proc Natl Acad Sci USA. 2002;99:10042–10047. doi: 10.1073/pnas.142005099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grewal PK, Uchiyama S, Ditto D, Varki N, Le DT, Nizet V, Marth JD. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat Med. 2008;14:648–655. doi: 10.1038/nm1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGrath RT, McKinnon TA, Byrne B, O’Kennedy R, Terraube V, McRae E, Preston RJ, Laffan MA, O’Donnell JS. Expression of terminal alpha 2–6-linked sialic acid on von Willebrand factor specifically enhances proteolysis by ADAMTS13. Blood. 2010;115:2666–2673. doi: 10.1182/blood-2009-09-241547. [DOI] [PubMed] [Google Scholar]
- 24.Stibenz D, Feurestein H, Halbhuber KJ, Linss W. Metabolic depletion of erythrocytes is accompanied by a decrease of sialic acid during blood bank storage: a reply. Vox Sang. 1982;42:93–96. doi: 10.1159/000460855. [DOI] [PubMed] [Google Scholar]
- 25.Barbalic M, Dupuis J, Dehghan A, et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Human Molecular Genetics. 2010;19:1863–1872. doi: 10.1093/hmg/ddq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paterson AD, Lopes-Virella MF, Waggott D, Boright AP, Hosseini SM, Carter RE, Shen E, Mirea L, Bharaj B, Sun L, Bull SB. Genome-wide association identifies the ABO blood group as a major locus associated with serum levels of soluble E-selectin. Arterioscler Thromb Vasc Biol. 2009;29:1958–1967. doi: 10.1161/ATVBAHA.109.192971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santoso S, Kiefel V, Mueller-Eckhardt C. Blood group A and B determinants are expressed on platelet glycoproteins IIa, IIIa, and Ib. Thromb Haemost. 1991;65:196–201. [PubMed] [Google Scholar]
- 28.Lefkovits J, Plow EF, Topol EJ. Platelet glycoprotein IIb/IIIa receptors in cardiovascular medicine. N Engl J Med. 1995;332:1553–1559. doi: 10.1056/NEJM199506083322306. [DOI] [PubMed] [Google Scholar]
- 29.Boersma E, Harrington RA, Moliterno DJ, White H, Théroux P, Van de Werf F, de Torbal A, Armstrong PW, Wallentin LC, Wilcox RG, Simes J, Califf RM, Topol EJ, Simoons ML. Platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: a meta-analysis of all major randomised clinical trials. Lancet. 2002;359:189–198. doi: 10.1016/S0140-6736(02)07442-1. [DOI] [PubMed] [Google Scholar]
- 30.Bussel JB, Kunicki TJ, Michelson AD. Platelets: New Understanding of Platelet Glycoproteins and Their Role in Disease. Hematology Am Soc Hematol Educ Program. 2000:222–240. doi: 10.1182/asheducation-2000.1.222. [DOI] [PubMed] [Google Scholar]
- 31.Stockelberg D, Hou M, Rydberg L, Kutti J, Wadenvik H. Evidence for an expression of blood group A antigen on platelet glycoproteins IV and V. Transfus Med. 1996;6:243–248. doi: 10.1111/j.1365-3148.1996.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 32.Berndt MC, Shen Y, Dopheide SM, Gardiner EE, Andrews RK. The vascular biology of the glycoprotein Ib-IX-V complex. Thrombosis and Haemostasis. 2001;86:178–188. [PubMed] [Google Scholar]
- 33.Shen Y, Romo GM, Dong J, Shade A, McIntire LV, Kenny D, Whisstock JC, Berndt MC, Lopez JA, Andrews RK. Requirement of leucine-rich repeats of glycoprotein (GP) Ibα for shear-dependent and static binding of von Willebrand factor to the platelet membrane GP Ib-IX-V complex. Blood. 2000;95:903–910. [PubMed] [Google Scholar]
- 34.Curtis BR, Edwards JT, Hessner MJ, Klein JP, Aster RH. Blood group A and B antigens are strongly expressed on platelets of some individuals. Blood. 2000;96:1574–1581. [PubMed] [Google Scholar]
- 35.Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arteriosclerosis Thrombosis and Vascular Biology. 2007;27:2514–2523. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- 36.Falati S, Patil S, Gross PL, Stapleton M, Merrill-Skoloff G, Barrett NE, Pixton KL, Weiler H, Cooley B, Newman DK, Newman PJ, Furie BC, Furie B, Gibbins JM. Platelet PECAM-1 inhibits thrombus formation in vivo. Blood. 2006;107:535–541. doi: 10.1182/blood-2005-04-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romaniuk MA, Negrotto S, Campetella O, Rabinovich GA, Schattner M. Identification of galectins as novel regulators of platelet signaling and function. International Union of Biochemistry and Molecular Biology Life. 2011;63:521–527. doi: 10.1002/iub.483. [DOI] [PubMed] [Google Scholar]
- 38.Pacienza N, Pozner RG, Bianco GA, D’Atri LP, Croci DO, Negrotto S, Malaver E, Gomez RM, Rabinovich GA, Schattner M. The immunoregulatory glycan-binding protein galectin-1 triggers human platelet activation. FAESEB Journal. 2008;22:1113–1123. doi: 10.1096/fj.07-9524com. [DOI] [PubMed] [Google Scholar]
- 39.Romaniuk MA, Tribulatti MV, Cattaneo V, Lapponi MJ, Molinas FC, Campetella O, Schattner M. Human platelets express and are activated by galectin-8. Biochem J. 2010;432:535–547. doi: 10.1042/BJ20100538. [DOI] [PubMed] [Google Scholar]
- 40.Martini F, Riondino S, Pignatelli P, Gazzaniga PP, Ferroni P, Lenti L. Involvement of GD3 in platelet activation. A novel association with Fcgamma receptor. Biochim Biophys Acta. 2002;1583:297–304. doi: 10.1016/s1388-1981(02)00250-0. [DOI] [PubMed] [Google Scholar]
- 41.Merten M, Thiagarajan P. Role for sulfatides in platelet aggregation. Circulation. 2001;104:2955–2960. doi: 10.1161/hc4901.100383. [DOI] [PubMed] [Google Scholar]
- 42.Merten M, Beythien C, Gutensohn K, Kühnl P, Meinertz T, Thiagarajan P. Sulfatides activate platelets through P-selectin and enhance platelet and platelet-leukocyte aggregation. Arterioscler Thromb Vasc Biol. 2005;25:258–263. doi: 10.1161/01.ATV.0000149675.83552.83. [DOI] [PubMed] [Google Scholar]
- 43.Guchhait P, Shrimpton CN, Honke K, Rumbaut RE, Lopez JA, Thiagarajan P. Effect of an anti-sulfatide single-chain antibody probe on platelet function. Thromb Haemost. 2008;99:552–557. doi: 10.1160/TH07-05-0351. [DOI] [PubMed] [Google Scholar]
- 44.Wang CT, Schick PK. The effect of thrombin on the organization of human platelet membrane glycosphingolipids. The sphingosine composition of platelet glycolipids and ceramides. J Biol Chem. 1981;256:752–756. [PubMed] [Google Scholar]
- 45.Santoro SA. Inhibition of platelet adhesion to fibronectin, fibrinogen, and von Willebrand factor substrates by complex gangliosides. Blood. 1989;73:484–489. [PubMed] [Google Scholar]
- 46.Cooling LL, Zhang D, Koerner TA. Human platelets express gangliosides with LKE activity and ABH blood group activity. Transfusion. 2001;41:504–516. doi: 10.1046/j.1537-2995.2001.41040504.x. [DOI] [PubMed] [Google Scholar]
- 47.Cooling LL, Kelly K, Barton J, Hwang D, Koerner TA, Olson JD. Determinants of ABH expression on human blood platelets. Blood. 2005;105:3356–3364. doi: 10.1182/blood-2004-08-3080. [DOI] [PubMed] [Google Scholar]
- 48.Hou M, Stockelberg D, Rydberg L, Kutti J, Wadenvik H. Blood group A antigen expression in platelets is prominently associated with glycoprotein Ib and IIb. Evidence for an A1/A2 difference. Transfus Med. 1996;6:51–59. doi: 10.1046/j.1365-3148.1996.d01-52.x. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto F, McNeil PD, Hakomori S. Human histo-blood group A2 transferase coded by A2 allele, one of the A subtypes is characterized by a single base deletion in the coding sequence, which results in an additional domain at the carboxyl terminal. Biochemical and Biophysical Research Communications. 1992;187:366–374. doi: 10.1016/s0006-291x(05)81502-5. [DOI] [PubMed] [Google Scholar]
- 50.Curtis BR, Edwards JT, Hessner MJ, Klein JP, Aster RH. Blood group A and B antigens are strongly expressed on platelets of some individuals. Blood. 2000;96:1574–1581. [PubMed] [Google Scholar]
- 51.Ogasawara K, Ueki J, Takenaka M, Furihata K. Study on the expression of ABH antigens on platelets. Blood. 1993;82:993–999. [PubMed] [Google Scholar]
- 52.Heal JM, Rowe JM, McMican A, Masel D, Finke C, Blumberg N. The role of ABO matching in platelet transfusion. European Journal of Haematology. 1993;50:110–117. doi: 10.1111/j.1600-0609.1993.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 53.Julmy F, Achermann F, Schulzki T, Carrel T, Nydegger U. PLTs of blood group A donors express increased surface A antigen owing to apheresis and prolonged storage. Transfusion. 2003;43:1378–1385. doi: 10.1046/j.1537-2995.2003.00526.x. [DOI] [PubMed] [Google Scholar]
- 54.Weiss EJ, Bray PF, Tayback M, Schulman SP, Kickler TS, Becker LC, Weiss JL, Gerstenblith G, Goldschmidt-Clermont PJ. A polymorphism of a platelet glycoprotein receptor as an inherited risk factor for coronary thrombosis. N Engl J Med. 1996;334:1090–1094. doi: 10.1056/NEJM199604253341703. [DOI] [PubMed] [Google Scholar]
- 55.Meisel C, López JA, Stangl K. Role of platelet glycoprotein polymorphisms in cardiovascular diseases. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:38–54. doi: 10.1007/s00210-003-0828-y. [DOI] [PubMed] [Google Scholar]
- 56.Fitzgerald DJ, Roy L, Catella F, FitzGerald GA. Platelet activation in unstable coronary disease. N Engl J Med. 1986;315:983–989. doi: 10.1056/NEJM198610163151602. [DOI] [PubMed] [Google Scholar]
- 57.Fitzgerald DJ, Catella F, Roy L, FitzGerald GA. Marked platelet activation in vivo after intravenous streptokinase in patients with acute myocardial infarction. Circulation. 1988;77:142–150. doi: 10.1161/01.cir.77.1.142. [DOI] [PubMed] [Google Scholar]
- 58.Collaboration AT. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moeller A, Weippert-Kretschmer M, Prinz H, Kretschmer V. Influence of ABO blood groups on primary hemostasis. Transfusion. 2001;41:56–60. doi: 10.1046/j.1537-2995.2001.41010056.x. [DOI] [PubMed] [Google Scholar]
- 60.Favaloro EJ, Soltani S, McDonald J, Grezchnik E, Easton L, Favaloro JW. Reassessment of ABO blood group, sex, and age on laboratory parameters used to diagnose von Willebrand disorder: potential influence on the diagnosis vs the potential association with risk of thrombosis. Am J Clin Pathol. 2005;124:910–917. [PubMed] [Google Scholar]
- 61.Feuring M, Harenberg J, Peiter A, Ganschow A, Ruf A, Losel R, Wehling M, Schultz A. Impact of ABO blood groups on tirofiban mediated inhibition of platelet function. Platelets. 2005;16:430–434. doi: 10.1080/09537100500181889. [DOI] [PubMed] [Google Scholar]
- 62.Feuring M, Ruf A, Schultz A, Wehling M. The PFA-100R cannot detect blood group-dependent inhibition of platelet function by eptifibatide or abciximab at therapeutic plasma concentrations. Platelets. 2010;21:176–182. doi: 10.3109/09537100903518260. [DOI] [PubMed] [Google Scholar]
- 63.Wandall HH, Rumjantseva V, Sorensen AL, Patel-Hett S, Josefsson EC, Bennett EP, Italiano JE, Clausen H, Hartwig JH, Hoffmeister KM. The origin and function of platelet glycosyltransferases. Blood. 2012;120:626–635. doi: 10.1182/blood-2012-02-409235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Take H, Tomiyama Y, Shibata Y, Furubayashi T, Honda S, Miztani H, Nishiura T, Tsubakio T, Kurata Y, Yonezawa T, Tarui S. Demonstration of the heterogeneity of epitopes of the platelet-specific alloantigen, Baka. Br J Haematol. 1990;76:395–400. doi: 10.1111/j.1365-2141.1990.tb06374.x. [DOI] [PubMed] [Google Scholar]
- 65.Goldberger A, Kolodziej M, Poncz M, Bennett JS, Newman PJ. Blood. 1991;78:681–687. [PubMed] [Google Scholar]
- 66.Davoren A, Curtis BR, Aster RH, McFarland JG. Human platelet antigen-specific alloantibodies implicated in 1162 cases of neonatal alloimmune thrombocytopenia. Transfusion. 2004;44:1220–1225. doi: 10.1111/j.1537-2995.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- 67.Weiss EJ, Bray PF, Tayback M, Schulman SP, Kickler TS, Becker LC, Weiss JL, Gerstenblith G, Goldschmidt-Clermont PJ. A polymorphism of a platelet glycoprotein receptor as an inherited risk factor for coronary thrombosis. N Engl J Med. 1996;334:1090–1094. doi: 10.1056/NEJM199604253341703. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, Jobe SM, Ding X, Choo H, Archer DR, Mi R, Ju T, Cummings RD. Platelet biogenesis and functions require correct protein O-glycosylation. Proc Natl Acad Sci U S A. 2012;109:16143–16148. doi: 10.1073/pnas.1208253109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith DF, Cummings RD. Application of microarrays for deciphering the structure and function of the human glycome. Mol Cell Proteomics. 2013;12:902–912. doi: 10.1074/mcp.R112.027110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mercado CP, Quintero MV, Li Y, Singh P, Byrd AK, Talabnin K, et al. A serotonin-induced N-glycan switch regulates platelet aggregation. Sci Rep. 2013;3:2795. doi: 10.1038/srep02795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fong KP, Barry C, Tran AN, Traxler EA, Wannemacher KM, Tang HY, Speicher KD, Blair IA, Speicher DW, Grosser T, Brass LF. Deciphering the human platelet sheddome. Blood. 2011;117:e15–e26. doi: 10.1182/blood-2010-05-283838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garcia BA, Smalley DM, Cho H, Shabanowitz J, Ley K, Hunt DF. The platelet microparticle proteome. J Proteome Res. 2005;4:1516–1521. doi: 10.1021/pr0500760. [DOI] [PubMed] [Google Scholar]