Abstract
Background
High-grade neuroendocrine carcinomas (HGNECs) of the colon and rectum are rare, constituting less than 1 % of colorectal cancers. The purpose of this study was to identify the natural history and oncologic outcomes of this disease, describe the use of surgery, and determine the clinical and pathological factors associated with outcomes.
Methods
Following Institutional Review Board approval, patients with HGNEC were identified from our institutional database. Patient charts and pathology reports were analyzed retrospectively for clinical and pathological factors.
Results
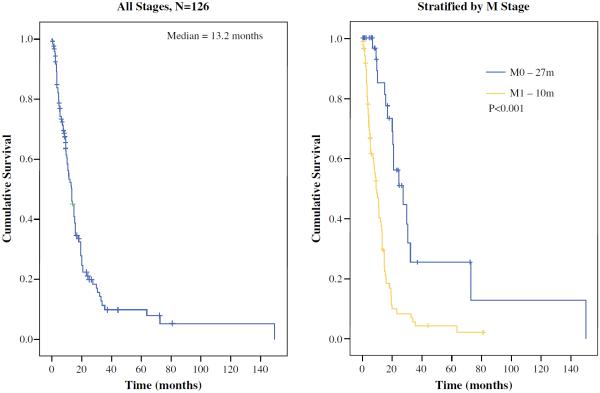
A total of 126 patients with a median follow-up of 9 months were identified. Median survival was 13.2 months, and 85 (67 %) patients had metastatic disease at diagnosis. Three-year overall survival (OS) was 5 and 18 % for patients with and without metastatic disease, respectively. Factors associated with improved OS on multivariable analysis were absence of metastatic disease and presence of an adenocarcinoma component within the tumor. In patients with metastatic disease, response to chemotherapy was the only factor associated with survival. In patients with localized disease, an adenocarcinoma component within the tumor was the only factor associated with survival. Resection of tumor was not associated with survival in either localized or metastatic disease.
Conclusion
High-grade colorectal NECs are extremely aggressive tumors with poor prognosis. Patients appear to have a marginally better prognosis if they present without metastatic disease, have an adenocarcinoma component within their tumor, or respond to chemotherapy. Surgery, particularly in the presence of metastatic disease, may not offer a survival benefit for the majority of patients.
High-grade (i.e. poorly differentiated) neuroendocrine carcinomas (HGNECs) constitute a spectrum of aggressive malignancies. HGNECs are clinically distinct from the more common well-differentiated neuroendocrine tumors (NETs; low and intermediate grade).1 Gastrointestinal HGNECs are morphologically and phenotypically related to pulmonary HGNECs (large and small cell types). Thus, HGNECs are managed primarily with platinum-based chemotherapy.2,3 Well and poorly differentiated NECs are classified together because of generic neuroendocrine marker expression (i.e. synaptophysin and chromogranin, detectable with immunohistochemistry).4 In contrast to HGNECs, most well-differentiated NETs (with the exception of some pancreatic NETs) are refractory to standard chemotherapy agents. Surgery is the mainstay of treatment for localized and/or resectable disease.5–7
The World Health Organization (WHO) and the European Neuroendocrine Tumor Society have devised grading systems to classify these tumors. Both allow the use of either mitotic rate or Ki-67 labeling index to define grade.8 An HGNEC is defined as a NEC with a high mitotic rate (>10 mitotic figures by 10 high-powered fields, or a Ki-67 proliferative index >20 %); extensive necrosis may also be present. The tumors commonly surpass these thresholds, expressing rates as high as 40–70 mitoses per 10 high-powered fields, or a Ki-67 index of 50–90 %.4 Recently, Sorbye et al. reviewed 305 patients with poorly differentiated gastrointestinal NECs, defined by WHO criteria. They identified Ki-67 <55 % as the best cutoff value for predicting prognosis and treatment response. Response to platinum-based chemotherapy was lower in patients with Ki-67 <55 % (14 vs. 44 %; p < 0.001), although these individuals had longer median survival (15 months) than those with Ki-67 >55 % (10 months; p < 0.001).9
Colorectal HGNECs are rare, accounting for <1 % of colorectal malignancies. Previous publications are limited to small retrospective series,10,11 and treatment regimens are extrapolated from published data on high-grade small cell carcinoma of the lung. However, some studies suggest a benefit for platinum-based chemotherapy.12,13 As with other gastrointestinal HGNECs, prognosis is generally poor, even in the setting of localized disease.14–16
This study reviews colorectal HGNECs treated at our institution. The goal was to better define the biology of and treatment options for these rare and challenging tumors.
METHODS
Patients
We obtained a waiver from the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSKCC) authorizing our review of electronic medical records (EMRs) and a prospective database, selecting for patients diagnosed with HGNEC, small cell carcinoma, poorly differentiated NEC, large cell carcinoma, and oat cell carcinoma of the colon, rectum, or anus, treated at MSKCC from January 1991 to October 2010. Patients were excluded if pathologic analysis was absent. Dedicated gastrointestinal pathologists, experienced in diagnosing HGNEC, reviewed all pathology. Resection of the primary tumor was considered definitive if it included the lymph node drainage basin. Endoscopic biopsy or resection and/or transanal excision were not considered definitive surgical treatment. Response to chemotherapy was based on data from the EMRs and defined by radiographic shrinkage on cross-sectional imaging. Recurrence was determined based on data from the EMRs of all study patients, including surgical and medical oncology clinic notes, endoscopy, radiology, operative and pathology reports. Tumor progression was defined as an increase in tumor size on radiological examination or if stated in surgical and medical oncology office notes.
Statistical Analysis
Overall survival (OS), measured from the date of diagnosis to the time of death, was the principle endpoint. Survival and recurrence distributions were compared using the log rank test, and illustrated with Kaplan–Meier curves. Two-year outcomes were reported in tabular format, with the log rank p value for corresponding stratified survival distribution. Proportions of categorical variables were compared using the χ2 test; if expected cell counts were <5, Fisher's exact test was used. A Cox proportional hazards model was constructed to test the measure of OS for all patients, and also for patients with metastatic disease only. Of 49 patients with known response to chemotherapy, 42 died; therefore, adequate information for an analysis of up to four covariates existed. The following potential predictors or confounder variables were included in our model, based on a priori clinical knowledge: site of tumor, response to chemotherapy, adenocarcinoma component in tumor, and whether the primary tumor was resected. Analyses were conducted using SAS version 9.1 (SAS Institute, Inc., Cary, NC, USA) and SPSS version 17.0 (SPSS, Chicago, IL, USA; Fig. 1).
FIG. 1.
Kaplan–Meier curves demonstrating overall survival and overall survival stratified for the presence of metastatic disease at presentation
RESULTS
Patient Demographics
A total of 126 patients were identified. Median follow-up was 9.4 months, and 67 % presented with metastatic disease (Table 1). Forty-seven percent had an anorectal primary, while 53 % had a colonic primary. More patients presenting with localized disease had an anorectal primary (61 %), while more patients presenting with metastatic disease had a colonic primary (62 %). As is typical of hindgut HGNECs, all but one patient had a non-functional tumor. The patient with a functional tumor had small cell carcinoma of the rectum with liver metastases, presenting with Cushingoid symptoms and elevated cortisol levels.
TABLE 1.
Patient demographics
| Number | 126 |
|---|---|
| Sex | |
| Male | 62 (48 %) |
| Female | 64 (52 %) |
| Age [years; median (range)] | 56 (30–91 %) |
| Histology | |
| SCC | 49 (39 %) |
| LCC | 23 (18 %) |
| Collision tumor (HGNEC with adenocarcinoma) | 18 (14 %) |
| Sites | |
| Anal | 5 (4 %) |
| Rectal | 53 (42 %) |
| Sigmoid | 9 (7 %) |
| Colon | 38 (30 %) |
| Cecum | 23 (18 %) |
| LVI | |
| Yes | 48 (84 %) |
| No | 9 (16 %) |
| PNI | |
| Yes | 18 (50 %) |
| No | 18 (50 %) |
| Median tumor size (range) | 4 (0.1–11) |
| AJCC stages | |
| 1 | 0 |
| 2 | 15 (12 %) |
| 3 | 26 (21 %) |
| 4 | 85 (67 %) |
| Resection of primary tumor | |
| Yes | 73 (58 %) |
| No | 53 (42 %) |
| Treatment of M0 diseases | |
| Surgery | 29 (71 %) |
| No surgery | 12 (29 %) |
| IBD | 8 (6 %) |
| Synchronous adenocarcinoma | 4 (4 %) |
| Metastatic disease | |
| Liver | 64 (51 %) |
| Lung | 11 (8 %) |
| Bone | 10 (7 %) |
| RPLN | 8 (6 %) |
| Peritoneal | 7 (5 %) |
| Inguinal lymph node | 5 (4 %) |
| Brain | 2 (2 %) |
Data are expressed as n (%) unless otherwise specified
SCC small cell carcinoma, LCC large cell carcinoma, HGNEC high-grade neuroendocrine carcinoma, LVI lymphovascular invasion, PNI perineural invasion, AJCC American Joint Committee on Cancer, IBD inflammatory bowel disease, RPLN retroperitoneal lymph nodes
Response to Chemotherapy
Response to treatment was identified in 48 of the 85 patients with metastatic disease receiving first-line chemotherapy. Eleven (23 %; four collision tumors, seven HGNECs) received fluorouracil (5-FU)-based chemotherapy [two folinic acid (leucovorin) + fluorouracil + oxaliplatin (FOLFOX), one capecitabine + oxaliplatin (XELOX), two folinic acid (leucovorin) + fluorouracil + irinotecan (FOLFIRI), one 5-FU/streptomycin, five FOLFOX/bevacizumab]. Of the seven patients with HGNECs, three (43 %) responded. Of the four with collision tumors, two (50 %) responded. Thirty-seven patients (77 %) received platinum-based chemotherapy (13 carboplatin/etoposide, 12 cisplatin/etoposide, 10 cisplatin/irinotecan, 1 cisplatin/etoposide/topotecan, 1 cisplatin/irinotecan/bevacizumab). Four of the 37 had collision tumors—three of these four (75 %) responded. Thirty-three had HGNECs; of these, 23 (70 %) responded.
Response to second-line chemotherapy was available for 35 patients. Regimens used were the same as those in first-line chemotherapy, as well as monotherapy with topotecan or paclitaxel alone. The response rate was 11 of 35 (31 %). Two patients with liver-only metastases, without an adenocarcinoma component, received hepatic artery infusion pumps delivering floxuridine. Both responded to treatment but eventually progressed and died of disease at 13 and 18 months post-diagnosis.
Of patients with localized anorectal disease, response data was available for eight receiving chemoradiotherapy and four receiving chemotherapy alone. One received 5-FU and radiotherapy, and the others received platinum-based chemotherapy or chemoradiotherapy. Eleven of 12 patients (92 %) responded to treatment. Of the five who underwent subsequent rectal resection, two had a pathological complete response. However, all eventually recurred systemically. One patient had a clinical complete response on examination, did not undergo rectal resection, and is currently disease-free 36 months post-diagnosis (Table 2).
TABLE 2.
Treatment of patients with localized colorectal HGNEC and metastatic colorectal HGNEC (%)
| Treatments | Localized (N = 41) |
Metastatic (N = 85) |
||
|---|---|---|---|---|
| Anorectal (61 %) | Colonic (39 %) | Anorectal (38 %) | Colonic (62 %) | |
| Chemotherapy only | 9 | 0 | 60 | 32 |
| CRT only | 38 | 0 | ||
| Surgery only | 0 | 19 | ||
| CRT and chemotherapy | 27 | 0 | ||
| CRT and surgery | 24 | 0 | 3 | 0 |
| Chemotherapy and surgery | 0 | 9 | ||
| Surgery and chemotherapy | 19 | 81 | 10 | 60 |
| Surgery and CRT | 10 | 0 | ||
HGNEC high-grade neuroendocrine carcinoma, CRT chemoradiotherapy
Of the patients with localized colon tumors who underwent surgical resection followed by chemotherapy, information was available for 11 (median follow-up 28 months). Four had collision tumors—two received FOLFOX and two received 5-FU/LV. Of these patients, three are currently no evidence of disease (NED) (median follow-up 37 months)—one died of other causes 72 months post-diagnosis. Of the seven patients with HGNEC alone, five received platinum-based adjuvant chemotherapy—three recurred, and two are free of disease (6 and 72 months post-diagnosis). Of the two patients who received FOLFOX, one is NED 25 months post-diagnosis; the other died of disease 20 months' post-diagnosis.
Overall Survival
Three-year OS was 8.7 %, with a median survival of 13.2 months. Patients presenting with metastatic disease did poorly, with median survival of 10 months (vs. 27 months in patients with localized disease; p<0.001). On multivariable analysis, the only factors associated with a favorable outcome were a collision tumor, and absence of metastatic disease (Table 3).
TABLE 3.
Univariable and multivariable analysis of factors associated with overall survival in patients with colorectal HGNEC
| Factors | Univariable |
Multivariable |
|||
|---|---|---|---|---|---|
| Median OS (months) | p value | Hazard ratio | 95 % CI | p value | |
| Sex | |||||
| Male | 10.6 | 0.16 | 0.66 | 0.42–1.02 | 0.64 |
| Female | 14.7 | ||||
| Age (years) | |||||
| <56 | 13.6 | 0.99 | 1.002 | 0.99–1.02 | 0.83 |
| ≥56 | 11.1 | ||||
| Site of tumor | |||||
| Colon | 10.8 | 0.54 | 0.81 | 0.49–1.31 | 0.39 |
| Rectum | 15.1 | ||||
| Metastases | |||||
| Yes | 10 | <0.001* | 0.37 | 0.20–0.67 | 0.001* |
| No | 24 | ||||
| Adenocarcinoma component | |||||
| Yes | 13.3 | 0.07 | 1.90 | 1.02–3.53 | 0.04* |
| No | 13.5 | ||||
| Resection of primary tumor | |||||
| Yes | 15 | 0.01* | 1.21 | 0.71–2.05 | 0.49 |
| No | 12 | ||||
| Inflammatory bowel disease | |||||
| Yes | 24.1 | 0.04* | 2.54 | 0.59–11.0 | 0.21 |
| No | 19.8 | ||||
| Radiotherapy (rectal only) | |||||
| Yes | 15.1 | 0.66 | |||
| No | 15.6 | ||||
| Histological types | |||||
| SCC | 14.8 | 0.88 | |||
| LCC | 9.5 | ||||
| Site of metastasis | |||||
| Liver | 9.2 | 0.47 | |||
| Lymph node | 12.9 | ||||
| Other | 9.4 | ||||
| Multiple sites of metastasis | |||||
| Yes | 8.3 | 0.11 | |||
| No | 12.9 | ||||
HGNEC high-grade neuroendocrine carcinoma, OS overall survival, CI confidence interval, LCC large cell carcinoma, SCC small cell carcinoma, LN lymph node
Statistical significance
For patients with localized disease, the only factor associated with survival on univariate analysis was presence of a collision tumor. Resection of the primary tumor was not associated with a statistically significant difference in survival (Table 4). For patients with metastatic disease, response to chemotherapy was the sole factor associated with better survival on both univariate and multivariable analysis (Table 5).
TABLE 4.
Univariable analysis of overall survival in patients with localized colorectal HGNEC
| Factors | Median OS (months) | p value |
|---|---|---|
| Sex | ||
| Male | 30.6 | 0.64 |
| Female | 20.9 | |
| Age (years) | ||
| ≤55 | 27.3 | 0.96 |
| >55 | 24.3 | |
| Site of tumor | ||
| Colon | 29.9 | 0.47 |
| Rectum | 24.3 | |
| Adenocarcinoma component | ||
| Yes | 72.8 | 0.009* |
| No | 21.0 | |
| Resection of the primary tumor | ||
| Yes | 27.4 | 0.17 |
| No | 20.3 | |
HGNEC high-grade neuroendocrine carcinoma, OS overall survival
Statistical significance
TABLE 5.
Univariable and multivariable analysis of overall survival in patients with metastatic colorectal cancer
| Factors | Univariable |
Multivariable |
|||
|---|---|---|---|---|---|
| Median OS (months) | p value | Hazard ratio | 95 % CI | p value | |
| Sex | |||||
| Male | 8.0 | 0.23 | |||
| Female | 11.5 | ||||
| Site of tumor | |||||
| Colon | 10.2 | 0.91 | 1.31 | 0.59–2.87 | 0.51 |
| Rectum | 8.4 | ||||
| Adenocarcinoma component | |||||
| Yes | 10.2 | 0.70 | 1.36 | 0.49–3.76 | 0.27 |
| No | 9.2 | ||||
| Response to chemotherapy | |||||
| Yes | 14.8 | <0.001* | 0.25 | 0.12–0.49 | <0.001* |
| No | 7.6 | ||||
| Resection of the primary tumor | |||||
| Yes | 10.2 | 0.44 | 0.62 | 0.26–1.45 | 0.27 |
| No | 9.5 | ||||
OS overall survival, CI confidence interval
Statistical significance
DISCUSSION
Colorectal HGNECs are extremely rare tumors, the largest previously reported series comprising only 38 patients.10 The results from that paper correlate quite closely with ours—5-year OS of 15 % and median survival of 10 months (13 months in our series). This review is the first to include the lesser-described large cell or non-small cell HGNEC following its classification by Shia et al.15 Similar to Shia's group, we report that the tumor cell type (i.e. small cell vs. large cell vs. mixed) does not predict OS or prognosis. However, in this cohort, the presence of a collision tumor was associated with better survival, particularly when patients presented with localized disease only.
The finding that two-thirds of patients with colorectal HGNEC present with metastatic disease is not unexpected and corresponds with data reported for other gastrointestinal HGNECs. Similarly, the fact that the liver is the main site of metastases for gastrointestinal HGNEC has been demonstrated previously.16 It is worth noting that brain metastases in this cohort of patients were rare; only two patients presented with brain metastases and an additional five subsequently developed them. This has been reported in other studies and, for that reason, the routine use of prophylactic whole brain radiation is not routinely practiced.16 Interestingly, patients with an anorectal HGNEC presented at an earlier stage, while patients with a colonic primary were more likely to present with measurable stage 4 disease. It is likely that location of the anorectal tumors may cause earlier symptoms of pain or bleeding per rectum. Unfortunately, earlier diagnosis of anorectal HGNEC did not lead to a better prognosis in this cohort.
Resection of the primary tumor was not associated with superior outcome in patients presenting with localized or metastatic disease. This finding is not surprising for patients with metastatic disease; however, it differs greatly from the results of surgery for primary colorectal adenocarcinoma, for which surgery is the principle treatment for localized disease.17,18 This corresponds to the observation that surgical resection of localized HGNEC of the lung is not associated with superior outcome compared with patients with the primary in place.19 Based on these data, resection of either the primary tumor or metastatic disease does not appear to be indicated when a definitive diagnosis of HGNEC has been made. The only exceptions to this non-operative approach may be for a localized collision tumor in the colon (as these have more favorable outcomes for localized disease) or a symptomatic primary tumor; however, obstructed patients could be considered for endoscopic self-expanding endoluminal stents or a defunctioning colostomy, facilitating a prompt start of chemotherapy or chemoradiotherapy. It is worth noting that although survival is better for standard metastatic colorectal adenocarcinomas, the primary tumor rarely causes symptoms necessitating resection during systemic chemotherapy.20 For patients with localized disease, when surgery can be expected to cause less morbidity (as with a colonic resection) surgical resection followed by adjuvant chemotherapy remains a reasonable treatment option, as almost all recurrences are systemic. In HGNEC of the lung, some data suggest that surgical resection may offer some benefit in very early-stage disease,21 and this may also be true for colorectal HGNEC. In situations where surgical resection can be expected to cause significant morbidity, as is the case with an abdominoperineal resection or a low anterior resection, chemoradiotherapy followed by cisplatin-based chemotherapy offers excellent local control with the added benefit of earlier initiation of systemic treatment.
In this study, we report a response rate to platinum-based chemotherapy similar to some previously reported rates in gastrointestinal HGNEC—75 % in a study of 12 gastric HGNECs using cisplatin/irinotecan;22 however, better than others—42 % in 41 gastrointestinal HGNECs treated with cisplatin/etoposide 12 and 14 % in hepatobiliary HGNEC treated again with cisplatin/etoposide.23 However, this is the first study to report response rates to modern 5-FU-based chemotherapy such as FOLFOX and FOLFIRI, which showed response rates of 45 %, which, while inferior to platinum-based chemotherapy, are reasonable. This treatment can be considered specifically in the case of collision tumors if there is a component not only of HGNEC but also adenocarcinoma. Treatment for pure HGNEC, however, should be platinum-based and extrapolated from published data on high-grade small cell carcinoma of the lung. We also report response rates of 31 % for second-line treatment. These results are difficult to interpret due to the heterogeneous treatment regimens used and the small sample size. However, they are similar to the only other data on second-line treatment in gastrointestinal HGNEC, a study by Welin et al. reporting on a series of 25 patients with mainly gastrointestinal HGNEC who had progressed on first-line platinum-based chemotherapy and were treated with temozolomide alone or in combination with capecitabine, with a response rate of 33 %.24
In this cohort, six patients were treated with the VEGF inhibitor bevacizumab—one in combination with cisplatin/irinotecan who responded to the treatment, and five others with FOLFOX, only one (20 %) of whom responded to treatment. It does not therefore appear that bevacizumab in addition to either 5-FU- or platinum-based CT can be expected to offer improved response rates, although these conclusions are based on very small numbers.
This is the first study to report on results of chemotherapy with or without chemoradiotherapy in patients with localized anorectal HGNECs. In this cohort, we report response rates of 92 % with one clinical complete response, while among the five patients who underwent rectal resection there were two pathological complete responses. This begs the question whether a radical rectal resection with substantial risk of morbidity and mortality is warranted. Chemoradiotherapy appears to obtain adequate control of the anorectal disease, especially given our data which illustrates 92 % of our patients recurring systemically. We believe surgery should be reserved for those patients who do not respond to treatment, if the goal is to achieve local control; however, one should not expect that surgery will alter the natural course of the disease.
The limitations of the present study are those typical of a retrospective analysis. The treatment regimens used were heterogeneous, with many different types of chemotherapy used as both first- and second-line treatment. There are treatment biases, as a proportion of patients underwent surgery prior to definitive treatment, affecting the subsequent decision-making process. As there were no standard surgical guidelines, the decision to proceed to surgery, particularly for localized anorectal tumors, may have been influenced by response to treatment, leading to biases when comparing these two groups. However, this review of 126 colorectal HGNECs provides the most comprehensive data currently available in the literature. In addition, this cohort was defined as poorly differentiated NEC, which is synonymous with HGNEC. The period of this study spanned the introduction of routine Ki-67 staining, which was therefore not assessed for each patient. By WHO classification, a well-differentiated NET with a Ki-67 of >20 % is currently defined as high grade. This retrospective cohort does not include those patients, as all of our patients had poorly differentiated NECs. This may be the reason for the higher response rates to chemotherapy we observed compared with previous studies.
CONCLUSIONS
High-grade colorectal NECs are extremely aggressive tumors with a poor prognosis. Patients appear to have a marginally better prognosis if they present without meta-static disease, have a component of adenocarcinoma within their tumor, or respond to chemotherapy. Surgery, particularly in the presence of metastatic disease, may not offer a survival benefit for the majority of patients with this disease.
ACKNOWLEDGMENT
This study was funded in part by the Cancer Center Core Grant P30 CA008748. The Core Grant provides funding to institutional cores, such as biostatistics and pathology, which were used in this study.
Footnotes
CONFLICT OF INTEREST None.
REFERENCES
- 1.Gardner GJ, Reidy-Lagunes D, Gehrig PA. Neuroendocrine tumors of the gynecologic tract: a Society of Gynecologic Oncology (SGO) clinical document. Gynecol Oncol. 2011;122(1):190–8. doi: 10.1016/j.ygyno.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Moertel C, et al. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin: evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68:227–32. doi: 10.1002/1097-0142(19910715)68:2<227::aid-cncr2820680202>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Rindi G, Inzani F, Solcia E. Pathology of gastrointestinal disorders. Endocrinol Metab Clin N Am. 2010;39(4):713–27. doi: 10.1016/j.ecl.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Strosberg JR, et al. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas. 2010;39(6):799–800. doi: 10.1097/MPA.0b013e3181ebb56f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrone C, Tang L, Tomplinson J, et al. Pancreatic neuroendocrine tumors: can the WHO staging system be simplified. J Clin Oncol. 2007;25(18S):15038. [Google Scholar]
- 6.Van Eeden S, Quadvlieg P, Babs G, et al. Classification of low-grade neuroendocrine tumors of midgut and unknown origin. Hum Pathol. 2002;33:1126–32. doi: 10.1053/hupa.2002.129204. [DOI] [PubMed] [Google Scholar]
- 7.Strosberg JR, et al. Prognostic validity of a novel American Joint Committee on Cancer staging classification for pancreatic neuroendocrine tumors. J Clin Oncol. 2011;29(22):3044–9. doi: 10.1200/JCO.2011.35.1817. [DOI] [PubMed] [Google Scholar]
- 8.Rindi G, Kloppel G, Couvelard A, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2007;451:757–62. doi: 10.1007/s00428-007-0452-1. [DOI] [PubMed] [Google Scholar]
- 9.Sorbye W, Welin S, Langer S, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal poorly differentiated neuroendocrine carcinoma: the NORDIC NEC study [abstract number 4015] J Clin Oncol. 2012;30(15S):4015. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]
- 10.Bernick PE, et al. Neuroendocrine carcinomas of the colon and rectum. Dis Colon Rectum. 2004;47(2):163–9. doi: 10.1007/s10350-003-0038-1. [DOI] [PubMed] [Google Scholar]
- 11.Staren ED, et al. Neuroendocrine carcinomas of the colon and rectum: a clinicopathologic evaluation. Surgery. 1988;104(6):1080–9. [PubMed] [Google Scholar]
- 12.Mitry E, et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer. 1999;81(8):1351–5. doi: 10.1038/sj.bjc.6690325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hainsworth JD, et al. Phase II trial of paclitaxel, carboplatin, and etoposide in advanced poorly differentiated neuroendocrine carcinoma: a Minnie Pearl Cancer Research Network Study. J Clin Oncol. 2006;24(22):3548–54. doi: 10.1200/JCO.2005.05.0575. [DOI] [PubMed] [Google Scholar]
- 14.Strosberg J, et al. Correlation between grade and prognosis in metastatic gastroenteropancreatic neuroendocrine tumors. Hum Pathol. 2009;40(9):1262–8. doi: 10.1016/j.humpath.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Shia J, et al. Is nonsmall cell type high-grade neuroendocrine carcinoma of the tubular gastrointestinal tract a distinct disease entity? Am J Surg Pathol. 2008;32(5):719–31. doi: 10.1097/PAS.0b013e318159371c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenner B, et al. Small-cell carcinoma of the gastrointestinal tract: a retrospective study of 64 cases. Br J Cancer. 2004;90(9):1720–6. doi: 10.1038/sj.bjc.6601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glimelius B, Oliveira J. Rectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann. Oncol. 2009;20(Suppl 4):54–6. doi: 10.1093/annonc/mdp128. [DOI] [PubMed] [Google Scholar]
- 18.Van Cutsem E, Oliveira J. Primary colon cancer: ESMO clinical recommendations for diagnosis, adjuvant treatment and follow-up. Ann. Oncol. 2009;20(Suppl 4):49–50. doi: 10.1093/annonc/mdp126. [DOI] [PubMed] [Google Scholar]
- 19.Sorensen M, Felip E. Small-cell lung cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann. Oncol. 2009;20(Suppl 4):71–2. doi: 10.1093/annonc/mdp133. [DOI] [PubMed] [Google Scholar]
- 20.Poultsides GA, et al. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J. Clin. Oncol. 2009;27(20):3379–84. doi: 10.1200/JCO.2008.20.9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider BJ, Saxena A, Downey RJ. Surgery for early-stage small cell lung cancer. J. Natl. Compr. Canc. Netw. 2011;9(10):1132–9. doi: 10.6004/jnccn.2011.0094. [DOI] [PubMed] [Google Scholar]
- 22.Okita NT, et al. Neuroendocrine tumors of the stomach: chemotherapy with cisplatin plus irinotecan is effective for gastric poorly-differentiated neuroendocrine carcinoma. Gastric. Cancer. 2011;14(2):161–5. doi: 10.1007/s10120-011-0025-5. [DOI] [PubMed] [Google Scholar]
- 23.Iwasa S, et al. Cisplatin and etoposide as first-line chemotherapy for poorly differentiated neuroendocrine carcinoma of the hepatobiliary tract and pancreas. Jpn. J. Clin. Oncol. 2010;40(4):313–8. doi: 10.1093/jjco/hyp173. [DOI] [PubMed] [Google Scholar]
- 24.Welin S, et al. Clinical effect of temozolomide-based chemotherapy in poorly differentiated endocrine carcinoma after progression on first-line chemotherapy. Cancer. 2011;117(20):4617–22. doi: 10.1002/cncr.26124. [DOI] [PubMed] [Google Scholar]