Abstract
Objective
We characterized functional impact of narcolepsy on patients using a general health status measure, the Sickness Impact Profile (SIP). It has 136 items grouped into 12 categories and 2 dimensions.
Methods
We ascertained patients with physician-diagnosed narcolepsy in King County, Washington using multiple overlapping methods over four years starting July 2001. We recruited 226 patients (mean age 48 years, 65% female) who underwent in-person interviews and completed: Epworth Sleepiness Scale (ESS), Ullanlinna Narcolepsy Scale (UNS), and SIP. Linear regression was used to assess correlations between measures.
Results
Mean percent of total dysfunction was higher for psychosocial dimension (13.2) and independent categories (13.4) than physical dimension (5.0). Mean percent of total dysfunction in descending order for categories was: Sleep and Rest (23.6), Alertness Behavior (22.6), and Recreation and Pastimes (20.6). Ten items were endorsed by at least a third of all patients but only two of them concerned sleep. Unexpectedly, among the top ten items were, “My sexual activity is decreased,” and “I forget a lot, for example, things that happened recently, where I put things, appointments.” Percent of overall dysfunction on SIP (mean 10.3) was significantly correlated with ESS (r=0.36, p<0.001) and UNS (r=0.47, p<0.001). In this population-based sample, mean percent of total dysfunction on SIP in patients with narcolepsy (10.3) was higher than previously reported in the general population (3.6) and similar to that in other chronic disabling conditions.
Discussion
The SIP correlated with ESS and UNS, and captured unique aspects of the impact of narcolepsy on patients.
Keywords: Narcolepsy, Cataplexy, HLA-DQB1*0602, Epidemiologic studies, Health status indicators, Sickness Impact Profile
1. Introduction
Scales that measure symptoms of narcolepsy emphasize rating clinical features of the disease such as excessive daytime sleepiness and cataplexy [1,2], but they may fail to capture the full functional impact of narcolepsy or its treatments. The Medical Outcomes Study Short Form-36 (SF-36) [3] has been used in patients with narcolepsy to characterize more fully the effect of the disease on physical and psychosocial spheres [4,5]. Another general health status measure, the Sickness Impact Profile (SIP) [6,7] has the potential to expand further our understanding of dysfunction in narcolepsy patients.
The SIP was designed to assess functional status of patients with any chronic disease [6,7]. Its reliability and validity have been extensively studied in neurologic [8,9] and non-neurologic diseases [7,10], but not in patients with narcolepsy, to our knowledge. The SIP contains 136 items grouped in 12 categories and requires about 30 min to complete. Each item describes a specific behavioral dysfunction, rather than a subjective self-evaluation. Patients are instructed to endorse only those items describing dysfunction due to their disease. Each item has a weight reflecting the relative severity of the dysfunction compared to other items [11]. The 12 category scores, expressed as percents, are the sum of the weighted scores for all the endorsed items in a particular category divided by the maximum score, which represents total dysfunction for that category. Some categories contribute to a physical dimension; some, to a psychosocial dimension; and some independent categories, to neither of these dimensions.
Our goals were to examine the performance of the SIP in a population-based study of patients with physician-diagnosed narcolepsy in King County, Washington. We wanted to compare the information obtained from the SIP to that from two measures used in patients with narcolepsy. We also wanted to generate a profile for patients with narcolepsy and see how this profile differed for various definitions of the disease.
2. Materials and methods
In the parent study, we attempted to identify all prevalent cases of physician-diagnosed narcolepsy who were 18 years and older and residing in King County, Washington as of July 1, 2001 [12]. Cases were recruited through multiple overlapping methods. For providers, we focused on clinicians working in sleep disorders centers but also contacted neurologists, family medicine physicians, psychiatrists, and community clinics where patients without financial resources often receive care. For patients, presentations were made at support groups and other regional meetings. Pharmacists in King County agreed to include an information sheet about the study with all prescriptions relevant to narcolepsy. Multimedia advertisements and public service announcements were also used and linked to telephone and online contact information.
Patients identified were contacted and asked to participate in an epidemiologic study. For those who provided written informed consent, trained professionals administered a structured interview. Consent to provide buccal specimens and to obtain medical records was also requested from each participant. A total of 425 patients were entered into the narcolepsy registry. Of these, 78 cases could not be located, 10 could not be interviewed due to language barriers or psychiatric illnesses, and 55 refused. Interviews were arranged for 282 cases, 279 of whom completed the interview and provided buccal specimens for deoxyribonucleic acid (DNA), which was tested for human leukocyte antigen (HLA) DQB1⁎0602 as detailed elsewhere [13]. The University of Washington Human Subjects Committee reviewed and approved the study.
3. Data collection
Trained professionals administered in-person interviews to patients using a standardized questionnaire that included demographic information, the Epworth Sleepiness Scale (ESS) [1], and the Ullanlinna Narcolepsy Scale (UNS) [2]. We defined cataplexy as present if indicated by medical record review, self-reported cataplexy, or an affirmative response to questions about experiencing muscle weakness when telling or hearing a joke, or when laughing [14]. After the interview, the SIP was explained to the patient, who was given the choice of completing the SIP while the interviewer waited or to complete it at a later date and return it by mail. Of the 279 patients who participated in the study, the 226 (81%) who completed the SIP, the ESS, and the UNS are the focus of this report.
4. Analysis
Percentages were calculated for the SIP׳s 12 categories, two dimensions, independent categories not included in the two dimensions, and overall score. Results were examined for all patients with a physician diagnosis of narcolepsy, the subgroups with and without cataplexy, and the subgroups with and without HLA DQB1⁎0602 positivity. We conducted a two-way analysis of variance (ANOVA) to assess the difference in mean dysfunction scores according to cataplexy and HLA DQB1⁎0602. Linear regression was also used to assess the association between the SIP scores and cataplexy status and HLA status while controlling for age and sex. Associations between the SIP and the ESS and UNS were assessed with partial correlation coefficients from linear regression models of the entire sample, which included age, sex, and HLA DQB1⁎0602 status. Findings with p-values less than 0.05 were considered significant, and all testing was two-tailed. All analyses were conducted in Stata (version 10.0 for Macintosh, StataCorp, College Station, TX).
5. Results
Considering the 226 patients included in these analyses, the mean and median age was 48 years old with a range from 18 to 92 with 47% over 50 years old and 65% women. Of the 226 patients, 152 (67%) had cataplexy and 113 (50%) had HLA DQB1⁎0602 positivity.
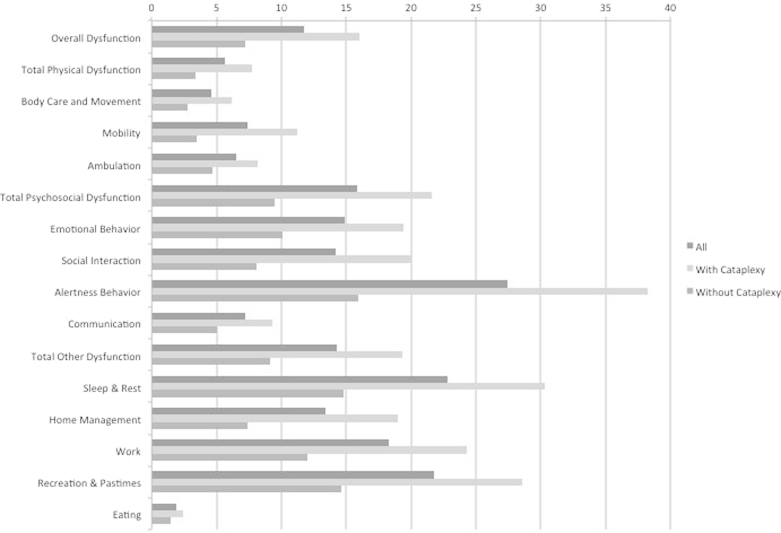
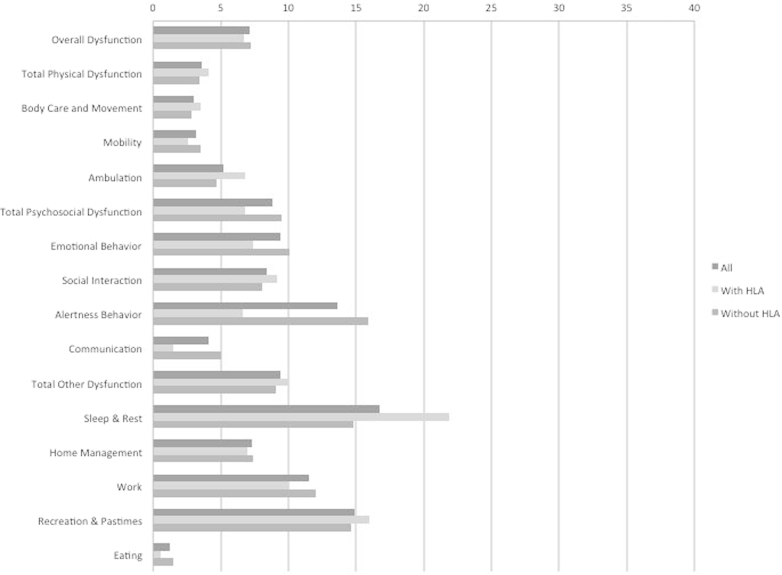
Among 113 who did not have the HLA marker, 58 (51.3%) had cataplexy. Among 113 who were positive for the HLA marker, 94 (83.2%) had cataplexy. Figs. 1 and 2 show the mean unadjusted percent of full dysfunction for the total SIP score, two dimensions, and 12 categories. Scores are shown for: everyone, those with and without cataplexy restricted to patients without HLA DQB1⁎0602 (Fig. 1), and those with and without HLA DQB1⁎0602 positivity restricted to patients without cataplexy (Fig. 2). In the absence of HLA DQB1⁎0602, dysfunction was highest in those with cataplexy. In the absence of cataplexy, those with HLA DQB1⁎0602 have lower or similar dysfunction except for sleep and rest component of the SIP, in which the HLA DQB1⁎0602 allele was associated with a much higher level of dysfunction (Table 1).
Fig. 1.
Results of the Sickness Impact Profile (SIP) in patients with physician-diagnosed narcolepsy without the HLA DQB1⁎0602 allele (n=113), according to cataplexy status. Scores for total SIP, two dimensions, independent categories, and 12 categories are the percent of total dysfunction.
Fig. 2.
Results of the Sickness Impact Profile (SIP) in patients with physician-diagnosed narcolepsy without cataplexy (n=74), according to presence or absence of the HLA DQB1⁎0602 allele. Scores for total SIP, two dimensions, independent categories, and 12 categories are the percent of total dysfunction.
Table 1.
Regression modelsa for dimensions and components of the SIP.
| Dimensions and components | Cataplexy | HLA DQB1⁎0602 |
|
|---|---|---|---|
| p-Value | p-Value | R2 | |
| Overall dysfunction | <0.001 | 0.001 | 0.10 |
| Total physical dysfunction | 0.005 | 0.004 | 0.15 |
| Body Care and Movement | 0.033 | 0.018 | 0.11 |
| Mobility | <0.001 | <0.001 | 0.11 |
| Ambulation | 0.078 | 0.109 | 0.17 |
| Total psychosocial dysfunction | <0.001 | 0.001 | 0.11 |
| Emotional Behavior | 0.008 | 0.026 | 0.07 |
| Social Interaction | 0.001 | 0.007 | 0.06 |
| Alertness Behavior | <0.001 | <0.001 | 0.14 |
| Communication | 0.053 | 0.012 | 0.04 |
| Total other dysfunction | <0.001 | 0.013 | 0.08 |
| Sleep and Rest | 0.001 | 0.617 | 0.05 |
| Home Management | <0.001 | 0.007 | 0.10 |
| Work | 0.015 | 0.013 | 0.05 |
| Recreation and Pastimes | 0.002 | 0.060 | 0.06 |
| Eating | 0.086 | 0.160 | 0.03 |
Adjusted for cataplexy, HLA DQB1⁎0602, age and sex.
In a two-way ANOVA, both cataplexy and HLA DQB1⁎0602 status were significantly and independently associated with total percent dysfunction (p<0.001 for both factors), total physical dysfunction (p<0.02 for cataplexy; p=0.05 for HLA DQB1⁎0602 allele), total psychological dysfunction (p<0.001 for both factors), and for other independent categories of dysfunction (p<0.001 for cataplexy; p=0.01 for HLA DQB1⁎0602 allele). In linear regression models with SIP scores as the dependent variable and age, sex, cataplexy and HLA status as the independent variables, those with cataplexy had higher scores than those without, and those with HLA DQB1⁎0602 positivity had lower scores than those without. Associations for cataplexy and HLA DQB1⁎0602 were statistically significant for both dimension and most of the categories (Table 2). Whether a patient had one (n=97) or two alleles (n=16) of HLA DQB1⁎0602 made little difference (data not shown). Those with cataplexy and without HLA DQB1⁎0602 positivity consistently showed the highest dysfunction (data not shown).
Table 2.
The 20 most commonly endorsed items on the Sickness Impact Profile (SIP) by (A) all patients, (B) those with cataplexy, and (C) those with HLA DQB1⁎0602 positivity.
| (A) Among all patients (n=226) | |||
|---|---|---|---|
| Itema | Weight | Dysfunctional behavior | Percent |
| sr7 | 60 | I sleep or nap more during the day | 46 |
| rp2 | 36 | I am going out for entertainment less often | 43 |
| hm2 | 44 | I am doing less of the regular daily work around the house than I would usually do | 39 |
| si9 | 51 | My sexual activity is decreased | 39 |
| rp1 | 39 | I do my hobbies and recreation for shorter periods of time | 39 |
| si6 | 36 | I am doing fewer social activities with groups of people | 37 |
| ab4 | 67 | I do not finish things I start | 37 |
| ab7 | 78 | I forget a lot, for example, things that happened recently, where I put things, appointments | 37 |
| sr6 | 61 | I sleep less at night, for example, wake up too early, don’t fall asleep for a long time, awaken frequently | 35 |
| hm1 | 54 | I do work around the house only for short periods of time or rest often | 35 |
| sr5 | 84 | I sit around half-asleep | 33 |
| sr2 | 49 | I sit during much of the day | 29 |
| si1 | 44 | I am going out less to visit people | 28 |
| a1 | 48 | I walk shorter distances or stop to rest often | 28 |
| ab10 | 80 | I have difficulty doing activities involving concentration and thinking | 28 |
| rp7 | 43 | I am cutting down on some of my usual physical recreation or activities | 28 |
| sr4 | 58 | I lie down more often during the day in order to rest | 27 |
| rp6 | 33 | I am doing fewer community activities | 26 |
| ab8 | 67 | I do not keep my attention on any activity for long | 23 |
| bcm12 | 30 | I change position frequently | 22 |
| (B) Among patients with cataplexy (n=152) | |||
|---|---|---|---|
| Itema | Weight | Dysfunctional behavior | Percent |
| si6 | 36 | I am doing fewer social activities with groups of people | 44 |
| rp2 | 36 | I am going out for entertainment less often | 44 |
| sr6 | 61 | I sleep less at night, for example, wake up too early, don׳t fall asleep for a long time, awaken frequently | 43 |
| sr7 | 60 | I sleep or nap more during the day | 43 |
| ab7 | 78 | I forget a lot, for example, things that happened recently, where I put things, appointments | 43 |
| hm2 | 44 | I am doing less of the regular daily work around the house than I would usually do | 42 |
| si9 | 51 | My sexual activity is decreased | 41 |
| rp1 | 39 | I do my hobbies and recreation for shorter periods of time | 41 |
| hm1 | 54 | I do work around the house only for short periods of time or rest often | 39 |
| ab4 | 67 | I do not finish things I start | 39 |
| sr2 | 49 | I sit during much of the day | 36 |
| sr5 | 84 | I sit around half-asleep | 36 |
| rp7 | 43 | I am cutting down on some of my usual physical recreation or activities | 35 |
| si1 | 44 | I am going out less to visit people | 34 |
| ab10 | 80 | I have difficulty doing activities involving concentration and thinking | 34 |
| a1 | 48 | I walk shorter distances or stop to rest often | 31 |
| rp6 | 33 | I am doing fewer community activities | 31 |
| ab8 | 67 | I do not keep my attention on any activity for long | 30 |
| bcm12 | 30 | I change position frequently | 28 |
| ab2 | 75 | I have more minor accidents, for example, drop things, trip and fall, bump into things | 27 |
| (C) Among patients with HLA DQB1⁎0602 positivity (n=113) | |||
|---|---|---|---|
| Itema | Weight | Dysfunctional behavior | Percent |
| sr7 | 60 | I sleep or nap more during the day | 44 |
| rp2 | 36 | I am going out for entertainment less often | 40 |
| si9 | 51 | My sexual activity is decreased | 39 |
| hm2 | 44 | I am doing less of the regular daily work around the house than I would usually do | 38 |
| rp1 | 39 | I do my hobbies and recreation for shorter periods of time | 37 |
| si6 | 36 | I am doing fewer social activities with groups of people | 36 |
| sr6 | 61 | I sleep less at night, for example, wake up too early, don’t fall asleep for a long time, awaken frequently | 35 |
| hm1 | 54 | I do work around the house only for short periods of time or rest often | 35 |
| ab4 | 67 | I do not finish things I start | 34 |
| ab7 | 78 | I forget a lot, for example, things that happened recently, where I put things, appointments | 32 |
| sr2 | 49 | I sit during much of the day | 31 |
| sr5 | 84 | I sit around half-asleep | 29 |
| sr4 | 58 | I lie down more often during the day in order to rest | 27 |
| si1 | 44 | I am going out less to visit people | 27 |
| a1 | 48 | I walk shorter distances or stop to rest often | 27 |
| ab10 | 80 | I have difficulty doing activities involving concentration and thinking | 26 |
| rp7 | 43 | I am cutting down on some of my usual physical recreation or activities | 26 |
| rp6 | 33 | I am doing fewer community activities | 25 |
| bcm12 | 30 | I change position frequently | 22 |
| rp3 | 59 | I am cutting down on some of my usual inactive recreation and pastimes, for example, watching TV, playing cards, reading | 21 |
The SIP items describe dysfunctional behavior endorsed by patient with narcolepsy. Each item has a weight reflecting the relative severity of the dysfunction compared to other items. The percent of patients endorsing an item out of all the patients is given in the final column. The letters stand for categories (see Figs. 1 and 2 and Table 3), and the number represents an item in the category.
Patients showed greater dysfunction in the psychological dimension and the independent categories than the physical dimension. Much of the dysfunction fell into the independent categories not included in either the physical or psychological dimension. Not surprisingly, the highest scores were in the categories for Sleep and Rest and Alertness Behavior, but high scores were also seen in categories for Recreation and Pastimes, Work, Emotional Behavior, and Social Interaction. The lowest scores were in the categories for Eating, Communication, and Body Care and Movement.
The 20 items most commonly endorsed by all patients are detailed in Table 2A, by those with cataplexy in Table 2B, and by those with HLA DQB1⁎0602 positivity in Table 2C. Considering all patients (Table 2A), 10 items were endorsed by at least a third of the patients, but only two concerned sleep. Tied for the third most commonly endorsed item was, “My sexual activity is decreased,” and tied for fourth was, “I forget a lot, for example, things that happened recently, where I put things, appointments.” The percent of men and women endorsing the item on sexual activity was not significantly different. Eighteen of the top 20 items were the same for all patients (Table 2A) and the two subgroups of those with cataplexy (Table 2B) and those with HLA DQB1⁎0602 positivity (Table 2C), although the order of items differed.
The partial correlation coefficients between the ESS and UNS and the SIP scores are presented in Table 3 controlling for age, sex, and HLA DQB1⁎0602 status. All correlations were significant. Correlations were stronger for the UNS than for the ESS for the total score, two dimensions and 11 of the 12 categories. As expected, the category with the strongest correlation with the ESS and UNS measures was Sleep and Rest, and the three categories with the weakest correlations were Ambulation, Communication, and Eating.
Table 3.
Partial correlations between ESS and UNS scales and a general health status measure, the Sickness Impact Profile (SIP).
|
Scales |
||||
|---|---|---|---|---|
| Epworth Sleepiness | Ullanlinna Narcolepsy | |||
| Percent of total dysfunction | Correlationa | p-Value | Correlationa | p-Value |
| Total SIP | 0.36 | <0.001 | 0.47 | <0.001 |
| Physical dimension | 0.26 | <0.001 | 0.35 | <0.001 |
| Body Care and Movement (bcm) | 0.24 | <0.001 | 0.29 | <0.001 |
| Mobility (m) | 0.27 | <0.001 | 0.36 | <0.001 |
| Ambulation (a) | 0.15 | 0.023 | 0.26 | <0.001 |
| Psychosocial dimension | 0.30 | <0.001 | 0.39 | <0.001 |
| Emotional Behavior (eb) | 0.23 | <0.001 | 0.28 | <0.001 |
| Social Interactions (si) | 0.30 | <0.001 | 0.37 | <0.001 |
| Alertness Behavior (ab) | 0.24 | <0.001 | 0.36 | <0.001 |
| Communication (c) | 0.17 | 0.009 | 0.27 | <0.001 |
| Independent categories | 0.40 | <0.001 | 0.52 | <0.001 |
| Sleep and Rest (sr) | 0.39 | <0.001 | 0.51 | <0.001 |
| Home Management (hm) | 0.31 | <0.001 | 0.42 | <0.001 |
| Work (w) | 0.25 | <0.001 | 0.29 | <0.001 |
| Recreation and Pastimes (rp) | 0.28 | <0.001 | 0.42 | <0.001 |
| Eating (e) | 0.24 | <0.001 | 0.24 | <0.001 |
Partial correlation coefficients from linear regression models including age, sex, and HLA DQB1⁎0602 status.
6. Discussion
Results from this general health status measure suggest that narcolepsy substantially impairs function, psychosocial more than physical. The impact of this disease on patients׳ daily lives may be underappreciated because the relatively preserved physical function gives others the impression that patients with narcolepsy are healthy or do not have a serious disease. Among patients with cataplexy, the impairment of function was greater. On the other hand, among patients with HLA DQB1⁎0602 positivity, the impairment of function was less. Whether cataplexy and HLA status identify subtypes of narcolepsy with possibly different etiologies remains to be determined. Recent publications have raised questions about the importance of cataplexy. In one, partial loss of hypocretin (orexin) cells was documented on neuropathologic examination in narcolepsy without cataplexy [15]. In another, a subset of patients with hypersomnia who did not meet criteria for narcolepsy had the same genetic profile described in patients with narcolepsy with cataplexy, namely positivity for HLA DQB1⁎0602 and T-cell receptor alpha (TCRA) locus [16].
The ESS and the UNS focus on clinical features. The ESS assesses sleepiness, while the UNS includes questions about cataplexy, perhaps explaining why correlations with the SIP were stronger for UNS than the ESS. Although these scales were correlated with the SIP, the correlation was not perfect suggesting that the SIP may be capturing other information about the impact of the disease on a person׳s function and providing a richer description of narcolepsy׳s profile. The SIP also allows comparison across diseases, although caution is needed because of the differences in populations studied. The mean overall SIP score in these patients was 10.3. The comparable figure was 3.6 in a general population; 15.6 in a clinic-based series of patients with rheumatoid arthritis [10]; and 16.8 in a clinic-based series of patients with Parkinson׳s disease [8]. The Psychosocial Dimension score was 13.2 with narcolepsy, 11.3 with rheumatoid arthritis, 19.4 with Parkinson׳s disease.
Many of the items endorsed in the SIP could be anticipated based on the key clinical feature of excessive daytime sleepiness. Less easily explained is that over a third of patients endorsed sexual and memory dysfunction. Sexual dysfunction has been noted previously [17] but not emphasized in descriptions of the disease. Although memory has been found to be preserved on neuropsychological testing, despite complaints of memory problems, executive function may be impaired in patients with narcolepsy [18]. Whether these dysfunctions relate to sleep deprivation, some direct effect of narcolepsy, or some medication side effect cannot be determined by this study, but the issue of cause deserves further consideration. Interventions aimed at improving these dysfunctions may improve the quality of life for patients with narcolepsy.
Although the study was population-based, we did not recruit all patients with physician-diagnosed narcolepsy into the full study so the responses of those who participated may have differed from those who did not participate. We did not perform standardized sleep studies in all patients, but the region׳s sleep medicine experts made most of the diagnoses [12], and our results were similar when restricted to patients with cataplexy. We also did not have biological specimens beyond DNA in these patients, such as hypocretin-1 levels in the cerebrospinal fluid. This study was cross-sectional, and longitudinal studies would be needed to judge the responsiveness of the SIP to changes judged important by the patients and providers.
The SIP is well validated but has been largely replaced by briefer screens such as the SF-36, which has been used in patients with narcolepsy. We would encourage others to explore using the SIP in populations of patients with narcolepsy because of the richness of descriptive information it provides and its simplicity. Inclusion of the SIP or some other general health status measures should be considered in clinical trials to detect unanticipated positive or negative effects of a treatment on daily function.
Financial support
The National Institute of Neurological Disorders and Stroke funded this study (NS038523).
Declaration of interest
None.
Acknowledgments
The National Institute of Neurological Disorders and Stroke funded this study (NS038523). This study would not have been possible without help on patient identification and recruitment from the many sleep medicine specialists in King County, especially Dr. Ralph Pascualy at the Swedish Sleep Medicine Institute, Seattle, WA and without help on HLA genotyping from Dr. Vivian Gersuk and Dr. Gerald Nepom at the Benaroya Research Institute at Virginia Mason, Seattle, WA.
Footnotes
Peer review under responsibility of Brazilian Association of Sleep.
Contributor Information
Thanh G.N. Ton, Email: thanhton@uw.edu.
Nathaniel F. Watson, Email: nwatson@uw.edu.
Thomas D. Koepsell, Email: koepsell@uw.edu.
William T. Longstreth, Email: wl@uw.edu.
References
- 1.Johns M.W. A new method for measuring daytime sleepiness: the epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 2.Hublin C., Kaprio J., Partinen M., Koskenvuo M., Heikkila K. The ullanlinna narcolepsy scale: validation of a measure of symptoms in the narcoleptic syndrome. J Sleep Res. 1994;3:52–59. doi: 10.1111/j.1365-2869.1994.tb00104.x. [DOI] [PubMed] [Google Scholar]
- 3.Stewart A.L., Hays R.D., Ware J.E., Jr. The mos short-form general health survey. Reliability and validity in a patient population. Med Care. 1988;26:724–735. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Reimer M.A., Flemons W.W. Quality of life in sleep disorders. Sleep Med Rev. 2003;7:335–349. doi: 10.1053/smrv.2001.0220. [DOI] [PubMed] [Google Scholar]
- 5.Ervik S., Abdelnoor M., Heier M.S., Ramberg M., Strand G. Health-related quality of life in narcolepsy. Acta Neurol Scand. 2006;114:198–204. doi: 10.1111/j.1600-0404.2006.00594.x. [DOI] [PubMed] [Google Scholar]
- 6.Bergner M., Bobbitt R.A., Carter W.B., Gilson B.S. The sickness impact profile: development and final revision of a health status measure. Med Care. 1981;19:787–805. doi: 10.1097/00005650-198108000-00001. [DOI] [PubMed] [Google Scholar]
- 7.de Bruin A.F., de Witte L.P., Stevens F., Diederiks J.P. Sickness impact profile: the state of the art of a generic functional status measure. Soc Sci Med. 1992;35:1003–1014. doi: 10.1016/0277-9536(92)90240-q. [DOI] [PubMed] [Google Scholar]
- 8.Longstreth W.T., Jr., Nelson L., Linde M., Muñoz D. Utility of the sickness impact profile in Parkinson׳s disease. J Geriatr Psychiatry Neurol. 1992;5:142–148. doi: 10.1177/002383099200500303. [DOI] [PubMed] [Google Scholar]
- 9.Petajan J.H., Gappmaier E., White A.T., Spencer M.K., Mino L., Hicks R.W. Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann Neurol. 1996;39:432–441. doi: 10.1002/ana.410390405. [DOI] [PubMed] [Google Scholar]
- 10.Deyo R.A., Inui T.S., Leininger J., Overman S. Physical and psychosocial function in rheumatoid arthritis. Clinical use of a self-administered health status instrument. Arch Intern Med. 1982;142:879–882. [PubMed] [Google Scholar]
- 11.Carter W.B., Bobbitt R.A., Bergner M., Gilson B.S. Validation of an interval scaling: the sickness impact profile. Health Serv Res. 1976;11:516–528. [PMC free article] [PubMed] [Google Scholar]
- 12.Longstreth W.T., Jr, Ton T.G.N., Koepsell T., Gersuk V.H., Hendrickson A., Velde S. Prevalence of narcolepsy in king county, washington, USA. Sleep Med. 2009;10:422–426. doi: 10.1016/j.sleep.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gersuk V.H., Nepom G.T. A real-time polymerase chain reaction assay for the rapid identification of the autoimmune disease-associated allele hla-dqb1⁎0602. Tissue Antigens. 2009;73:335–340. doi: 10.1111/j.1399-0039.2009.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anic-Labat S., Guilleminault C., Kraemer H.C., Meehan J., Arrigoni J., Mignot E. Validation of a cataplexy questionnaire in 983 sleep-disorders patients. Sleep. 1999;22:77–87. [PubMed] [Google Scholar]
- 15.Thannickal T.C., Nienhuis R., Siegel J.M. Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy. Sleep. 2009;32:993–998. doi: 10.1093/sleep/32.8.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyagawa T., Honda M., Kawashima M., Shimada M., Tanaka S., Honda Y. Polymorphism located in tcra locus confers susceptibility to essential hypersomnia with hla-drb1⁎1501-dqb1⁎0602 haplotype. J Hum Genet. 2009 doi: 10.1038/jhg.2009.118. [DOI] [PubMed] [Google Scholar]
- 17.Karacan I. Erectile dysfunction in narcoleptic patients. Sleep. 1986;9:227–231. doi: 10.1093/sleep/9.1.227. [DOI] [PubMed] [Google Scholar]
- 18.Naumann A., Bellebaum C., Daum I. Cognitive deficits in narcolepsy. J Sleep Res. 2006;15:329–338. doi: 10.1111/j.1365-2869.2006.00533.x. [DOI] [PubMed] [Google Scholar]