Abstract
Sleep medicine in general and psychology in particular have recently developed cognitive behavioral treatment for narcolepsy (CBT-N). Despite a growing interest in this topic, most studies since 2007 have reviewed CBT applications for other sleep disorders. Currently, 6 reviews have been published on narcolepsy, with an expert consensus being reached that CBT represented an important adjunctive treatment for the disease. The current paper reviews the need for CBT applications for narcolepsy by generalizing the application of multicomponent treatments and performing studies that extrapolate the results obtained from multicenter studies. Nineteen studies were found in which the need-for-treatment guidelines identified the use of CBT for narcolepsy. Three additional studies were identified that evaluated the effectiveness of cognitive behavioral measures and multicomponent treatments for which treatment protocols have been proposed.
Keywords: Cognitive behavioral treatment, Narcolepsy, Behavior modification, Clinical formulation, Non-pharmacological treatment
1. Introduction
Narcolepsy is a disabling sleep disorder that significantly affects overall patient functioning. One study reported that this disease affects 1 in 2000 individuals [1]. Narcolepsy consists of a set of core symptoms known by many authors as “narcoleptic tetrad”. These symptoms include excessive daytime sleepiness (EDS; i.e., sudden sleep attacks during the day), cataplexy (i.e., the loss of muscle tone during intense emotions), sleep paralysis (i.e., feeling unable to move when waking up), hypnagogic hallucinations (i.e., active hallucinations prior to the onset of sleep), and dream pattern alterations [2] as well as secondary symptoms that accompany the disorder, such as automatic behaviors and cognitive deficits [3–6].
Different consequences of narcolepsy have been described [7,8]. Because this disease is chronic, patients and their families have trouble coping with it. Furthermore, narcolepsy is associated with an increased risk of work-related and transit accidents [9], sexual dysfunctions [10], neuropsychological alterations [9,11,12] (increased reaction time, decreased executive functioning), and an overall significant reduction in quality of life [10,13–15].
The treatment of choice for narcolepsy consists of prescribing stimulants to control EDS and antidepressants to treat parasomnias and associated cataplexy. Drug therapy has been highly recommended and supported by well-designed research that shows its effectiveness.
Conversely, the implementation of CBT (i.e., the systematic application of the principles and learning techniques needed to evaluate and improve behavior) has not been well studied with regard to narcolepsy, most likely because
-
(a)
the studies performed have specific methodological weaknesses or are case reports;
-
(b)
the low prevalence of narcolepsy draws little attention from psychologists; and
-
(c)
psychologists have limited interest in working with patients who have diseases that “are the exclusive domain of medical doctors”.
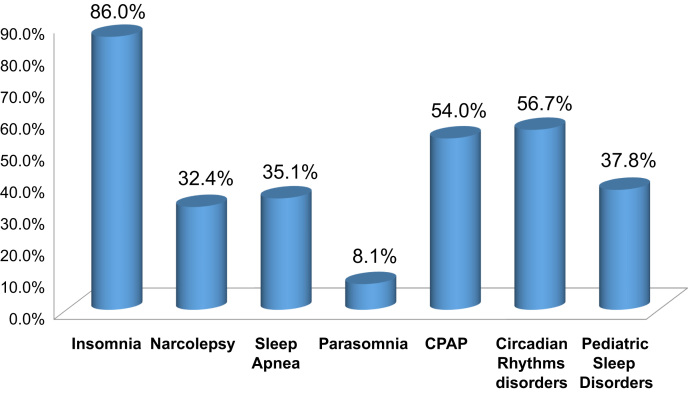
As Fig. 1 shows, however, 32.4% of psychologists who are trained in CBT are interested in applying it to cases of narcolepsy (American Association of Sleep Medicine AASM, [16]). This figure is similar to the interest in the use of CBT for other sleep disorders (e.g., sleep-disordered breathing and circadian rhythm disorders), and is surpassed only by insomnia.
Fig. 1.
Training rates by interest in cognitive behavioral intervention for sleep disorders in the United States 2007.
In another important sense, the control of hypersomnia through behavioral interventions was included in the conference organized by the American Society of Behavioral Sleep Medicine. Thus, a growing interest exists in developing comprehensive and effective treatments for narcolepsy.
The primary aim of this review is to describe the background regarding the use of CBT for patients with narcolepsy. It also seeks to advance specific proposals and questions, putting forward an agenda concerning where research on this subject should be directed.
2. Methodology
A search was conducted using MEDLINE, PsycINFO, ScienceDirect, and Springer databases as well as conference proceedings and relevant journals. Several key sources of non-indexed literature were also searched.
The papers cited in the reference lists of previous reviews on this topic were also examined. CBT and narcolepsy were used as keywords. When necessary, certain authors and associations were contacted for additional published and unpublished materials. In all, 30 papers were collected, with an additional 7 which were discarded because they were not related to CBT.
The remaining papers were classified based on the type of study and behavioral measures used as well as the sample, method, and results.
An assessment of the level of evidence was not included because some studies were not adequate. Thus, descriptions of the studies and the relevant conclusions with regard to their quality, conclusions, and contributions were preferred.
The analysis did not examine a study design effect because of the disparity between the measures and methods used across the papers included.
3. Discussion
3.1. Explanatory psychological models of narcolepsy
During this review, two theoretical psychological models were identified that provide the rationale for CBT’s use for narcolepsy.
The cognitive-behavioral model of narcolepsy first identifies the basic elements which make up the clinical syndrome, and then describes the causal factors (both intrinsic and extrinsic) that either precede or modify the production of symptoms [17].
This model provides the basis for designing a structured intervention program by identifying the interaction between external risk factors (i.e., those associated with disease symptoms that might be precipitants and triggers) and internal risk factors (i.e., predictors of narcolepsy such as biological factors, orexinergic neuron deficits, and so on). The model presumes that the internal factors (i.e., predisposing factors) are constant while it is the external risk factors which chronically maintain the illness.
Cognitive factors are also taken into account. The model notes that while some individuals may manifest symptoms under specific circumstances, others might be asymptomatic in the presence of the same stimuli. It attributes these response differences to the unique individual learning histories whereby some patients are able control their cataleptic symptoms because they have learned to regulate their emotions. Thus, while recognizing the important contribution of precipitating factors (e.g., conditions that trigger cataplexy, passive situations, sleepiness, sleep deprivation, and so on) to the overall illness, the model also permits the possibility of symptom modification through learning.
The second model [18] attempts to explain the relationship between REM sleep disorders and cataplexy. This model assumes that REM sleep is an anti-stress filter that is affected by narcolepsy, such that it does not fully filter the activating information of these processes, which thereby increase as a result of the occurrence of high emotional arousal that cannot be processed. As an incomplete survival process, the body acts in the same way during sleep (but not in a waking state) to counteract the anti-stress production deficit that generates cataplexy during an emotional event.
In this model of narcolepsy, afferent sensations of the patient׳s emotional state cause catalepsy, similar to what would happen in extreme situations (e.g., similarly to paralysis resulting from intense fear, patients with narcolepsy want to move but cannot). Patients with narcolepsy cannot perform movements after the initial paralysis. This suggests that a type of neuronal interference may be present, in which prior programming blocks later processing; this in turn inhibits or prevents motor movements.
3.2. Consensus, expert reviews, and clinical evidence
The literature reports that stimulants are the treatment of choice for narcolepsy; however, the clinical guidelines of several countries recommend the use of cognitive and behavioral measures as adjunctive therapies to lessen the effect of overall patient dysfunction and obtain the best response with the lowest possible prescription dosage.
3.2.1. Consensus in Latin America
No formal consensus exists in Latin America concerning approaches to narcolepsy compared with Europe and the United States. Only the Brazilian Society of Sleep Medicine has provided an official set of guidelines on this subject (in 2009 and 2010) [19,20].
These guidelines recommend that clinicians obtain 4 and 5 types of evidence (e.g., non-randomized controlled trials, retrospective case reports, clinical case studies, or expert consensus) prior to rendering a diagnosis or initiating therapy. Therapeutic strategies emphasize behavioral interventions such as CBT sleep hygiene recommendations (based on the literature and clinical experience of a specialist committee and approved by majority vote) Additionally, it is recommended that clinicians prescribe a regimen of patient naps, employ psychosocial measures (e.g., adapt work shifts based on specific patient needs), and administer psychological support therapy.
3.2.2. Consensus in Europe
Two consensuses were found in Europe. The following measures are recommended in the United Kingdom [21]: education regarding the disease (grade C, level IV), scheduled naps (grade B, level IIb), nocturnal sleep duration (grade C, level IV), support in planning work (grade C, level IV), and psychosocial support (grade C, level IV).
In most cases, the “levels of evidence” refer to expert-obtained consensus (except with regard to naps); levels III and IV refer to evidence obtained from non-experimental studies (except IIb, which refers to quasi-experimental studies).
The second consensus [22], reached by the European Association of Neurology in 2006, presents a symptomatological approach that cites behavioral measures to manage narcolepsy in the same manner.
This consensus considers the role of naps with levels of evidence II and III and recommendation grade B. With regard to cataplexy, the aim is to avoid precipitating factors and increase social contact (but without grades of recommendation). These measures and other psychological interventions were not reviewed nor recommended, because they lacked support from well-controlled studies, although they were used for other symptoms.
3.2.3. Consensus in North America
The 2007 consensus and review of the general management of narcolepsy by the American Academy of Sleep Medicine [23] considered only one study with evidence level II behavioral measures.
This consensus did not describe behavioral measures for other symptoms. Importantly, however, they highlighted the need for more evidence-based studies to improve the quality of life of patients with narcolepsy who are undergoing drug treatment.
3.3. Narcolepsy reviews
In addition to the foregoing consensus statement, six reviews of behavioral treatments for narcolepsy were found.
The first review [24] described non-pharmacologic interventions, such as behavior modification (e.g., planning structured sleep schedules, naps, dietary measures, and support with regard to issues related to mental and physical health to address social factors).
Another study surveyed practitioners and found that 63.2% used behavioral to reduce cataplexy, 54.5% did so for sleep attacks, and 35.5% did so for EDS [25]. The management in the survey of specific coping strategies was included, which augmented the behavioral measures as measured by reductions in drug treatments.
The third review was published in 2003 [26]. This paper stressed the importance of sleep hygiene, scheduled naps, and their use in combination with pharmacological strategies. Moreover, dietary recommendations and other psychological techniques, such as hypnosis and progressive muscle relaxation, were included. In addition, this review was the first to report effects similar in size to meta-analyses in two studies conducted with regard to scheduled naps, where the effects were 0.15 for short naps, 0.43 for 15 min, and 0.7 for long naps. These results indicate that long naps had a greater effect on the control group in studies that used this measure for naps.
The fourth review [27] addressed the behavioral management of narcolepsy, making recommendations for the symptomatic treatment of nocturnal and daytime symptoms. This review regarded behavioral management of narcolepsy as being important for different reasons. First, although symptoms are usually well controlled with medications, which causes severe psychosocial distress, EDS remains present in most cases. Second, behavioral measures should assist the patient in gaining some control of the disease to reduce its effect on quality of life.
This review classified measures as either nocturnal or diurnal and investigated the studies that addressed each type.
Finally, the fifth review [28] argued that the central goal of behavioral measures must point to the involvement of many psychosocial problems commonly experienced by patients with narcolepsy. The review recognized that a shortage of controlled studies existed which focused on examining to examine the effectiveness of non-pharmacologic measures. Furthermore, it suggested that despite this deficiency, the use of these therapies was increasing, with approximately 15% of UK physicians reporting that they had used them, either as an adjuvant or a first-choice treatment, and further reporting specific benefits following their use.
Overall, the reviews recognized that a number of behavioral therapies were being increasingly used, and were coming to be regarded as key adjunctive elements in comprehensive treatment programs for narcolepsy (See Table 1).
Table 1.
Behavioral approaches to treat narcolepsy, adapted from Monderer [28].
| Technique used | Description of the technique |
|---|---|
| Structuring nocturnal sleep habits | Maintain a structured sleep schedule and set time according to need, despite the quality or continuity of nocturnal sleep |
| Avoid sleep deprivation and changes in sleep time; maintain a regular schedule of nocturnal sleep (e.g., from 10:30 p.m. to 7 a.m.) | |
| If patients awaken during the night and have difficulty returning to sleep, then they can take a short break and perform a sedentary activity such as reading for a short period of time. However, they should return to bed and attempt to sleep | |
| Relaxation techniques before nocturnal sleep prevent intense stimulation before sleep | The estimated time to sleep at night should be 8 h or more, depending on individual differences |
| Planning naps | Naps during the day are a fundamental aspect of the treatment of the daytime sleepiness associated with narcolepsy. Naps can range from 15 to 20 min to over 1 h. For many patients, short naps (<30 min) are helpful, but others require longer naps |
| Fifteen-minute naps between 12:30 p.m. and 5:00 p.m. are highly effective | Overall, people with narcolepsy show no significant effects related to sleep inertia after taking a nap; however, if the duration of the nap is longer (>15 min), then it does not provide additional benefits. |
| Take 15-min naps | A single nap (or even two) benefits virtually all patients with narcolepsy |
| Plan nap strategies before using drugs | Moreover, adding a brief morning nap can reduce deterioration in the morning (i.e., continual performance decreases since waking). |
| Diet | Little is known regarding the effects of diet with regard to alertness and sleep among patients with narcolepsy; overall, however, healthy eating habits are useful to ensure sleep hygiene |
| Over-the-counter stimulants (e.g., tea, coffee, mate, and so on) should only be used on a planned schedule and according to doctor׳s recommendations | |
| The caffeine content of six cups of strong coffee has the same stimulating effect as 5 mg of dexamphetamine | Certain over-the-counter stimulants (e.g., tea and coffee) are not accepted drug treatments; thus, these drinks should be consumed responsibly to allow for more accurate schedule tracking, and they should be alternated with accepted drug treatments |
| Sweets and carbohydrates should be avoided from the time of awakening in the morning until 12:00 noon. | |
| Abstinence or minimal use of alcohol | |
| Avoidance of REM suppressants and drugs that increase daytime sleepiness | |
| Counseling or other assistance | A recent study revealed that over 500 patients with narcolepsy suffered from declining quality of life, which is similar to the experience of patients with Parkinson׳s disease. Special considerations at work or school are required for most patients with narcolepsy |
| Counseling for lifestyle reorganization | It is extremely difficult for patients with narcolepsy who work late shifts or have changes in their working hours to maintain work productivity. Work during the day is highly recommended |
| Counseling to review the type of work or individual or group psychotherapy | |
| Help with programming the mental alertness required by everyday activities | Advice concerning the psychosocial effects of this syndrome should be provided so that patients can optimize their adaptation to the disease and are realistic in their expectations when making decisions regarding daily activities |
| Professional advocacy for office workers |
3.4. Studies reviewed
Analyses were performed on the studies found in the databases and strategies were reviewed regarding the use of behavioral techniques to address narcolepsy. The following 19 behavioral studies were found (see Table 2).
Table 2.
Non-pharmacological therapies (including CBT) to treat narcolepsy.
| Technique | Study | Year | Sample | Procedure | Results |
|---|---|---|---|---|---|
| Sleep satiation | Volk S, Schulz H, Yassouridis A, Wilde-Frenz J, Simon O [29] | 1990 | N=14 | • Thirty-two hours of polygraphic recording | • Different amounts of slow-wave sleep (SWS) |
| • Patients were free to nap whenever they wanted. | • Decreased SWS compared with the control group | ||||
| • Patients remained at rest in bed, sleeping between 2–3 times more during the day than the control group. | • Intact SWS homeostatic regulation in patients with narcolepsy | ||||
| Hishikawa Y, Wakamatsu H, Furuya E, Sugita Y, Masaoka S, Kaneda H, Sato M, Nan׳no H, Kaneko Z [30] | 1976 | N=20 | • Polysomnogram for one night and the next day | • Not significantly different from the control group | |
| Yuchiyama MG, Mayer G, Meier-Ewert K [31] | 1994 | N=10 | • Sleep program for 2 weeks (nocturnal sleep period: 10:00 p.m. to 6:00 a.m. and one nap during the day) | • Extending sleep latencies >10 minutes compared with baseline | |
| • Reference PSG | • Longer latencies; positive correlations with sleep duration of N3; 54% found another PSG record the next morning | ||||
| • Multiple sleep latency test (MSLT; lying in bed for 20 minutes with lights out at 9:30 a.m., 11:30 a.m., 1:30 p.m., 3:30 p.m., and 5:30 p.m.. | |||||
| Billiard M [32] | 1976 | N=5 | • Case-control study; sleep time scheduling and measurement of NREM and REM sleep ratio recoveries | • No differences were found with regard to the restoration of the REM and NREM sleep ratios. | |
| • Alertness improved | |||||
| Scheduled naps | Helmus T, Rosenthal L, Bishop C, Roehrs T, Syron ML, Roth T [33] | 1997 | N=11 | • One night of 8 hours of sleep | • Sleep-deprived participants improved performance with a 120-minute nap. |
| • Two nap conditions: 15 or 120 minutes | • Patients with narcolepsy were benefitted by naps spaced between 15 minutes and three hours. | ||||
| • Sleep-deprived participants and patients with narcolepsy | |||||
| Rogers AE, Aldrich MS [34] | 1993 | N=16 | • ABA design (assessment and post-intervention assessment) in which measurement was performed before, during, and after using a program of regular naps | • Sleep latency increased significantly after 1 month of therapy. | |
| • No sleeping habits or medication changes during the study | • No significant changes were observed in the frequency of sleep attacks or the severity of other symptoms. | ||||
| • Participants who reported more severe symptoms and had taken more daily naps prior to the study period received the greatest benefit from treatment. | |||||
| Mullington J, Broughton R [35] | 1993 | N=8 | • Experimental study | • One 180-minute nap added to nocturnal sleep improved sustained performance compared with no nap. | |
| • Long-period sleep | • Performance response time significantly improved with a long nap. | ||||
| • Short naps for 24 hours | • The greatest improvements were with regard to implementing tasks in the afternoon and evening. | ||||
| • Measurement of performance tests | |||||
| • EEG endpoint | |||||
| Roehrs T, Zorick F, Wittig R, Paxton C, Sicklesteel J, Roth T [36] | 1986 | N=45 | • Case-control study; the controls were patients with EDS and other sleep disorders (SDs) | • A 15-minute nap at 4:00 p.m. (condition 1) | |
| • Patients were assigned to three nap conditions | ○ Increased latency at stage 1 sleep within a 15-minute latency test. | ||||
| • MSLT | ○ The largest increase was found for patients with narcolepsy. | ||||
| • A 30-minute nap at 4:00 p.m. (condition 2) | |||||
| ○ Increased latency of 15 minutes | |||||
| ○ The increase was greatest for patients with other SDs | |||||
| Rogers AE, Aldrich MS, Lin X [37] | 2001 | N=29 | Randomized into three treatment groups | • The addition of two 15-minute naps does not alter symptom severity or the duration of unscheduled daytime sleep. | |
| • (1) Two 15-minute naps per day | • Standard hours of reduced nocturnal sleep reduce the perceived severity of symptoms but do not reduce the amount of unscheduled daytime sleep. | ||||
| • (2) A regular schedule of nocturnal sleep | • Only the combination of scheduled naps and the regulation of nocturnal sleep hours significantly reduced symptom severity and the amount of unscheduled daytime sleep among participants treated with narcolepsy. | ||||
| • (3) A combination of bedtime and scheduled naps | • Regular sleep periods are useful only for those patients who stay deeply asleep despite stimulant medications; these drugs should not be prescribed for all patients with narcolepsy. | ||||
| • Symptom severity was measured at the start and end of a two-week treatment. | |||||
| Hypnosis | Nardi TJ [38] | 1981 | N=2 | • Case studies | • Data monitoring suggests that this approach might reduce the frequency of sleep paralysis attacks among patients with narcolepsy. |
| • Self-hypnosis to desensitize patients from the anxiety that accompanies sleep paralysis | |||||
| • Self-hypnosis also provides a means of ending sleep paralysis attacks. | |||||
| Price R [39] | 1981 | N=1 | • Case report | • A follow-up assessment at two months showed improvement in symptoms of sleepiness. | |
| Schneck JM [40] | 1980 | N=1 | • Case report | • The hypnotic measures in combination with self-instruction techniques decreased sleep attacks among patients. | |
| • Hypnotherapy protocol | |||||
| • Measures including post-hypnotic suggestions for voluntary and automatic hand movements, acting as signals to prevent sleep attacks | |||||
| Lucid dreams | Brulowski A [41] | 1987 | N=1 | • Case study; one-month training in lucid dreaming | • The patient reported a decrease in sleep paralysis and hypnagogic hallucinations. |
| • Non-significant decrease in sleepiness | |||||
| Cognitive therapy | Rogers AE [42] | 1984 | N=12 | • Advice on problem solving | • Better adherence to drug treatment |
| Bruck D, Broughton R [43] | 2001 | N=20 | • Sleep- and wake-cycle control analysis | • Identification of factors to control and regulate the sleep/wake cycle | |
| • Scheduling of productivity for circadian levels of alertness. | |||||
| Chen SY, Clift SJ, Dahlitz MJ, Dunn G, Parkes JD [44] | 1995 | N=124 | • Analysis of sleepiness after treatment with dexamphetamine and clomipramine | • Little therapeutic efficacy and frequent drug side effects compared with those receiving counseling regarding adherence to treatment. | |
| • Monitored using the Epworth sleepiness scale and the scale of postural tone | |||||
| Wilson SJ, Frazer DW, Lawrence JA, Bladin PF [45] | 2007 | N=33 | • Qualitative psychosocial assessment with a semi-structured validated interview | • Better psychosocial adjustment after treatment compared with controls (P<0.05) | |
| • Quantitative measures of anxiety (State-Trait Anxiety Inventory) and depression (Beck Depression Inventory-II) were also administered. | |||||
| • Structured program of psychosocial adjustment. | |||||
| Muscle relaxation | Kolko DJ [46] | 1984 | N=1 | • Behavioral treatment consisting of progressive muscle relaxation training | • Improvement was observed in |
| • Fluid restriction | ○ Sleep attacks at home | ||||
| • The use of a rubber band to hit one׳s wrist to facilitate arousal during the day. | ○Sleep attacks while driving | ||||
| ○ Episodes of cataplexy | |||||
| ○ Nocturnal awakenings | |||||
| ○ Nocturnal enuresis | |||||
| • Results were maintained at 6- and 12-month follow-up assessments. | |||||
| • The integration of medical and behavioral strategies was highlighted. | |||||
| Diet | Pollak CP, Green J [47] | 1990 | N=6 | • Case-control study | • Participants with narcolepsy ate more frequently than controls when meals were available on a 24-hour schedule. |
| • Laboratory without temporal cues | • Food intake among narcoleptic participants was preceded by a decreased 90-minute nap and a state of greater subjective arousal when meals were scheduled | ||||
| • Food was given immediately after awakening | |||||
| ○ Breakfast one to two hours after awakening | |||||
| ○ A snack before bedtime | |||||
| ○ Lunch and dinner at equal intervals after breakfast |
Seven behavioral intervention techniques were identified within the above 19 studies. These methods converge with regard to the study quality but not quantity of the five tests listed in Table 1.
The first technique described is sleep satiation, which consists of the scheduled extension of nocturnal sleep and increasing equilibrium during the day. This technique, which is based on the theory of sleep homeostasis, is effective for certain patients when combined with other measures.
Importantly, the studies reviewed differ with regard to the benefits provided to patients with narcolepsy because improvement is typically found with regard to alertness during the day but not the quantity and quality of sleep.
Short naps benefited certain patients, whereas long naps resulted in increased nocturnal sleep in others. Overall, a clear consensus exists with regard to scheduling 15-min naps in the afternoon depending on the difficulty and level of disruption that patients experience.
This nap schedule has also led to a considerable decrease in the consumption of drugs among patients with narcolepsy [37].
In three of the cases reported, hypnosis showed some level of effectiveness; however, the description of the protocols used in each study differed. A clear hypnosis protocol has not been established to address narcolepsy. Furthermore, control studies are lacking that discriminate whether it is hypnosis itself or the sleep induced by hypnosis that improves patient functioning.
In addition, lucid dreaming decreased episodes of hypnagogic hallucinations; however, this technique should be used with caution because of the sleep fragmentation caused by training in this technique.
The studies using cognitive behavioral techniques showed that education about the disease and psychosocial support were associated with increased adherence to drug treatment and improved patient quality of life.
The progressive muscle relaxation and additional measures used in one case report were useful in addressing an acute problem, but this study did not yield significant information regarding the duration of the effect of these techniques. Thus, a larger controlled study should be performed to test their effectiveness.
Finally, healthy eating is important, but more studies are needed to empirically support its effectiveness. Overall, studies have addressed the symptoms of narcolepsy, but they are not designed to extrapolate their results to the patient׳s daily life and are limited in use. However, naps are useful.
The behavioral measures reported here are not meant for a multi-component or systematic approach. Furthermore, they lack cognitive-behavioral theoretical foundations; for this reason, it is understandable that they have not been considered the treatments of choice in consensus agreements, although experts continue to have positive expectations regarding these method as a result of their experience and intuition.
Against this background, the question becomes, “Where should the aims of psychological interventions for narcolepsy be directed?”
In contrast to CBT for insomnia and the growing interest in the application of CBT for sleep-disordered breathing (as in the case of narcolepsy), few advances have been made. In a recent review Conroy et al. [51] summarized a number of studies that had used non-pharmacological therapies for managing hypersomnia. Although not focused specifically on narcolepsy, the review described the functional aspects of the techniques used for EDS intervention. Among the referenced therapies were general sleep hygiene techniques, physical exercise, distraction techniques, and naps; information was gathered from a previous consensus. Also included were specific cognitive-behavioral measures such as behavioral analysis, behavior experiments, motivational therapy, and psychoeducation. In these studies, however, the levels of evidence to help understand this problem were not reported.
3.5. What is necessary to know regarding cognitive behavioral therapy for narcolepsy (CBT-N)?
The studies that have been reported thus far have used a variety of techniques and programs to treat other sleep and mood disorders with physiological etiologies. An appropriate clinical formulation should be established initially to serve as a starting point for preparing formal (multi-component) protocols because of the multi-symptomatic manifestation of narcolepsy and the multiple effects on patient quality of life.
From this point of view, (a) CBT for narcolepsy (CBT-N) should start from a clinical formulation based on symptoms and their effects on social life; (b) the intervention process should be contextualized according to the patient and not preclude a systematic follow-up; and (c) CBT should have elements common to any psychological treatment.
3.5.1. Characteristics of CBT-N
Based on previous findings, it is proposed that CBT for narcolepsy should have three components.
-
(a)
The behavioral component begins with specific techniques aimed at changing sleep-disordered behaviors or sleep-related disorder variables that are not compatible (e.g., sleep satiation and nap training).
-
(b)
The cognitive component is aimed at modifying beliefs, motivations, and emotions that might play an important role in maintaining narcolepsy (thereby increasing the frequency of symptoms) and emphasizing the psychosocial effect of the disorder.
-
(c)
The educational component seeks to instruct the patient regarding the nature of the disease, the mechanism of drug action, and precautions regarding the use of medication to achieve an overall understanding of the problem. The acceptance of treatment and the therapeutic relationship become important aspects for treatment success, and the use of strategies becomes a type of therapeutic contract.
The full protocol assumes the use of psychometric assessment self-report strategies that facilitate follow-up evaluations of their effectiveness, in addition to the objective measures used in sleep medicine.
3.5.2. Other intervention strategies
Within the CBT approach, symptomatic strategies that have served for other disorders closely related to an emotional and physiological component have been proposed. For example, systematic desensitization is used to address cataplexy. The main characteristic of this cognitive-behavioral technique is the successive approximation of situations that increase the frequency and intensity of dysfunctional behaviors, emotions, or cognitions. Given the characteristics of cataplexy, CBT becomes a starting point for patients with narcolepsy to cope with emotions.
Another technique for potential use in CBT with regard to cataplexy is stimulus satiation; this procedure uses the reinforcer that maintains cataplectic behavior continuously until its effect is lost.
Another technique is sleep satiation. As described in previous reviews, the frequency of the behavior (i.e., daytime sleep attacks) is detected to determine the degree of sleepiness, and a sleep diary is used to determine the number of sessions. Afterwards, continuous one-day sleep episodes are scheduled without light-dark cues. This behavioral disposition (in addition to sleep regulation) explains the effectiveness of naps and scheduled nocturnal sleep extension.
Imagery rehearsal therapy is used effectively for nightmares and might be used among patients with narcolepsy to control sleep paralysis and hypnagogic hallucinations. Table 3 shows the major cognitive behavioral intervention techniques in narcolepsy based on the findings of this review.
Table 3.
CBT techniques among patients with narcolepsy.
| Therapeutic technique | Description | Therapeutic target |
|---|---|---|
| Sleep satiation | Detecting sleep satiation behavioral frequency (in this case, daytime sleep attacks) to determine the degree of sleepiness using a sleep diary and sleepiness scales as well as the number of sessions. Afterward, longer episodes of nocturnal sleep and daytime naps with no light-dark cues were scheduled. | Reduction of daytime sleepiness attacks |
| Scheduled naps | • Sleep times during the day | Reduction of daytime sleepiness attacks |
| • Strategically timed | ||
| • Fifteen-minute naps at 12:30 p.m. and 5:00 p.m. | ||
| • Daytime naps provide a critical portion of treatment for daytime sleepiness. | ||
| • Naps ranged from 15 to 20 min to over 1 h. | ||
| • Many short naps (<30 min) are beneficial, but other patients require longer naps. | ||
| Systematic desensitization | Systematic desensitization involves the application of a hierarchy of previously identified stressful visual stimuli through which the patient feels that his or her cataplexy is relieved. That is, the patient and therapist imagine a set of situations that the former typically fears, specifying as many details as possible. Then, while the patient is in a deep state of relaxation, he or she is guided to imagine these scenes based on the degree of anxiety associated with them. | Reduction of cataplexy attacks |
| Stimulus control | This technique uses the reinforcer that maintains cataplectic behavior in a continuous manner until its effect is lost. | Reduction of cataplexy attacks |
| Imagery rehearsal therapy. | • Nightmares likely represent a form of negative imagination that happens during sleep. | Reduction of hypnagogic hallucinations and the ability to cope with them |
| • Working with the imagination while awake affects daydreaming due to the continuity of fantasies. | ||
| • Nightmare imagery can be changed while awake. | ||
| • Testing or reviewing a new dream while awake reduces nightmares. | ||
| Hypnosis | A physiological mechanism through which a direct suggestion is accepted by internalized self-instructions. For this to happen, four things are needed: | Reduction of sleep paralysis |
| • A focus of attention; | ||
| • A shock; | ||
| • The suggestion itself; and | ||
| • No criticism of the suggestion. | ||
| When these requirements are met, the suggestion takes root and is externalized in motor function. Thus, the suggestion has overcome the mind. | ||
| Lucid dreams | • Remember recent hallucinations. | Reduction of hypnagogic hallucinations and the ability to cope with them |
| • Develop intention through self-instruction. | ||
| • Visualize recent hallucinations. | ||
| • Repeat the above steps. | ||
| • “Bring back” the mind to the present moment. | ||
| Imagery rehearsal therapy | • Select and write about a particular nightmare. | Reduction of hypnagogic hallucinations and the ability to cope with them |
| • Change it according to preference, thereby generating a new sequence of images. | ||
| • Review the new sequence of images during 15 to 20 minutes of wakefulness. | ||
| • Generate a state of relaxation. | ||
| Cognitive therapy | This technique seeks to identify and modify the dysfunctional cognitions of a patient using different techniques, highlighting the negative effect that symptoms have on the daily life, emotions, and other functional areas of patients with narcolepsy. | Reduction of the consequences that maintain symptoms and affect the patient׳s daily life |
| Muscle relaxation | This technique seeks to relax the muscle groups, starting with the distal part of a limb, passing after a few seconds to another segment. The process proceeds to cover the whole body. | Reduction of anxious situations that can assist in maintaining symptoms or impair patient quality of life |
| Diet | Food is provided after awakening; specifically, | |
| • Breakfast one to two hours after awakening; | ||
| • A snack before bedtime; and | ||
| • Lunch and dinner are offered at equal intervals after breakfast. |
3.5.3. Structure of therapy sessions
Although all recommendations are aimed at addressing the symptoms of narcolepsy, this perspective proposes dealing with both symptomatic factors and their effect on patient quality of life. The following recommendations are suggested for handling sessions (see Table 3).
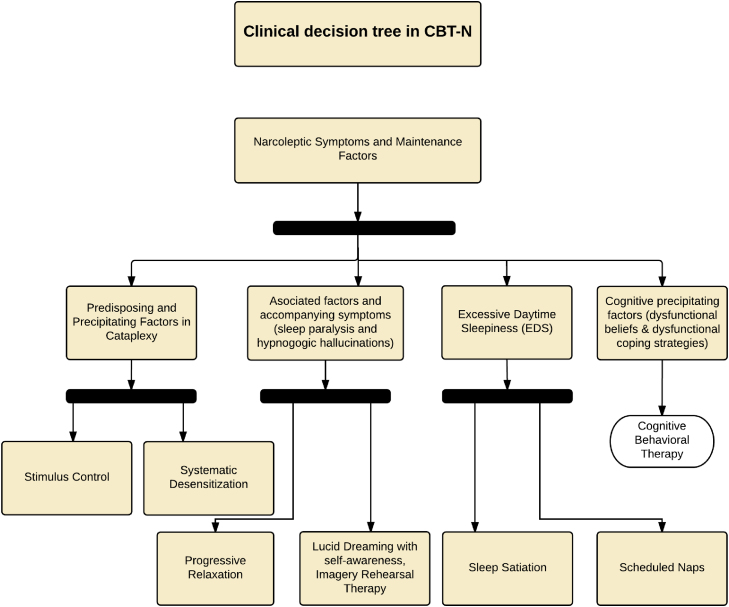
Given the technical provisions in the field of group therapy, this protocol can be applied as a group. Importantly, this procedure is not a prescription and can have particularities; nevertheless, it organizes the multicomponent elements of the CBT approach to narcolepsy. It is important to establish which techniques can be used through a process of clinical formulation and apply those best suited to the problematic context of the patient [48,49]. Fig. 2 shows a flow chart as an example of the use of these techniques to treat narcolepsy and other sleep disorders (Table 4).
Fig. 2.
CBT flow chart for treating narcolepsy-cataplexy syndrome.
Table 4.
Sessions and activities structuring CBT among patients with narcolepsy.
| Sessions | Activities |
|---|---|
| Treatment session 1 | An overview of the treatment program is presented during the first therapy session. Each therapy component is discussed briefly, but specific procedures are not described. |
| The agenda of subsequent sessions is reviewed. At the end of the session, the objectives form is jointly completed by the patient and his or her clinician, and the therapeutic approach is determined. | |
| Treatment session 2 | The behavioral component of therapy (systematic desensitization, nap training, stimulus satiation, or some combination thereof) is introduced during the second session. |
| The methods are described with their theoretical foundations. Patient resistance and obstacles with regard to implementing the procedures are briefly discussed; however, the patient is encouraged to experiment with these techniques first and realize the problems during the next session. | |
| Treatment session 3 | This session is primarily dedicated to solving the difficulties found during the first week of practice at home. |
| Each procedure is reviewed, and the patient is asked whether he or she has met the requirements. Specific methods are reviewed to facilitate treatment acceptance. | |
| Treatment session 4 | The cognitive therapy component is introduced during this session. The basic principles, objectives, and foundation of cognitive restructuring are discussed. The importance of this conceptual framework for understanding narcolepsy and its effect on the patient׳s life is emphasized. |
| Furthermore, imagery rehearsal therapy is introduced in the event that hypnagogic hallucinations occur. | |
| Treatment session 5 | After the issues related to the implementation of behavioral procedures have been reviewed, this session continues with cognitive therapy. |
| Patient-reported cognitions during the preceding week are reviewed; if they are considered dysfunctional, then they are replaced by others that are more appropriate. During this treatment phase, the progress made toward the proposed objectives is discussed with the patient, and the summary data sheet is reviewed. | |
| Treatment session 6 | This session is dedicated to the sleep hygiene education that is similar to what is recommended among patients with insomnia; however, little evidence supports its use among patients with narcolepsy. This education prescribes improved sleep habits and constant sleep patterns among patients with narcolepsy, thereby increasing their quality of sleep. The stimulus satiation, nap training, and systematic desensitization techniques are checked. |
| Treatment session 7 | The clinician comprehensively reviews therapy components. Patient progress is reviewed and discussed with the patient, highlighting the areas that need more attention. The therapeutic relationship, process, and intervention outcomes are discussed. |
3.6. Effectiveness of multicomponent treatments
Since 2011, several studies have describes the effectiveness of CBT for narcolepsy. The first randomized study [50–52] compared results with a control group after six months and one year of treatment; self-report measures (i.e., the Epworth Sleepiness Scale, Stanford Sleepiness Scale, Scale Ullanlina Narcolepsy, SF 36, and MSLT) were applied to evaluate the effectiveness of a multicomponent (sleep satiation, nap training, cognitive restructuring, and problem-solving techniques) treatment. Significant differences (p<0.001) between the two groups were found. Specifically, the treatment group demonstrated a significant improvement with regard to quality of life (physical function, social function, vitality and emotional role) and significant improvements were observed in subjective and objective reports of EDS (p<0.005).
A second study [52] compared decreased sleepiness and cataplexy with nap training and systematic desensitization. The study found significant post-intervention differences in p300 wave latency (p<0.001) as well as the amplitude and latency of electrodermal signal recovery after treatment (p<0.001). The use of both techniques explained all changes.
The last study [53] was based on a treatment that evaluated the effectiveness of the cognitive measures that cope with narcolepsy. Techniques and behavioral experiments including cognitive restructuring and intervention were aimed at reducing the safety behaviors that maintain the problem. The characteristics of patient quality of life were previously measured, and a questionnaire of beliefs and attitudes toward narcolepsy was used. The cognitive-behavioral therapy group had significantly superior (p<0.005) post-treatment assessment scores when compared to the control and drug treatment groups.
4. Conclusions
Despite the limitations of the reviewed publications, psychological interventions are both ancillary and primary therapeutic strategies to control the symptoms of narcolepsy when presented in an organized way. Specifically,
-
(a)
Systematic studies using cognitive behavioral measures as symptomatic treatments of choice for other sleep disorders have shown some clinical efficacy when compared to drug treatments.
-
(b)
Behavioral measures and strategies for narcolepsy intervention are the most frequently reported in the literature, but cognitive and multicomponent approaches remain rare; however, studies describing their importance and effectiveness are beginning to appear.
-
(c)
Effective cognitive behavioral techniques and measures currently exist for treating symptoms that overlap or are similar to the manifestation of narcolepsy, such as hypersomnia and depression.
-
(d)
The literature consistently shows that impaired quality of life and dysfunction are major complaints among patients with narcolepsy. Thus far the psychometric tests which have been used to support this conclusion have however not been sufficiently used to evaluate the effectiveness of cognitive behavioral techniques. The addition of these psychometric tests would importantly contribute to advancing efforts to evaluate CBT’s effectiveness in improving patients’ quality of life and thus to hasten its addition to multimodal therapy programs for narcolepsy.
-
(e)
To date, intervention measures extrapolated to patients׳ daily lives lack multicenter studies and follow-up procedures to support their benefit. These findings should be a starting point for research and a validation process of the intervention model proposed in multicenter studies to develop related research regarding CBT for narcolepsy.
In clinical settings, several factors make non-pharmacological treatments indispensable for narcolepsy.
First stimulants (amphetamines) are the drugs of choice to control sleepiness, and are primarily used for their stimulating effect. Although they effectively control EDS, their high risk for addiction, which is related to the phenomena of tolerance, abuse, and abstinence should be mentioned. In the context of narcolepsy, it is common for patients not receiving a follow-up assessment to alter their daily consumed dose, especially in the case of methylphenidate.
This drug results in increased side effects (e.g., headache, tremor, palpitations, and anxiety) that hinder adherence to treatment and reduce patient quality of life. However, the use of amphetamine derivatives is contraindicated among patients with comorbidities such as systemic arterial hypertension or acquired diabetes mellitus. Thus, patients with comorbid narcolepsy are suitable candidates for CBT-N.
5. Potential Competing Interest / Disclosure Statement
The authors have read the journal’s policy and have the following potential conflicts: All authors declare that they have no proprietary, financial, professional, nor any other personal interest of any nature or kind in any product or services and/or company that could be construed or considered to be a potential conflict of interest that might have influenced the views expressed in this manuscript.
Acknowledgments
Thanks go to Vanessa Marín, Ulises Jiménez Jr., and Félix Jiménez as well as Drs. Maritza Pedemonte, María José Ramos, and Rosa Peraita Adrados for their invaluable support.
Footnotes
Peer review under responsibility of Brazilian Association of Sleep
References
- 1.Ohayon M.M. From wakefulness to excessive sleepiness: what we know and still need to know. Sleep Med Rev. 2008;12(2):129–141. doi: 10.1016/j.smrv.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiménez-Correa U., Haro R., González R., Velázquez-Moctezuma J. Correlations between subjective and objective features of nocturnal sleep and excessive diurnal sleepiness in patients with narcolepsy. Arq Neuropsiquiatr. 2009;67(4):995–1000. doi: 10.1590/s0004-282x2009000600006. [DOI] [PubMed] [Google Scholar]
- 3.Schenck CH, Bassetti CL, Arnulf I, Emmanuel Mignot E. English translations of the first clinical reports on narcolepsy by Gelineau and on cataplexy by Westphal in the late 19th century, with commentary. p. 7–24 [PMC free article] [PubMed]
- 4.American Academy of Sleep Medicine. International classification of sleep disorders revised: diagnostic and coding manual. Rochester, Minnesota: Autor; 2005.
- 5.American Psychiatric Association. Manual diagnóstico y estadístico de lostrastornos mentales, DSM-IV-TR (texto revisado). Barcelona: Masson; 2002. [Diagnostic and statistical manual of mental disorders, DSM-IV-TR (revised text)].
- 6.American Academy of Sleep Medicine. International classification of sleep disorders, revised: diagnostic and coding manual. Chicago, Illinois: American Academy of Sleep Medicine; 2001.
- 7.Goswami Meeta. Humana Press; New York: 2008. Sleep and quality of life in narcolepsy. En: Joris C. Verster JC, Pandi-Perumal SR and Streiner DL. Sleep and quality of life in clinical medicine; pp. 93–100. [Google Scholar]
- 8.Goswami Meeta. Quality of life and psychosocial issues in narcolepsy. In: Goswami Meeta, Pandi-Perumal Michael S.R., Thorpy J., editors. Narcolepsy: a clinical guide. Springer Humana Press; New York: 2010. pp. 189–204. [Google Scholar]
- 9.Donjacour Claire E.H.M., Mets Monique A.J., Verster Joris C. Narcolepsy, driving and traffic safety. In: Goswami Meeta, Pandi-Perumal S.R., Thorpy Michael J., editors. Narcolepsy: a clinical guide. Springer Humana Press; New York: 2010. pp. 217–222. [Google Scholar]
- 10.Lindsley Gila. Narcolepsy, intimacy and sexuality. In: Goswami Meeta, Pandi-Perumal S.R., Thorpy Michael J., editors. Narcolepsy: a clinical guide. Springer Humana Press; New York: 2010. pp. 205–216. [Google Scholar]
- 11.Naumann A., Bellebaum C., Daum I. Cognitive deficits in narcolepsy. J. Sleep Res. 2006;15:329–338. doi: 10.1111/j.1365-2869.2006.00533.x. [DOI] [PubMed] [Google Scholar]
- 12.Bellebaum Christian, Daum Irene. Memory and cognition in narcolepsy. In: Goswami Meeta, Pandi-Perumal S.R., Thorpy Michael J., editors. Narcolepsy: a clinical guide. Springer Humana Press; New York: 2010. pp. 223–230. [Google Scholar]
- 13.Ingravallo Francesca, Plazzi Giuseppe. Medico-legal aspects of disability in narcolepsy. In: Goswami Meeta, Pandi-Perumal S.R., Thorpy Michael J., editors. Narcolepsy: a clinical guide. Springer Humana Press; New York: 2010. pp. 231–238. [Google Scholar]
- 14.Shneerson John. Narcolepsy and mental health. In: Goswami Meeta, Pandi-Perumal S.R., Thorpy Michael J., editors. Narcolepsy: a clinical guide. Springer Humana Press; New York: 2010. pp. 239–250. [Google Scholar]
- 15.Vignatelli L., Plazzi G., Peschechera F., Delaj L., D׳Alessandro R. A 5-year prospective cohort study on health-related quality of life in patients with narcolepsy. Sleep Med. 2014 doi: 10.1016/j.sleep.2010.07.008. (in press) [DOI] [PubMed] [Google Scholar]
- 16.McCrae C, Nau, S. Behavioral sleep medicine training roster 2007 update. Department of Clinical and Health Psychology, University of Florida, Sleep Research Project, University of Alabama; 2007. p. 3–51
- 17.Marín-Agudelo H.y., Vinaccia S. Modelo cognitivo comportamental del síndrome de narcolepsia-cataplejía: exposición teórica. [Cognitive behavioral model of the narcolepsy-cataplexy syndrome: theoretical exposition] Rev Psicopatol Psicol Clín. 2005;10(3):153–172. [Google Scholar]
- 18.Carretero-Dios H., Roig M.y., Buela Casal G. Narcolepsia, emoción, conciencia y su hipotética relación [Narcolepsy, emotion, consciousness and their hypothesized relationship] Rev Ecuat Neurol. 2001;10(1–2):12–19. [Google Scholar]
- 19.Alóe F. Elsevier editora; Rio de Janeiro: 2009. Directices clínicas para el diagnostico y tratamiento de la narcolepsia. [Clinical guidelines for the diagnosis and treatment of narcolepsy] pp. 79–81. [Google Scholar]
- 20.Alóe F., Cardoso R., Araújo J.F., Azevedo A., Bacelar A., Bezerra M. Vol. 32. 2010. Brazilian guidelines for the treatment of narcolepsy; pp. 305–314. (Rev Bras Psiquiatr). [PubMed] [Google Scholar]
- 21.Britton T., Hansen A., Hicks J., Howard R., Meredith A. Taylor Patten Communications Ltd; Ashtead, UK: 2002. Guidelines on the diagnosis and management of narcolepsy in adults and children. Evidence-based guidelines for the UK with graded recommendations. [Google Scholar]
- 22.Billiard M., Bassetti C., Dauvilliers Y., Dolenc-Groselj L., Lammers G.J., Mayerf G. EFNS guidelines on management of narcolepsy. Eur J Neurol. 2006;13:1035–1048. doi: 10.1111/j.1468-1331.2006.01473.x. [DOI] [PubMed] [Google Scholar]
- 23.Morgenthaler T.I., Kapur V.K., Brown T.M., Swick T.J., Alessi C., Aurora R.N. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705–1711. doi: 10.1093/sleep/30.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garma L., Marchand F. Non-pharmacological approaches to the treatment of narcolepsy. Sleep. 1994;17:S97–S102. doi: 10.1093/sleep/17.suppl_8.s97. [DOI] [PubMed] [Google Scholar]
- 25.Cohen F.L., Nehring W.M., Cloninger L. Symptom description and management in narcolepsy. Holist Nurs Pract. 1996;10(4):44–53. doi: 10.1097/00004650-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Rogers A.E., Mullington J. The symptomatic management of narcolepsy. In: Perlis M.L., Lichstein K.L., editors. Treating sleep disorders: principles and practice of behavioral sleep medicine. John Wiley & Sons; Hoboken, NJ: 2003. pp. 118–135. [Google Scholar]
- 27.Broughton R.J., Murray B.J. The behavioral management of narcolepsy. In: Bassetti C.L., Billard M., Mignot E., editors. Narcolepsy and hypersomnia. Informa Healthcare; New York: 2007. pp. 497–512. [Google Scholar]
- 28.Monderer R., Freedman H.S., Thorpy M.J. Non-pharmacologic treatments of narcolepsy. In: Goswami Meeta, Pandi-Perumal S.R., Thorpy Michael J., editors. Narcolepsy: a clinical guide. Springer Humana Press; New York: 2010. pp. 313–322. [Google Scholar]
- 29.Volk S., Schulz H., Yassouridis A., Wilde-Frenz J., Simon O. The influence of two behavioral regimens on the distribution of sleep and wakefulness in narcoleptic patients. Sleep. 1990;13(2):136–142. doi: 10.1093/sleep/13.2.136. [DOI] [PubMed] [Google Scholar]
- 30.Hishikawa Y., Wakamatsu H., Furuya E., Sugita Y., Masaoka S., Kaneda H. Sleep satiation in narcoleptic patients. Electroencephalogr Clin Neurophysiol. 1976;41:1–18. doi: 10.1016/0013-4694(76)90210-8. [DOI] [PubMed] [Google Scholar]
- 31.Yuchiyama M.G., Mayer G., Meier-Ewert K. Differential effects of extended sleep in narcoleptics patients. Electroencephalogr Clin Neurophysiol. 1994;91:212–218. doi: 10.1016/0013-4694(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 32.Billiard M., QueraSalva M., de Koninck J., Besset A., Touchon J., Cahilhac J. Daytime sleep characteristics and night sleep in the narcoleptic patient. Sleep. 1986;9:167–174. doi: 10.1093/sleep/9.1.167. [DOI] [PubMed] [Google Scholar]
- 33.Helmus T., Rosenthal L., Bishop C., Roehrs T., Syron M.L., Roth T. The alerting effects of short and long naps in narcoleptic, sleep deprived, and alert individuals. Sleep. 1997;20(4):251–257. doi: 10.1093/sleep/20.4.251. [DOI] [PubMed] [Google Scholar]
- 34.Rogers A.E., Aldrich M.S. The effect of regularly scheduled naps on sleep attacks and excessive daytime sleepiness associated with narcolepsy. Nurs Res. 1993;42:111–117. [PubMed] [Google Scholar]
- 35.Mullington J., Broughton R. Scheduled naps in the management of daytime sleepiness in narcolepsy cataplexy. Sleep. 1993;16:444–456. doi: 10.1093/sleep/16.5.444. [DOI] [PubMed] [Google Scholar]
- 36.Roehrs T., Zorick F., Wittig R., Paxton C., Sicklesteel J., Roth T. Alerting effects of naps in patients with narcolepsy. Sleep. 1986;9:194–199. doi: 10.1093/sleep/9.1.194. [DOI] [PubMed] [Google Scholar]
- 37.Rogers A.E., Aldrich M.S., Lin X. A comparison of three different sleep schedules for reducing sleepiness in narcolepsy. Sleep. 2001;24:385–391. doi: 10.1093/sleep/24.4.385. [DOI] [PubMed] [Google Scholar]
- 38.Nardi T.J. Treating sleep paralysis with hypnosis. Int J Clin Exp Hypn. 1981;9:358–365. doi: 10.1080/00207148108409169. [DOI] [PubMed] [Google Scholar]
- 39.Price R. Hypnotherapy in the control of cataplexy in a narcoleptic subject. Am J Clin Hypn. 1981;29:201–205. doi: 10.1080/00029157.1987.10734352. [DOI] [PubMed] [Google Scholar]
- 40.Schneck J.M. Hypnotherapy for narcolepsy. Int J Clin Exp Hypn. 1980;28:95–100. doi: 10.1080/00207148008409832. [DOI] [PubMed] [Google Scholar]
- 41.Brulowski A. The role of lucid dreaming in the treatment of narcolepsy and nightmares: a case study. Sleep Res. 1987;16:319. [Google Scholar]
- 42.Rogers A.E. Problems and coping strategies identified by narcoleptic patients. J Neurosurg Nurs. 1984;16:326–334. doi: 10.1097/01376517-198412000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Bruck D., Broughton R. Achieving control over sleepiness in narcolepsy. Aust J Prim Health. 2001;7:16–24. [Google Scholar]
- 44.Chen S.Y., Clift S.J., Dahlitz M.J., Dunn G., Parkes J.D. Treatment of the narcoleptic syndrome: self-assessment of the action of dexamphetamine and clomipramine. J Sleep Res. 1995;4:113–118. doi: 10.1111/j.1365-2869.1995.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 45.Wilson S.J., Frazer D.W., Lawrence J.A., Bladin P.F. Psychosocial adjustment following relief of chronic narcolepsy. Sleep Med. 2007;8(3):252–259. doi: 10.1016/j.sleep.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Kolko D.J. Behavioral treatment of excessive daytime sleepiness in an elderly woman with multiple medical problems. J Behav Ther Exp Psychiatry. 1984;15(4):341–345. doi: 10.1016/0005-7916(84)90099-5. [DOI] [PubMed] [Google Scholar]
- 47.Pollak C.P., Green J. Eating and its relationships with subjective alertness and sleep in narcoleptic subjects living without temporal cues. Sleep. 1990;13:467–478. doi: 10.1093/sleep/13.6.467. [DOI] [PubMed] [Google Scholar]
- 48.Martin G., Pear J. Pearson; Madrid: 2007. Modificación de conducta. Qué es y cómo aplicarla. [Behavior modification. What it is and how to apply it] p. 7. [Google Scholar]
- 49.Méndez F.X., Olivares J. Biblioteca Nueva; Madrid: 2001. Técnicas de modificación de conducta. [Behavior modification techniques] pp. 160–165. [Google Scholar]
- 50.Marin-Agudelo H. Multicomponent Cognitive Behavioral treatment efficacy for narcolepsy (MCBT-N) Sleep Med. 2011;12(Suppl 1):S55. [Google Scholar]
- 51.Conroy D.A., Novick D.M., Swanson L.M. Behavioral management of hypersomnia. Sleep Med Clin. 2012;7(2):325–331. [Google Scholar]
- 52.Marín-Agudelo H., Jiménez Correa U. Scheduled naps and systematic desensitization in the emotional processing in patients with narcolepsy: a comparative study of autonomic and cognitive evoked potentials. Sleep. 2012;35 (Suppl: A275) [Google Scholar]
- 53.Marín-Agudelo H., Jiménez Correa U. Beliefs and dysfunctional attitudes in patients with narcolepsy; double-blind study of treatment efficacy. Sleep. 2013;36 (Suppl: A256) [Google Scholar]