Abstract
Purpose
To determine the maximum tolerated dose (MTD) of topotecan in combination with ifosfamide, mesna, and etoposide (TIME), followed by autologous hematopoietic cell transplant (HCT), in patients with chemotherapy-refractory malignancies.
Experimental Design
Patients were treated with (in mg/m2/d) ifosfamide 3,333, mesna 3,333, and topotecan 3.3 to 28.3 during days-8 through-6 and etoposide 500 (days-5 through-3) followed by HCT on day 0. Once MTD was defined, we expanded this dosing cohort to include patients with high-risk lymphoma due to activity seen during dose escalation. Topotecan pharmacokinetic analyses were carried out, and topoisomerase I levels and activity were measured.
Results
The topotecan MTD in this regimen was 64 mg/m2 (21.3 mg/m2/d). Mucositis was dose limiting and correlated with topotecan dose level and area under the curve (AUC). Dose level was also correlated with length of hospitalization, number of days of parenteral nutrition, and neutrophil and platelet engraftment. Topotecan AUC was significantly correlated with time to platelet recovery. The baseline peripheral blood mononuclear cell topoisomerase I level was found to be a significant positive predictor for overall and progression-free survival. Topotecan AUC was positively correlated with dose level, with a trend toward decreasing clearance with increasing dose.
Conclusion
Topotecan can be a useful drug in the high-dose setting given its activity in some malignancies when given in standard dose. Pharmacokinetic monitoring may be a valuable tool for optimizing the use of topotecan and to avoid toxicity seen with high-systemic exposures. Baseline topoisomerase I levels may have an important role in predicting topotecan efficacy.
Introduction
High-dose chemotherapy followed by autologous hematopoietic cell transplantation (HCT) is used in the treatment of a wide variety of relapsed and refractory malignancies. Despite the many reports of positive outcomes of HCT, patients continue to relapse posttransplant. One strategy to reduce posttransplant relapse is the development of novel conditioning regimens that take advantage of synergistic mechanisms of cytotoxicity. Most high-dose chemotherapy regimens include at least one alkylating agent because of the steep dose–response curves exhibited by these agents and because the dose-limiting toxicity in the standard dose setting is myelosuppression, allowing dose escalation in the HCT setting. Other drugs have been added to alkylating agents because of complementary mechanisms of cytotoxicity. Studies in several human cell lines and xenografts have shown that inhibition of DNA repair by a topoisomerase I (topo I) inhibitor may potentiate cytotoxicity induced by an alkylating agent (1–5). In addition, the sequential treatment with atopo I inhibitor followed by a topoisomerase II (topo II) inhibitor has resulted in synergistic cytotoxicity (6, 7). These preclinical and in vivo data suggest a paradigm for sequencing these antitumor agents: an alkylating agent followed by a topo I inhibitor and then a topo II inhibitor. This specific sequence serves as the basis for the design of this trial.
In this phase I study, our aim was to define the dose-limiting toxicity of topotecan in combination with ifosfamide and etoposide. Our starting dose of topotecan was approximately 60% of the maximum tolerated dose (MTD) reported in standard dose phase I trials with every 3- to 4-week administration schedule. The doses of ifosfamide and etoposide were 50% of the MTD determined in a previous dose escalation study done at our institution (8). Due to activity seen during the present topotecan dose escalation study, we expanded the MTD cohort to include 16 patients with high-risk lymphoma. Preliminary clinical results have been previously described (9–11), and the mature and correlative data are presented here.
Patients and Methods
Patients
Patients 18 to 64 years of age with histologically confirmed cancer and an Eastern Cooperative Oncology Group performance status of less than 2 were eligible. Patients were required to have adequate organ function and to have had no prior treatment with topotecan. Patients who had active infections, major metabolic disease (e.g., insulin-dependent diabetes or uncompensated adrenal or thyroid dysfunction), a positive antibody test for HIV, or leptomeningeal involvement were not eligible. All patients provided written informed consent. The research was reviewed and approved by the University of South Florida Institutional Review Board.
Patients with breast cancer were required to have meta-static disease that had failed to respond to an anthracycline-containing regimen and 1 other salvage regimen. Patients with intermediate or high-grade non-Hodgkin lymphoma (NHL) who had relapsed and failed to respond to more than 2 salvage chemotherapy regimens or had failed to achieve a complete remission after first-line induction chemotherapy and failed to respond to more than 1 salvage regimen were eligible. Patients with Hodgkin lymphoma were eligible if they had failed at least 3 chemotherapy regimens. Patients with other malignancies were considered if they had failed standard therapy for their disease.
Patients who were less than 55 years of age were eligible for the expanded MTD cohort if they had (i) intermediate or high-grade NHL and if they had relapsed and/or failed to respond to at least 2 chemotherapy regimens or had failed to achieve a complete remission after first-line induction chemotherapy and failed to respond to 1 or more salvage regimen, or (ii) low-grade or indolent histologies and had relapsed or failed to achieve a complete remission after first-line induction chemotherapy and had failed to respond to less than 2 salvage chemotherapy regimens, or (iii) had Hodgkin lymphoma and had received 2 or more salvage regimens. Patients who were 55 years of age or more were eligible if they had NHL with any histology or Hodgkin lymphoma if they had relapsed and/or failed to achieve a complete remission after first-line induction chemotherapy.
Treatment plan
Autologous hematopoietic cell collection and reinfusion
Hematopoietic cells were mobilized, harvested, and transplanted per standard operating procedures of the Moffitt Cancer Center Stem Cell Processing Laboratory. Most patients received standard dose chemotherapy followed by granulocyte colony-stimulating factor (G-CSF) for the purposes of tumor cytoreduction and mobilization of hematopoietic cells. Mobilizing regimens consisted of either cyclophosphamide, paclitaxel, and G-CSF (patients with breast or ovarian cancer) orcyclophosphamide, etoposide, and G-CSF (lymphoma patients). Patients who did not require further cytoreduction or in whom further chemotherapy was not appropriate received G-CSF alone.
Conditioning regimen
Patients were treated with ifosfamide 3,333 mg/m2/d via a 2-hour intravenous infusion on days -8 through -6 followed immediately by topotecan 3.3 to 28.3 mg/m2/d (10–85 mg/m2 total over 3 days) via a 30-minute intravenous infusion on days -8 through -6. Mesna dosing was 1,111 mg/m2 via 30-minute intravenous infusion 30 minutes before and 4 and 8 hours after ifosfamide on days -8 through -6. Etoposide 500 mg/m2/d was given by intravenous infusion over 24 hours on days -5 through -3 beginning exactly 24 hours after the completion of the last topotecan infusion. Patients underwent HCT 48 hours after infusion of the chemotherapy regimen was completed (day 0).
Topotecan was dose escalated in cohorts of at least 4 patients each; patients were evaluated for 30 days posttransplant for toxicity. If, at a given dose level, 3 of 4 patients developed severe nonhematologic toxicities (with the exception of mucositis or enteritis), no further dose escalation and no further accrual at this level were allowed. Six more patients were entered at the preceding dose level to establish safety. If, at a given dose level, 1 or 2 of 4 patients developed severe toxicities, then 4 more patients were entered at that dose level. Only if 1 or 2 of 8 patients reported severe toxicity was the dose escalated further. If 5 or 6 of 8 patients experienced severe toxicity, then 6 more patients were entered at the previous dose level. Finally, if severe toxicity was observed in 3 or 4 of 8 patients, then 4 more patients were entered at this dose level for a total of 12 patients at that level. Patients could be treated at lower dose levels that had been declared to be “safe” while accrual was halted at a higher dose level until toxicity was evaluated. The MTD was defined as the dose level immediately preceding that which caused severe toxicity in more than 50% of patients. Severe toxicity was defined as any grade 3 or 4 toxicity based on a toxicity scale adapted for bone marrow transplant patients from the World Health Organization and the Eastern Cooperative Oncology Group (see Supplementary Table S1).
Supportive care
All patients received G-CSF 5 μg/kg/d beginning the day after transplant until an absolute neutrophil count (ANC) of more than 10 × 109 cells/L was achieved. Prophylactic antibiotics included an oral quinolone, fluconazole, and, in patients with elevated herpes simplex virus titers, acyclovir. Lymphoma patients also received prophylactic therapy for Pneumocystitis pneumonia.
Safety assessment
Routine laboratory evaluations included complete blood count, metabolic panels, and renal/hepatic function tests which were carried out after the conditioning regimen was started. The frequency and duration of tests were at the discretion of the treating physician and based on the clinical status of each patient. Patients were monitored for adverse events at least weekly until discharged posttransplant and during each clinic visit thereafter through day 90 posttransplant.
Tumor response
Response was assessed at 30 days, at 90 days, and at 1 year and then yearly post-HCT. A complete response (CR) was defined as no evidence of disease on physical examination, X-rays, or computed tomography scans for a minimum of 4 weeks. In patients with bone metastasis as their only evaluable disease, a CR was defined as recalcification of osteolytic lesions or complete resolution of bone scan abnormalities. A partial response (PR) was defined as a 50% or more decrease in the sum of the products of the diameters of all measurable lesions for 1 month or more with no concomitant appearance of new lesions for a minimum of 4 weeks. Stable disease (SD) was defined as a less than 50% decrease in measurable lesions without appearance of new lesions. Progressive disease was defined as a 25% or more increase in preexisting tumor or appearance of any new lesions.
For patients with nonmeasurable disease, responses were defined as follows: a CR was normalization of carcino-embryonic antigen (CEA) and/or cancer antigen 15-3 (CA 15-3) without the appearance of measurable tumor, a PR was a 50% or more decrease in CEA and/or CA 15-3, and SD was a less than 50% decrease in CEA and/or CA 15-3. Progressive disease was defined as an increase in CEA or CA 15-3 in patients with stable bone scans.
Topotecan pharmacokinetics
Pharmacokinetic parameters were calculated by model-independent methods by WinNonlin software. For the phase I portion of the study, blood samples (4 mL) were collected at 15 minutes prior to the first and third topotecan infusions and 20, 35, 45, 60, and 90 minutes and 2.5, 4.5, 8.5, 12.5, 18.5, and 24.5 hours after the initiation of first and third topotecan infusion. Patients in the expanded MTD cohort had samples drawn 30 minutes before and then at the end of the first infusion and then 15 minutes and 6 hours after the end of the infusion (12). Blood samples drawn from a lumen of the indwelling central venous catheter not being used for topotecan infusion were immediately centrifuged, with plasma separated, placed in a heparinized tube, and immediately frozen at –80°C. Ice-cold methanol was added to stop the closed ring (lactone) from opening. Plasma concentrations of both total topotecan and the closed lactone ring of topotecan were measured by high-pressure liquid chromatography (13, 14).
Topo I activity and levels (phase I patients only)
Band depletion assay for topo I activity
Cleavable complex formation was used to assess topo I activity, which we analyzed as previously described with few modifications (15, 16). Details of these methods can be found in the Supplementary Material. Values reported are concentrations of topotecan required to induce DNA-topo I complexes such that 50% of the topo I could not enter a 10% polyacrylamide gel.
Peripheral blood processing for topo I levels
Thirty-five milliliters of whole blood were collected in tubes containing sodium citrate immediately before the start of the topotecan infusion. Blood was also collected at 35 minutes and 45 minutes and at 1, 1.5, 2.5, 4.5, 8.5, and 24 hours after the start of the 30-minute infusion and kept on ice throughout the procedure. These samples were collected on days -8 and -6 of high-dose chemotherapy. Details of the subsequent analyses can be found in the Supplementary Material.
Statistical analysis
Response rates were reported with 95% CIs by the Clopper-Pearson method. The Spearman correlation coefficient was used to estimate correlation between continuous variables. The Kaplan–Meier method was used to estimate overall survival and progression-free survival (PFS) from time of initiation of the treatment. PFS was defined by the absence of progression or death. The potential predictor variables were evaluated by the Cox proportional hazard model, using univariate and multi-variate analysis with backward elimination method with P out value of 0.15. Band depletion was not involved in the multivariate analysis because of missing observations for all patients in dose level 1. The Cox proportional hazard model was also used to evaluate the effects of pharmacokinetic variables on time to recovery of platelets or neutrophils. The cumulative incidence of transplant-related mortality was estimated by the Gray method (17); death and progression were considered event and competing risk, respectively.
Results
Patients were enrolled between September 1996 and October 2002. Patient demographics and hematopoietic cell collection data are shown in Table 1. The majority of patients in the phase I study had refractory metastatic breast cancer. Of the 8 NHL patients, 2 with low-grade/indolent histologies were transplanted in chemosensitive relapse (second or greater); the remaining NHL patients all had chemoresistant disease. Of the 11 patients with intermediate grade or aggressive NHL in the expanded MTD cohort, 8 patients had diffuse large B-cell histology and 1 patient each had angioimmunoblastic T-cell lymphoma, anaplastic large cell lymphoma, and mantle cell lymphoma. Six patients had failed primary induction therapy, and 5 had relapsed disease. Of the 3 patients in the expanded MTD cohort who had low-grade/follicular histologies, one had chemosensitive disease and 2 had refractory disease. Both patients with Hodgkin lymphoma had failed primary induction therapy. The majority of patients received peripheral blood hematopoietic cells. Two patients received both bone marrow and peripheral blood–derived cells as they had inadequate collections from peripheral blood alone.
Table 1. Patient demographics and hematopoietic cell collections.
| Parameter | Phase I (n = 45) | Expanded MTD cohort (n = 16) |
|---|---|---|
| Median age, y (range) | 46 (29–63) | 58 (35–64) |
| Sex, n (%) | ||
| Female | 40 (89) | 6 (38) |
| Male | 5 (11) | 10 (63) |
| Race, n (%) | ||
| White | 38 (84) | 14 (88) |
| Black | 3 (7) | 1 (6) |
| Hispanic | 3 (7) | 1 (6) |
| Other | 1 (2) | |
| Primary tumor type, patients, n (%) | ||
| Breast cancer | 29 (64) | |
| NHL | 8 (18) | 14 (88) |
| Low grade/indolent | 4 (9) | 3 (19) |
| Intermediate grade/aggressive | 4 (9) | 11 (69) |
| Hodgkin lymphoma | 0 | 2 (12) |
| Ovarian cancer | 7 (16) | |
| Testicular cancer | 1 (2) | |
| Hematopoietic cell source, n (%) | ||
| Bone marrow (no priming) Peripheral blood | 1 (2) | |
| Cyclophosphamide/taxol/G-CSF | 33 (73) | |
| Cyclophosphamide/etoposide/G-CSF | 7 (16) | 16 (100) |
| G-CSF | 2 (4) | |
| Both | ||
| Cyclophosphamide/taxol/G-CSF (PB) | 1 (2) | |
| G-CSF + GM-CSF (PB) | 1 (2) | |
| CD34+ cells/kg collected, ×106; median (range) | 4.01 (0.11–72.10) | 3.01 (2.05–8.48) |
Abbreviation: PB, peripheral blood.
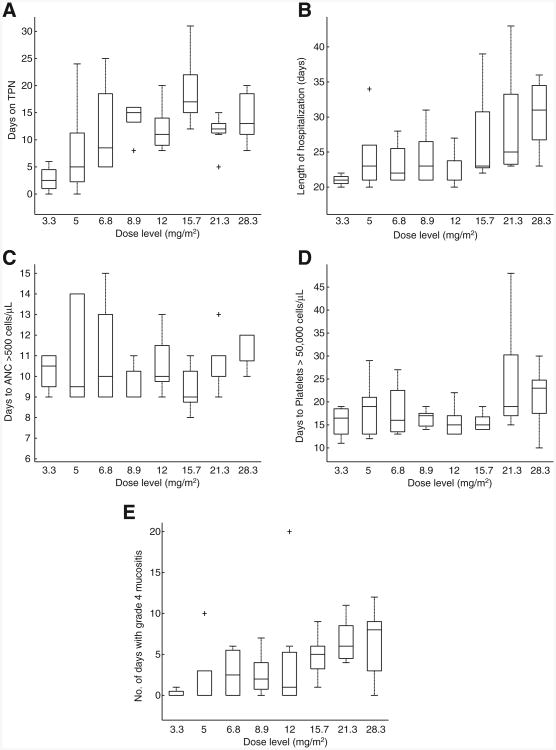
Table 2 shows grade 3/4 toxicity per topotecan dose level. Although initially not recognized as a potential dose-limiting toxicity, the severity and duration of grade 4 mucositis at dose level 8 (85 mg/m2 over 3 days) were felt to be unacceptable, especially given the lack of improvement in efficacy seen at this level; further dose escalation was not undertaken and the MTD was dose level 7 (64 mg/m2 over 3 days). Other grade 3 or 4 toxicities were randomly observed across all dose levels. The cumulative incidence of transplant-related mortality for all phase I patients at 100 days was 4%. Both deaths were a result of infectious complications. The length of hospitalization (from day of transplant to day of discharge), days of total parenteral nutrition (TPN), and median time to hematologic recovery by dose level are presented in Fig. 1. The dose level of topotecan was significantly correlated with days of grade 4 mucositis (Spearman correlation coefficient = 0.53, P = 0.0002), length of hospitalization (Spearman correlation coefficient = 0.51, P = 0.0004), and the number of days patients received TPN (Spearman correlation coefficient = 0.44, P = 0.0032). Engraftment of neutrophils and platelets was also significantly correlated with topotecan dose level (HR = 0.51, P < 0.001 and HR = 0.96, P = 0.03, respectively).
Table 2. Number of patients with grade 3 or 4 nonhematologic toxicities per topotecan dose level.
| Dose level | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Expanded MTD cohort | |
| 10 mg/m2 | 15 mg/m2 | 20 mg/m2 | 27 mg/m2 | 36 mg/m2 | 48 mg/m2 | 64 mg/m2 | 85 mg/m2 | ||
| n = 4 | n = 6 | n = 4 | n = 5 | n = 9 | n = 5 | n = 7 | n = 5 | N = 16 | |
| Mucositis (grade 4) | 1 (25%) | 2 (33%) | 2 (50%) | 4 (80%) | 5 (56%) | 5 (100%) | 7 (100%) | 4 (80%) | 15 (94%) |
| Number of days with grade 4 mucositis. median (range) | 0 (0-1) | 0 (0-10) | 2.5 (0-6) | 2 (0-7) | 1 (0-20) | 5 (1-9) | 6 (4-11) | 8 (0-12) | 6 (0-20) |
| Enteritis | 0 | 1 (17%) | 3 (75%) | 3 (60%) | 4 (44%) | 2 (40%) | 4 (57%) | 3 (60%) | 5 (31 %) |
| Hematuria | 0 | 1 (17%) | 1 (25%) | 0 | 0 | 0 | 1 (14%) | 1 (20%) | 0 |
| Cortical function | 0 | 1 (17%) | 0 | 1 (20%) | 1 (11 %) | 0 | 0 | 0 | 2 (13%) |
| Peripheral nerve function | 0 | 1 (17%) | 0 | 0 | 0 | 0 | 1 (14%) | 0 | 0 |
| Reduction in ejection fraction | 0 | 0 | 0 | 1 (20%) | 0 | 0 | 0 | 1 (20%) | 1 (6%) |
| Pulmonary clinical status | 1 (25%) | 1 (17%) | 0 | 1 (20%) | 0 | 0 | 0 | 0 | 1 (6%) |
| Nonrelapse deaths | 0 | 0 | 0 | 0 | 0 | 0 | 1 (14%) | 1 (20%) | 2 (13%) |
| Fungal infection | Septic shock | Arrhythmia (1) and bacterial sepsis (1) | |||||||
Figure 1.
Relationship between topotecan dose level and toxicity outcomes and engraftment for patients in the phase I trial. A, dose level versus days on TPN. B, dose level versus length of hospitalization. C, dose level versus days to ANC more than 500 cells/μL. D, dose level versus days to platelets more than 50,000 cells/μL. E, dose level versus number of days with grade 4 mucositis. The horizontal line in the middle of each box indicates the median, whereas the top and bottom of each box represent the 75th and 25th percentiles, respectively. The whiskers above and below the box represent the 90th and 10th percentile, respectively.
Patients in the expanded MTD cohort had a nonhematologic toxicity profile similar to that seen in patients in the phase I study. The cumulative incidence of transplant-related mortality in the expanded cohort was 13%. One patient died 21 days after transplant as a result of hypokalemia-induced ventricular fibrillation resulting in myocardial infarction; another patient expired on day 11 after transplant of gram-negative bacterial sepsis. The median length of time patients received TPN was 12 days (6–24 days). The median time to hematologic recovery (engraftment) was 11 days (range, 10–13 days) for ANC more than 500 per microliter and 17 days (range, 12–32 days) for platelets more than 50,000 per microliter.
Efficacy
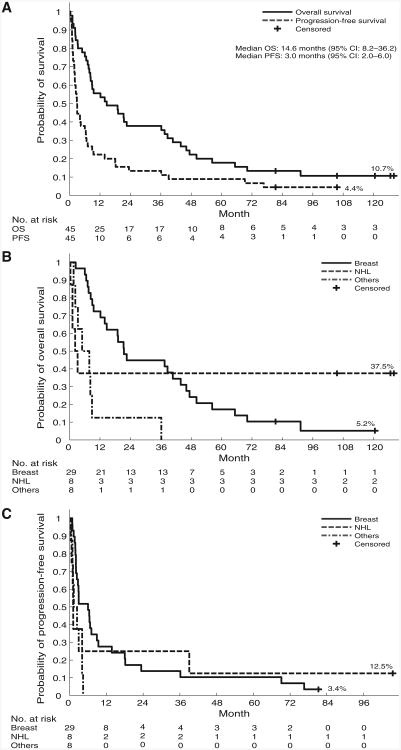
The overall response rate in the phase I study was 31%, with 5 CR (11%; 3 patients with breast cancer and 2 with NHL) and 9 PR (20%; 6 patients with breast cancer, 2 with NHL, and 1 with ovarian cancer). An additional 17 patients had SD (39%). Responses did not seem to correlate with dose level: 2 CRs were seen at dose level 3 (20 mg/m2 over 3 days) and 1 each occurred at dose levels 4, 5, and 7 (27 mg/m2, 36 mg/m2, and 64 mg/m2, respectively, over 3 days). For patients on the phase I trial, overall survival at 1, 3, 5, and 10 years was 56%, 38%, 18%, and 11%, respectively; PFS at 1, 3, 5, and 7 years was shown to be 22%, 13%, 9%, and 4%, respectively (Fig. 2A). At present, 5 patients are alive for more than 6 years after transplant (2 progression free); 3 of these 5 patients have survived more than 10 years. Three of the survivors had NHL (low grade) and 2 had chemorefractory metastatic breast cancer; 1 patient of each diagnosis is progression free. Four of these long-term survivors were treated at dose levels 1 to 3, and one was treated at dose level 7. There was a significant difference in overall survival between diagnoses (breast cancer vs. NHL vs. other; P = 0.009; Fig. 2B and C). By Cox proportional hazards modeling, both breast cancer and NHL patients had significantly improved survival compared with patients who had other diagnoses.
Figure 2.
Overall survival and PFS for patients in the phase I trial. A, overall survival and PFS for all phase I patients. B, overall survival for phase I patients by disease type (breast, NHL, and other). C, PFS for phase I patients by disease type (breast, NHL, and other).
Results of univariate and multivariate analyses for overall survival and PFS are shown in Table 3. Baseline topo I levels, topotecan (total) area under the curve (AUC), diagnosis, and age were significant predictors in the univariate analysis of overall survival. In the multivariate analysis, baseline topo I levels and diagnosis remained the only significant predictors, with increasing topo I levels correlating with increased overall survival and breast cancer patients having better survival than those being transplanted with diagnosis other than breast cancer or NHL. When PFS was the outcome, baseline topo I, age, and diagnosis were significant in the univariate analysis, but only topo I levels in peripheral blood mononuclear cells (PBMC) appeared to be important in the multivariate analysis.
Table 3. Results of univariate and multivariate analysis.
| Overall survival | ||||
|---|---|---|---|---|
|
| ||||
| Variable | Univariate | Multivariate | ||
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Topotecan AUC (total) | 1.31 (1.08–1.59) | 0.006 | 0.146 | |
| Band depletion (topo I activity) | 0.98 (0.93–1.04) | 0.501 | ||
| Topo I levels | 0.94 (0.89–0.98) | 0.007 | 0.90 (0.86–0.95) | <0.001 |
| Maximum decrease in topo I levels | 1.02 (1.00–1.04) | 0.071 | 0.465 | |
| Age | 0.96 (0.92–0.99) | 0.031 | 0.897 | |
| Diagnosis | ||||
| Othersa | ||||
| Breast | 0.30 (0.13–0.70) | 0.006 | 0.19 (0.06–0.68) | 0.009 |
| NHL | 0.23 (0.07–0.77) | 0.017 | 0.388 | |
| PFS | ||||
|
| ||||
| Variable | Univariate | Multivariate | ||
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
|
| ||||
| Topotecan AUC (total) | 1.43 (0.95–2.15) | 0.089 | 0.495 | |
| Band depletion (topo I activity) | 0.99 (0.95–1.04) | 0.799 | ||
| Topo I levels | 0.95 (0.91–0.99) | 0.033 | 0.93 (0.89–0.97) | <0.001 |
| Maximum decrease in topo I levels | 1.01 (0.99–1.03) | 0.273 | 0.562 | |
| Age | 0.96 (0.92–1.00) | 0.057 | 0.502 | |
| Diagnosis | ||||
| Othersa | ||||
| Breast | 0.38 (0.17–0.88) | 0.024 | 0.253 | |
| NHL | 0.44 (0.15–1.31) | 0.140 | 0.154 | |
Others refers to diagnoses other than breast cancer. This group served as the reference group in the analyses (HR = 1).
Fourteen patients in the expanded MTD cohort were evaluable for response (2 patients died before disease reevaluation posttransplant). The overall response rate was 29%, with 4 CR (29%); there were no PR. One additional patient achieved SD (7%). The 1-, 2-, 5-, and 9-year PFS rates were 19%, 6%, and 6%, respectively; the 1-, 2-, 5-, and 10-year overall survival rates were 44%, 25%, 25%, and 19%, respectively. Three patients remain alive 10 years posttransplant; one has been progression free since transplant.
Pharmacokinetics
The pharmacokinetic parameters for topotecan (lactone and total concentrations) are summarized in Table 4. There were no significant differences between day 8 and 6 parameters or between phase I and expanded MTD cohort patients; thus only day 8 results for phase I patients are shown. Maximum concentrations (Cmax) and AUC of topotecan (total) were positively correlated with dose level (r = 0.9 and 0.93, respectively, with both P < 0.0001). Clearance appeared to decrease with increasing dose level (r = −0.32; P = 0.04); however, this could have been due to lower variability in clearance at higher dose levels as there was substantial variability in clearance at the lower dose levels, most likely caused by concentrations that were below quantifiable limits of the assay. Therefore, the clinical significance of this finding is questionable. Similar relationships were seen for the lactone assays. Higher total topotecan AUCs were associated with increasing duration of grade 4 mucositis; time to platelet recovery was also longer in patients with higher AUCs. No correlation was found between any of the pharmacokinetic parameters and maximum reduction in topo I levels, as measured by the band depletion assay.
Table 4. Pharmacokinetic parameter medians for topotecan.
| Dose level | Daily dose, mg/m2 | Cmax, μmol/L (range) | T1/2, h (range) | Cl, L/h/m2 (range) | Vdss, L/m2 (range) | AUC, μmol/L × h (range) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| Total | Lactone | Total | Lactone | Total | Lactone | Total | Lactone | Total | Lactone | ||
| 1 | 3.3 | 0.18 | 0.18 | 2.7 | 2.6 | 12.97 | 27.95 | 49.2 | 56.6 | 0.56 | 0.26 |
| n = 4 | (0.14–0.26) | (0.14–0.20) | (1.7–5.1) | (0.6–4.8) | (11.50–21.67) | (21.28–63.95) | (31.1–72.4) | (28.8–81.6) | (0.33–0.63) | (0.11–0.34) | |
| 2 | 5.0 | 0.21 | 0.20 | 3.0 | 2.7 | 15.44 | 41.19 | 66.8 | 111 | 0.65 | 0.27 |
| n = 6 | (0.13–0.75) | (0.09–0.68) | (2.0–9.9) | (1.5–5.1) | (10.12–26.17) | (29.27–46.04) | (33.8–118) | (39.6–183) | (0.42–0.87) | (0.24–0.37) | |
| 3 | 6.8 | 0. 33 | 0.23 | 2.6 | 3.4 | 17.14 | 39.59 | 57.5 | 113 | 0.86 | 0.38 |
| n = 4 | (0.25–0.43) | (0.15–0.43) | (2.0–3.4) | (2.0–8.9) | (9.32–21.50) | (28.07–49.01) | (43.1–74.5) | (81.9–302) | (0.68–1.57) | (0.30–0.52) | |
| 4 | 8.9 | 0.52 | 0.48 | 3.3 | 3.1 | 12.05 | 28.1 | 53.7 | 91.6 | 1.63 | 0.70 |
| n = 5 | (0.34–1.1) | (0.34–0.89) | (2.9–4.2) | (2.6–4.0) | (10.14–23.96) | (25.61–46.73) | (39.1–79.7) | (67.9–120) | (0.82–1.93) | (0.42–0.77) | |
| 5 | 12.0 | 0.65 | 0.61 | 2.9 | 3.2 | 16.43 | 30.11 | 53.3 | 94.4 | 1.86 | 0.88 |
| n = 9 | (0.42–1.21) | (0.30–1.12) | (1.7–10.7) | (1.8–7.2) | (5.85–20.45) | (18.06–40.77) | (35.8–96.8) | (48.7–168) | (1.28–4.47) | (0.64–1.45) | |
| 6 | 15.7 | 0.87 | 0.80 | 3.1 | 3.3 | 12.62 | 23.53 | 48.1 | 76.6 | 2.77 | 1.48 |
| n = 5 | (0.84–1.47) | (0.63–1.46) | (2.3–4.1) | (2.0–3.9) | (9.61–16.90) | (20.38–34.04) | (34.5–65.7) | (54.3–114) | (2.07–3.64) | (1.03–1.71) | |
| 7 | 21.3 | 1.08 | 0.92 | 2.6 | 2.7 | 13.43 | 29.10 | 63.9 | 95.8 | 3.47 | 1.60 |
| n = 7 | (0.83–4.17) | (0.57–4.06) | (2.1–6.3) | (1.9–7.0) | (7.93–17.79) | (14.47–35.17) | (36.2–98.1) | (48.4–199) | (2.62–5.87) | (1.32–3.22) | |
| 8 | 28.4 | 2.12 | 1.67 | 2.4 | 2.3 | 10.78 | 25.77 | 41.1 | 81.5 | 5.73 | 2.40 |
| n = 5 | (1.32–3.03) | (1.21–2.70) | (2.0–5.8) | (2.1–4.4) | (6.20–12.34) | (20.39–27.85) | (38.3–64.0) | (41.2–107) | (5.02–9.98) | (2.22–3.03) | |
Note. Daily dose was given each day for 3 days.
Abbreviations: Cl, clearance; Cmax, maximal concentration; T1/2, half life; Vdss, volume of distribution.
Topo I activity and levels
Thirty-five patients on the phase I study had band depletion assays conducted before the start of their conditioning regimen. The median value for all patients was 4.23 μmol/L (range 1.7–40 μmol/L). Lower values of this variable indicate that lower concentrations of topotecan were required to stabilize cleavable complexes and thus induce cytotoxicity. To evaluate the results of the assay, we calculated the ratio of topotecan Cmax to the band depletion assay results, postulating that the higher the ratio, the more likely topotecan levels would be over the amount required to have a cytotoxic effect. Indeed, this ratio was found to be significantly correlated with the duration of grade 4 mucositis (Spearman correlation coefficient = 0.41; P = 0.016). There was no correlation between this ratio and PFS (HR = 2.99; 95% CI = 0.35–25.61; P = 0.32).
Baseline concentrations of topo I were measured in PBMCs of 34 phase I study patients (in arbitrary units: median 20,748; range 5,230–49,937). These concentrations were not significantly correlated with days of grade 4 mucositis or time to recovery of platelets or neutrophils. Topo I concentrations were also measured over time after the administration of topotecan. The maximum reduction in topo I levels did not seem to be correlated with either maximum concentrations or AUC of topotecan. We did not evaluate reduction in topo I levels as a function of time above a particular topotecan level.
Discussion
Several other phase I/II trials have evaluated topotecan in combination with other cytotoxic agents as conditioning therapy followed by autologous HCT. Schilder and colleagues treated 20 patients who had chemo-naïve solid tumors with 3 or 4 cycles of paclitaxel (250 mg/m2 over 24 hours), carboplatin (AUC 16), and topotecan (10–15 mg/m2 over 24 hours) supported by peripheral blood hematopoietic cells and G-CSF (18). Dose-limiting toxicities were hemorrhagic stomatitis and prolonged hematopoietic recovery. Median days to neutrophil and platelet recovery were similar to those seen in our study. The MTD of topotecan when given in 3 cycles was 12.5 mg/m2; 4 cycles could be given when topotecan was dosed at 10 mg/m2. Topotecan pharmacokinetics was linear over the dose range tested. Stomatitis was highly correlated with topotecan AUC (P = 0.005); 10 of 12 patients who developed grade 4 stomatitis had topotecan AUC of more than 1,000 ng hour/mL.
Three reports have been published by an MD Anderson group headed by Donato; this group evaluated the use of high-dose topotecan, melphalan, and cyclophosphamide as conditioning therapy before HCT in the treatment of ovarian cancer and multiple myeloma. In the first report, 53 patients were treated with topotecan (1.25 to 4 mg/m2/d from days −6 to −2), cyclophosphamide (1 g/m2/d on days -6 to -4), and melphalan (70 mg/m2/d on days −3 to −2; ref. 19). The dose selected for further testing was 4 mg/m2/d for 5 days. Toxicity was limited to mucositis and diarrhea. In the second report, 18 patients with multiple myeloma were treated with the same regimen except the dosing for topotecan was 3 to 3.5 mg/m2/d (20). Similarly, the toxicity was limited to mucositis and diarrhea. This series was recently updated to include 60 myeloma patients with 3.5 mg/m2/d for 5 days (21). The most common nonhematologic grade 3 and 4 toxicity was mucositis/stomatitis (22% of patients). Response rates and survival were comparable with local historical controls treated with high-dose melphalan as a single agent prior to HCT.
Lotz and colleagues evaluated escalating doses of single-agent topotecan with hematopoietic cell support. Topotecan was given at doses of 4 to 10 mg/m2/d for 5 days. Forty-nine patients were treated. Dose-limiting toxicity was diarrhea, and the MTD was determined to be 9.5 mg/m2/d × 5 days. A linear relationship between dose and AUC was observed (22). Litzow and colleagues from the Mayo Clinic dose escalated topotecan in combination with cyclophosphamide (1.5 g/m2/d) and carboplatin (200 mg/m2/d) all as 4-day continuous infusions prior to HCT in 16 patients with relapsed or persistent ovarian or primary peritoneal carcinoma (23). The initial dose of topotecan was 1.5 mg/m2/d for 4 days with subsequent escalation to 6 mg/m2/d. Pretreatment biopsies were analyzed for topo I by immunohistochemistry, and topotecan pharmacokinetics was also evaluated. The dose-limiting toxicity was severe stomatitis requiring parenteral nutrition and narcotic administration; this was seen at 6 mg/m2/d. These authors defined the MTD as 4.5 mg/m2/d. Median topotecan clearance was 184 mL/min/m2, which was very similar to the values reported in our evaluation. Also in concordance with our study was the finding that patients with tumors showing weak or undetectable topo I had a shorter median time to progression than patients with tumors having readily detectable topo I staining.
Single-agent topotecan was used in the second of 3 cycles of high-dose therapy followed by hematopoietic cell support in women with advanced ovarian cancer (24). The starting dose of topotecan was 5 mg/m2 given as a 72-hour continuous infusion starting on day 8. The largest dose given was 11 mg/m2, but the authors stated that the MTD was not reached. Grade 3 or 4 nonhematologic toxicity included mucositis and increased amylase. Steady-state topotecan levels were similar for the 5 and 7 mg/m2 doses. However, greater increases in steady-state levels were detected for the 9 and 11 mg/m2 dose levels, consistent with reports of nonlinear pharmacokinetics with high doses of topotecan administered by continuous infusion. The terminal half-life of total topotecan following the end of the 72-hour infusion ranged from 1.8 to 4.7 hours.
We determined the MTD of topotecan to be 64 mg/m2 when given over3daysincombination with ifosfamide and etoposide in the treatment of refractory and heavily pre-treated patients undergoing autologous HCT. This is higher than previous reports of topotecan given in this setting. The ability to dose escalate to this level may have been due to a variety of factors: greater threshold for mucositis as a dose-limiting toxicity, use in a different combination of chemotherapy agents, or difference in scheduling. As seen with other studies, gastrointestinal toxicity was dose limiting, and, in our study, the duration of grade 4 mucositis was significantly correlated with dose level of topotecan as were the number of days that TPN was administered and length of hospitalization. We were also able to show that engraftment of neutrophils and platelets was correlated with dose level; these correlations have not been previously reported in the high-dose setting, although others have shown similar correlations in the nontransplant setting (25, 26).
Others have shown a relationship between topotecan AUC and toxicity (18); our data also show that higher AUCs are associated with longer duration of severe mucositis as well as time to platelet recovery. Topotecan AUC appeared to be a significant negative predictor of overall survival in the univariate analysis. However, this effect was attenuated in the multivariate analysis. One explanation for this unexpected finding could be that patients having higher exposures to topotecan could have suffered subclinical toxicities, which would have prohibited effective therapy for post-transplant relapse, especially in this group of relatively heavily pretreated patients. Inability to receive salvage therapy would have affected overall survival, as the majority of these patients relapsed after transplant. Alternatively, this finding could have been an artifact of an unequal distribution of diagnoses across dose levels as diagnosis was a significant covariate in the multivariate analysis. We saw no benefit with respect to response rate or PFS with increasing topotecan AUC. A definitive dose–response relationship could not be established due to lack of power in this phase I trial. The recommended phase II dose is 64 mg/m2 topotecan. Consideration could be given to adding palifermin to attenuate mucositis.
The other significant predict or of overall survival (and the only independent predictor for PFS) was baseline topo I levels, suggesting that higher levels of the pharmacologic target of topotecan enhanced sensitivity. This observation should be viewed with caution, however, as topo I levels in our study were measured in PBMCs. Whether baseline topo I in cancer cells would also be predictive is only speculative based on our results. Litzow and colleagues were able to show an association between topo I expression in pretreatment biopsies and time to progression (23), whereas others have failed to show a correlation between higher topo I levels and clinical response (27). Baseline topo I levels were not associated with indicators of toxicity. However, we did see acorrelation between toxicity and the ratio of maximum topotecan levels and results of the band depletion assay.
During the phase I portion of the trial, we observed potential activity of this regimen in lymphoma patients and treated an expanded cohort of 16 patients at the MTD. We saw similar toxicity as that seen in the phase I study. The CR rate in this refractory disease population was 29% with a 2-year PFS of 13%. Because the regimen did not seem to be superior to currently used regimens with respect to toxicity or efficacy, further study of the regimen was not pursued.
Extensive work has been done by investigators at St. Jude's Children's Research Hospital using pharmacokinetic modeling to develop more effective scheduling of topotecan in the standard dose setting (28, 29). Their findings have shown that pharmacokinetically targeting topotecan is feasible and may lead to improved response rates in children with neuroblastoma. This same approach should be studied when topotecan is used in the high-dose setting due to the variability of topotecan exposure and increased likelihood of toxicity at higher systemic exposures. In addition, baseline topo I levels may have an important role in predicting the efficacy of topotecan and should be explored further. To this end, our transplant program is conducting a clinical trial of high-dose topotecan in combination with melphalan in the HCT setting for treatment of myeloma. Both topotecan pharmacokinetics and topo I levels are being evaluated with the aim of corroborating the findings of the ifosfamide, mesna, and etoposide (TIME) study.
Supplementary Material
Translational Relevance.
Preclinical and in vivo data support sequencing antitumor agents as alkylating agent → topoisomerase I inhibitor → topoisomerase II inhibitor. This paradigm was the basis for this phase I high-dose chemotherapy regimen in relapsed/refractory malignancies amenable to autologous stem cell transplantation. The topoisomerase I inhibitor topotecan given intravenously was dose escalated to a maximum tolerated dose of 64 mg/m2 given over three days (21.3 mg/m2/d). Mucositis was the dose-limiting toxicity and correlated with topotecan pharmacokinetics. Topoisomerase I levels in peripheral blood mononuclear cells correlated with overall and progression-free survival in univariate and multivariate analyses; higher levels predicted better survival. Our results show that topotecan in combination with high-dose etoposide (topoisomerase II inhibitor) and ifosfamide is tolerable, resulting in a response rate of 31% in the phase I part of this trial and 29% in the expanded cohort of lymphoma patients and 5-year overall survival rates of 18% and 25%, respectively.
Acknowledgments
The authors thank GlaxoSmithKline for partial clinical trial support and for providing topotecan for the clinical trial and also thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Kano Y, Suzuki K, Akutsu M, Suda K, Inoue Y, Yoshida M, et al. Effects of CPT-11 in combination with other anti-cancer agents in culture. Int J Cancer. 1992;50:604–10. doi: 10.1002/ijc.2910500420. [DOI] [PubMed] [Google Scholar]
- 2.Cheng MF, Chatterjee S, Berger NA. Schedule-dependent cytotoxicity of topotecan alone and in combination chemotherapy regimens. Oncol Res. 1994;6:269–79. [PubMed] [Google Scholar]
- 3.Goldwasser F, Valenti M, Torres R, Kohn KW, Pommier Y. Potentiation of cisplatin cytotoxicity by 9-aminocamptothecin. Clin Cancer Res. 1996;2:687–93. [PubMed] [Google Scholar]
- 4.Kaufmann SH, Peereboom D, Buckwalter CA, Svingen PA, Grochow LB, Donehower RC, et al. Cytotoxic effects of topotecan combined with various anticancer agents in human cancer cell lines. J Natl Cancer Inst. 1996;88:734–41. doi: 10.1093/jnci/88.11.734. [DOI] [PubMed] [Google Scholar]
- 5.Ma J, Maliepaard M, Nooter K, Boersma AW, Verweij J, Stoter G, et al. Synergistic cytotoxicity of cisplatin and topotecan or SN-38 in a panel of eight solid-tumor cell lines in vitro. Cancer Chemother Pharmacol. 1998;41:307–16. doi: 10.1007/s002800050744. [DOI] [PubMed] [Google Scholar]
- 6.Whitacre CM, Zborowska E, Gordon NH, Mackay W, Berger NA. Topotecan increases topoisomerase II α levels and sensitivity to treatment with etoposide in schedule-dependent process. Cancer Res. 1997;57:1425–8. [PubMed] [Google Scholar]
- 7.Grabowski D, Ganapathi R. Cytotoxic efficacy with combinations of topoisomerase I and topoisomerase II inhibitors in sensitive and multidrug-resistant L1210 mouse leukemia cells. Ann N Y Acad Sci. 1996;803:306–7. doi: 10.1111/j.1749-6632.1996.tb26400.x. [DOI] [PubMed] [Google Scholar]
- 8.Fields KK, Elfenbein GJ, Lazarus HM, Cooper BW, Perkins JB, Creger RJ, et al. Maximum tolerated doses of ifosfamide, carboplatin, and etoposide given over six days followed by autologous stem cell rescue: toxicity profile. J Clin Oncol. 1995;13:323–32. doi: 10.1200/JCO.1995.13.2.323. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan DM, Partyka JS, Hernandez AN, Trudeau WL, Fields KK, Goldstein SC, et al. Phase I/II study of intensive dose topotecan (TPT), ifosfamide (IFOS)/mesna and etoposide (TIME) followed by autologous stem cell rescue in refractory malignancies [abstract]. Proceedings of the 89th AACR Annual Meeting; 1998; New Orleans, LA: AACR. 1998. Abstract nr 2212. [Google Scholar]
- 10.Sullivan DM, Partyka JS, Hernandez AN, Trudeau WL, Fields KK, Goldstein SC, et al. A phase I/II study of intensive-dose topotecan (TPT), ifosfamide (IFOS)/mesna and etoposide (TIME) followed by autologous stem cell rescue in refractory malignancies [abstract]. Proceedings of the 35th ASCO Annual Meeting; 1999; Atlanta, GA. 1999. Abstract nr 171. [Google Scholar]
- 11.Sullivan D, Partyka J, Fields K, Goldstein S, Field T, Djulbegovic P, et al. A phase I study of high-dose topotecan, ifosfamide/mesna and etoposide (TIME) followed by autologous stem cell rescue in refractory malignancies (Abstract) Exp Hematol. 2000;28:248. [Google Scholar]
- 12.Minami H, Beijnen JH, Verweij J, Ratain MJ. Limited sampling model for area underthe concentration time curve of total topotecan. Clin Cancer Res. 1996;2:43–6. [PubMed] [Google Scholar]
- 13.Loos WJ, Stoter G, Verweij J, Schellens JH. Sensitive high-performance liquid chromatographic fluorescence assay for the quantitation of topotecan (SKF 104864-A) and its lactone ring-opened product (hydroxy acid) in human plasma and urine. J Chromatogr B Biomed Appl. 1996;678:309–15. doi: 10.1016/0378-4347(95)00529-3. [DOI] [PubMed] [Google Scholar]
- 14.Beijnen JH, Smith BR, Keijer WJ, van Gijn R, ten Bokkel Huinink WW, et al. High-performance liquid chromatographic analysis of the new antitumour drug SK&F 104864-A (NSC 609699) in plasma. J Pharm Biomed Anal. 1990;8:789–94. doi: 10.1016/0731-7085(90)80122-6. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann SH, Svingen PA, Gore SD, Armstrong DK, Cheng YC, Rowinsky EK. Altered formation of topotecan-stabilized topoisomerase I-DNA adducts in human leukemia cells. Blood. 1997;89:2098–104. [PubMed] [Google Scholar]
- 16.Engel R, Valkov NI, Gump JL, Hazlehurst L, Dalton WS, Sullivan DM. The cytoplasmic trafficking of DNA topoisomerase IIα correlates with etoposide resistance in human myeloma cells. Exp Cell Res. 2004;295:421–31. doi: 10.1016/j.yexcr.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. [Google Scholar]
- 18.Schilder RJ, Gallo JM, Millenson MM, Bookman MA, Weiner LM, Rogatko A, et al. Phase I trial of multiple cycles of high-dose carbo-platin, paclitaxel, and topotecan with peripheral blood stem cell support as front-line therapy. J Clin Oncol. 2001;19:1183–94. doi: 10.1200/JCO.2001.19.4.1183. [DOI] [PubMed] [Google Scholar]
- 19.Donato ML, Gershenson DM, Wharton JT, Ippoliti CM, Aleman AS, Bodurka-Bevers D, et al. High-dose topotecan, melphalan, and cyclophosphamide (TMC) with stem cell support: A new regimen for the treatment of advanced ovarian cancer. Gynecol Oncol. 2001;82:420–6. doi: 10.1006/gyno.2001.6326. [DOI] [PubMed] [Google Scholar]
- 20.Donato ML, Aleman A, Champlin RE, Weber D, Alexanian R, Ippoliti CM, et al. High dose topotecan, melphalan and cyclophosphamide (TMC) with stem cell support: a new regimen for the treatment of myeloma. Leuk Lymphoma. 2004;45:755–9. doi: 10.1080/10428190310001603957. [DOI] [PubMed] [Google Scholar]
- 21.Kazmi SM, Saliba RM, Donato M, Wang M, Hosing C, Qureshi S, et al. Phase II trial of high-dose topotecan, melphalan, and CY with autol-ogous stem cell support for multiple myeloma. Bone Marrow Transplant. 2011;46:510–5. doi: 10.1038/bmt.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lotz JP, Pautier P, Selle F, Viens P, Fabbro M, Lokiec F, et al. Groupe d′ Intensification des traitements des Tumeurs Ovariennes (ITOV Group) Phase I study of high dose topotecan and haematopoietic stem cell support in the treatment of ovarian carcinomas: the ITOV 01 protocol. Bone Marrow Transplant. 2006;37:669–75. doi: 10.1038/sj.bmt.1705310. [DOI] [PubMed] [Google Scholar]
- 23.Litzow MR, Peethambaram PP, Safgren SL, Keeney GL, Ansell SM, Dispenzieri A, et al. Phase I trial of autologous hematopoietic SCT with escalating doses of topotecan combined with Cy and carboplatin in patients with relapsed or persistent ovarian or primary peritoneal carcinoma. Bone Marrow Transplant. 2010;45:490–7. doi: 10.1038/bmt.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiersten A, Selleck M, Smith DH, Wertheim I, Kaufman E, Hershman D, et al. Phase I/II study of tandem cycles of high-dose chemotherapy followed by autologous hematopoietic stem cell support in women with advanced ovarian cancer. Int J Gynecol Cancer. 2006;16:57–64. doi: 10.1111/j.1525-1438.2006.00278.x. [DOI] [PubMed] [Google Scholar]
- 25.Cooper BW, Veal GJ, Radivoyevitch T, Tilby MJ, Meyerson HJ, Lazarus HM, et al. A phase I and pharmacokinetic study of fludarabine, carboplatin, and topotecan in patients with relapsed, refractory, or high-risk leukemia. Clin Cancer Res. 2004;10:6830–9. doi: 10.1158/1078-0432.CCR-04-0097. [DOI] [PubMed] [Google Scholar]
- 26.Furman WL, Baker SD, Pratt CB, Rivera GK, Evans WE, Stewart CF. Escalating systemic exposure of continuous infusion topotecan in children with recurrent acute leukemia. J Clin Oncol. 1996;14:1504–11. doi: 10.1200/JCO.1996.14.5.1504. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann SH, Karp JE, Letendre L, Kottke TJ, Safgren S, Greer J, et al. Phase I and pharmacologic study of infusional topotecan and carboplatin in relapsed and refractory acute leukemia. Clin Cancer Res. 2005;11:6641–9. doi: 10.1158/1078-0432.CCR-05-0817. [DOI] [PubMed] [Google Scholar]
- 28.Panetta JC, Schaiquevich R, Santana VM, Stewart CF. Using pharmacokinetic and pharmacodynamic modeling and simulation to evaluate importance of schedule in topotecan therapy for pediatric neuroblastoma. Clin Cancer Res. 2008;14:318–25. doi: 10.1158/1078-0432.CCR-07-1243. [DOI] [PubMed] [Google Scholar]
- 29.Schaiquevich P, Panetta JC, Iacono LC, Freeman BB, III, Santana VM, Gajjar A, et al. Population pharmacokinetic analysis of topotecan in pediatric cancer patients. Clin Cancer Res. 2007;13:6703–11. doi: 10.1158/1078-0432.CCR-07-1376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.