Abstract
In 1998, a group of phenotypically distinct neurons were discovered in the postero-lateral hypothalamus which contained the neuropeptides hypocretin 1 and hypocretin 2 (also called orexin A and orexin B), which are excitatory neuromodulators. Hypocretinergic neurons project throughout the central nervous system and have been involved in the generation and maintenance of wakefulness. The sleep disorder narcolepsy, characterized by hypersomnia and cataplexy, is produced by degeneration of these neurons.
The hypocretinergic neurons are active during wakefulness in conjunction with the presence of motor activity that occurs during survival-related behaviors. These neurons decrease their firing rate during non-REM sleep; however there is still controversy upon the activity and role of these neurons during REM sleep. Hence, in the present report we conducted a critical review of the literature of the hypocretinergic system during REM sleep, and hypothesize a possible role of this system in the generation of REM sleep.
Keywords: Hypothalamus, Peptides, Paradoxical sleep, Narcolepsy, Cataplexy, MCH
1. Introduction
The basis for one of the most paradigmatic sleep disorders, narcolepsy, has been partially solved. Nowadays, based on animal and human research, i.e., a prolific interaction between basic and clinical data, it is known that the pathogenesis of narcolepsy with cataplexy occurs as a result of the degeneration of hypocretinergic (orexinergic) neurons within the hypothalamus [1]. An autoimmune process may be responsible for the degeneration of hypocretinergic neurons; in fact, a recent study demonstrated the presence of CD4+T cells that are reactive to hypocretin in patients with narcolepsy–cataplexy, which suggests a T cell-driven autoimmune response [2]. However, there are still many questions about the physiology of the hypocretinergic system.
The physiopathology of narcolepsy spins around two main axes: the difficulty in maintaining wakefulness (hypersomnia, mainly in the form of sleep attacks) and an increase in rapid eyes movements (REM) sleep. This is manifested by a decrease in REM sleep latency or in intrusion of partial aspects of this state into wakefulness (cataplexy, sleep paralysis, hypnagogic hallucinations) [1]. However, in spite of the presence of hypersomnia, night sleep is also disrupted.
It is well-known that in the mesopontine tegmentum is located the “necessary” and “sufficient” neuronal network for REM sleep generation [3–5]. This network is strongly modulated by hypothalamic projections from the postero-lateral hypothalamus, where hypocretinergic neurons are located (see below). Consequently, in the present report, we will focus on the role of the postero-lateral hypothalamus and its hypocretinergic neurons in the generation and control of REM sleep.
2. Hypocretinergic neurons, hypocretins and receptors
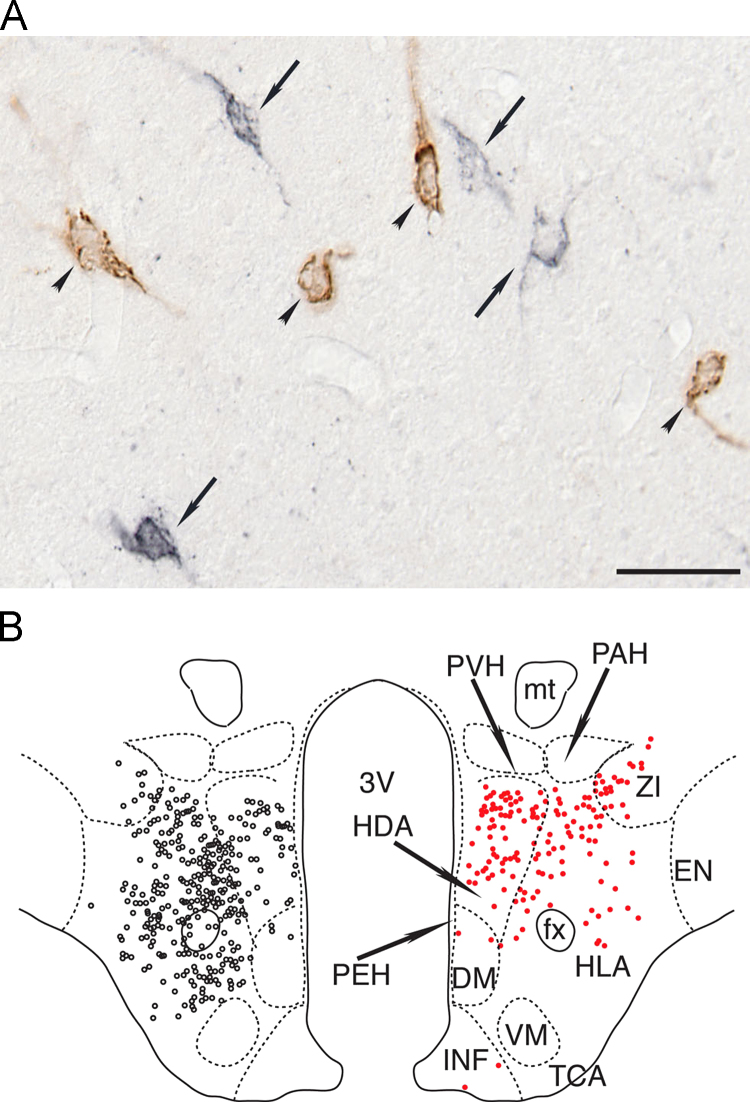
Hypocretin (Hcrt) 1 and Hcrt 2 (also called orexin A and B) were discovered in 1998 by two independent groups [6,7]. These neuropeptides are synthesized in a discrete group of neurons (~5000 in rodents, ~11,000 in cat and 20–50,000 in humans) in the postero-lateral hypothalamus [8,9]. Fig. 1 shows the characteristics and distribution of the hypocretinergic neurons in the postero-lateral hypothalamus. Hcrts exert their biological function through two metabotropic receptors Hcrt-R1 and Hcrt-R2 (also known as orexin 1 and 2 receptors) that have broad and partially overlapping but distinct patterns of distribution throughout the brain and body. Through these receptors, Hcrts produce an excitatory effect at the presynaptic and postsynaptic sites [1,8].
Fig. 1.
Hypocretinergic neurons are intermingled with MCHergic neurons in the postero-lateral hypothalamus. (A) Photomicrographs of the postero-lateral hypothalamic area of the cat. The sections were immunostained for hypocretin (black, arrows) and MCH (brown, arrowheads). Sections were processed utilizing the ABC method and the DAB–H2O2 reaction to detect peroxidase activity. This reaction was enhanced with nickel to label hypocretinergic cells. Calibration bars: 50 μm. (B) Location of MCHergic and hypocretinergic neurons in the postero-lateral hypothalamus of a representative cat. Camera lucida drawings of MCHergic (on the left, black circles) and hypocretinergic neuronal bodies (on the right, red circles) in the postero-lateral hypothalamus. The neurons are from the same hemi-hypothalamus (reflected in the figure). Camera lucida drawings were obtained from adjacent sections that were immunostained for MCH for Hcrt-2, respectively; these sections were counterstained with Pyronin-Y. The demarcation and nomenclature of cell groups in the cat hypothalamus are based on Berman and Jones, as well as Bleier׳s work [105,106]. DM, dorsomedial nucleus; EN, entopeduncular nucleus; fx, fornix; HDA, dorsal hypothalamic area; HLA, lateral hypothalamic area; INF, infundibular nucleus; mt, mammillothalamic tract; PAH, paraventricular nucleus; PEH, periventricular complex; PVH, parvocellular nucleus; TCA, area of the tuber cinereum; VM, ventromedial nucleus; ZI, zona incerta; 3V, third ventricle. Modified from [9]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3. Postero-lateral hypothalamus
A logical first step in determining the function of any discrete, highly localized group of neurons, is to examine the behaviors and processes that are controlled by the site in the brain wherein they reside. The postero-lateral hypothalamic area is the key brain site that, for decades, has been identified as being responsible for initiating, coordinating and maintaining goal-oriented survival-type behaviors such as fight, flight and food consumption among others [10–15]. In addition, and in accordance with the preceding concept, experimental studies have demonstrated that this area is critically involved in controlling the following functions:
Sleep and wakefulness: Electrical or chemical activation of the postero-lateral hypothalamus area results in wakefulness accompanied by different patterns of somatomotor activity (see below). Interestingly, chemical inhibition by muscimol (a GABAA agonist) in this area decreases wakefulness and leads to non-REM (NREM) sleep hypersomnia [16]. In addition, it abolishes REM sleep. The preceding data suggests the presence of REM sleep inducing neurons within this area.
Somatomotor activity: Electrolytic lesions or chemical inhibition of this region have been shown to produce a marked decrease in locomotor activity, while electrical or chemical activation produces an increase in motor activity [17–22]. It is also well established that stimulation within the perifornical lateral hypothalamic region initiates predatory activities and that these behaviors are abolished or attenuated when this hypothalamic site is lesioned [12].
The “pleasure” or “reward” zone of the hypothalamus [13,23–25] is congruent with the site of the greatest concentration of hypocretinergic neurons [26,27].
It is important to consider that, in addition to the hypocretinergic neurons, other groups of neurons such as the melanin concentrating-hormone (MCH) containing neurons are present within this area [9]. The anatomical interrelation between these groups of neurons is shown in Fig. 1.
4. Afferents to hypocretinergic neurons
Hypocretinergic neurons receive inputs from several regions such as the allocortex, many hypothalamic nuclei, periaqueductal gray matter, the dorsal raphe nucleus (DRN), and parabrachial regions, which suggest that these neurons integrate a variety of interoceptive, exteroceptive and homeostatic signals [28].
Several neurotransmitters act on hypocretinergic neurons [29]. Glutamate depolarizes these neurons acting through AMPA and NMDA receptors, and GABA inhibits these neurons through GABAA and GABAB receptors. Neurotransmitters used by neurons that form part of the activating systems [30], such as serotonin, noradrenaline, acetylcholine and dopamine, modulate the activity of hypocretinergic neurons. An in vitro study in mice has shown that serotonin and noradrenaline hyperpolarize hypocretinergic neurons through 5HT1A and α2 receptors, respectively [29]. A weak depolarization mediated by α1 receptor was also observed in the presence of a α2-receptor antagonist. In rats, both noradrenaline and acetylcholine have a predominant excitatory effect [31]. Carbachol (a mixed cholinergic agonist) depolarizes 27% and hyperpolarizes 6% of the population of hypocretinergic neurons in mice [29]; these effects are mediated by different muscarinic receptors. Interestingly, histamine has almost no effect on hypocretinergic neurons. D1 and D2 dopamine receptors have opposing effects on excitatory presynaptic terminals that impinge on hypocretinergic neurons [32].
The sleep-promoting factor, melanin-concentrating hormone (MCH), inhibits hypocretinergic neurons (see below).
5. Projections of the hypocretinergic neurons
Hypocretinergic neurons project throughout the central nervous system [26]; furthermore, the activity of the peripheral organs is also influenced by the Hcrts [33]. Hypocretinergic neurons also have the potential to mediate complex functions since they exhibit the morphology of prototypical “command” neurons, which are small groups of highly specialized cells that coordinate and integrate, in a complementary fashion, the activities of a vast number of neural and hormonal systems [34–36].
Sensory and motor nuclei are directly innervated by hypocretinergic neurons [37–40]. These neurons also project to the thalamus and cortex [26], wherein they directly influence thalamo-cortical activities that support cognitive functions. Dense concentrations of hypocretin-containing axon terminals are located in the tuberomammillary nucleus of the hypothalamus, a waking-promoting area [41], as well as in brainstem areas such as the laterodorsal and pedunculopontine tegmental nucleus (LDT–PPT), locus coeruleus (LC) and DRN [26,42–44], that are known to participate in the control of sleep and wakefulness [30].
We demonstrated that hypocretinergic neurons project to the nucleus pontis oralis (NPO), which is considered to exert executive control over the initiation and maintenance of REM sleep [45]. A single injection of a cholinergic agonist, such as carbachol, within the NPO of the cat, results in the generation of a state that consists of all the behavioral and electrographic signs of REM sleep with a very short latency (30 s to a few minutes); this state lasts up to two hours (see below).
6. Hypocretinergic neurons and wakefulness
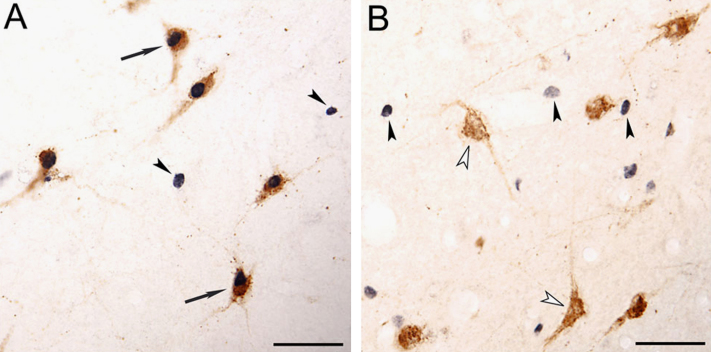
Early studies revealed that the intraventricular injection of Hcrt promotes wakefulness [46,47]. These data, as well as the fact that the lack of hypocretinergic neurons in narcoleptic patients induces hypersomnia [1], strongly suggested that this system promotes wakefulness. However, by means of Fos technology (the Fos protein is a marker of neuronal activity), we demonstrated in the cat, that hypocretinergic neurons are not active during wakefulness per se [48]. On the contrary, hypocretinergic system becomes active during aroused wakefulness when the animal is moving. In the absence of motor activity during alert wakefulness, quiet wakefulness or quiet sleep, the hypocretinergic system is not activated to any significant extent [49]. In this regard, several reports confirmed that the hypocretinergic neurons are involved in the promotion of somatomotor activity. The data in Fig. 2 demonstrates that while Hcrt-containing neurons do not express Fos during quiet wakefulness, they are active during active wakefulness.
Fig. 2.
Photomicrographs illustrating hypocretin and Fos immunoreactive neurons from the postero-lateral area of the hypothalamus. (A) Hypocretinergic neurons that express c-fos during active wakefulness with motor exploratory activity (arrows). Hypocretinergic neurons are stained in brown. Fos immunoreactivity, which is shown in black, is restricted to nuclei. Hcrt-Fos+ neurons (arrowheads) are also intermingled with Hcrt+Fos+neurons. (B) Group of hypocretinergic neurons during quiet wakefulness. Hypocretinergic neurons did not express c-fos (unfilled arrowheads), although Hcrt-Fos+neurons are intermingled with these neurons (filled arrowheads). All photomicrographs were taken from 20 µm-thick sections and were processed with the diaminobenzidine method enhanced by nickel. Calibration bars: (A and B) 50 µm. Modified from [48]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
A detailed analysis of hypocretinergic neuronal activity shows that these neurons are strongly activated when animals are exploring an unknown environment (exploratory motor activity) [50]. In fact, in this study, the number of Hcrt+Fos+neurons was approximately 10 times greater during exploratory motor activity than during repetitive motor activity that occurred during forced locomotion, even though in both paradigms there was a comparable amount of motor activity. Therefore, neither wakefulness nor motor activity per se, were critical with respect to the activation of hypocretinergic neurons. Therefore, we conclude that the hypocretinergic system is engaged when animals are performing goal (reward)-directed behaviors. In agreement with our results, it was found that Hcrt knock-out mice were unable to work for food or water reward during the light phase [51].
Recently, Chase presented the “Unified Survival Theory for the Functioning of the Hypocretinergic System” [52]. The basis of this theory is that the main role of the hypocretinergic system is to initiate, coordinate and maintain survival behaviors and survival-related processes.
7. Hypocretinergic neurons and NREM sleep
The hypocretinergic neurons, as a component of the activating systems [30], do not participate in NREM sleep. In fact, we demonstrated a lack of Fos expression in these neurons during this behavioral state [49]. Subsequently, unit recordings confirmed our early findings [53–55].
8. Hypocretinergic neurons and REM sleep
There are presentations that argue that hypocretinergic neurons are REM-OFF neurons and that these neurons inhibit REM sleep. The REM-OFF profile concept of the hypocretinergic neurons arose based upon electrophysiological recordings of identified hypocretinergic neurons [53–55]. Lee et al. [54] recorded six hypocretinergic neurons in the hypothalamus of the rat during unanesthesized semi-restricted conditions. They found that the six neurons exhibited the largest firing rate during wakefulness, the rate decreased during NREM sleep, and a nadir was reached during REM sleep. Another study in rats [53], and a study in mice [55], demonstrated similar firing profiles.
However, an in-depth analysis of the preceding data reveals that hypocretinergic neurons also discharge during some “phasic” components of REM sleep. Fig. 2 of Lee et al.׳s [54] study illustrates the discharge of one representative neuron; its firing rate increases in relation to muscle twitches during REM sleep as well as during REM sleep that precedes awakening. In addition, their Fig. 2 shows that there is a burst of firing in a short period (just a couple of seconds) of “wakefulness” that is between two REM sleep periods (without a preceding NREM sleep epoch). Amici et al., consider this kind of episodes as a “cluster” type of REM sleep, instead of two independent REM sleep episodes interrupted by an episode of wakefulness [56,57]. Another study in rats also acknowledged that hypocretinergic neurons “occasionally discharge in phasic REM” [53]. In addition, in freely moving mice, hypocretinergic neurons display transient discharges during REM sleep [55]. Hence, at least some hypocretinergic neurons increase their firing rate during the phasic periods of REM sleep. It would be important to examine the pattern of activity of these neurons in an animal that exhibits robust phasic periods of REM sleep, such as the cat [58].
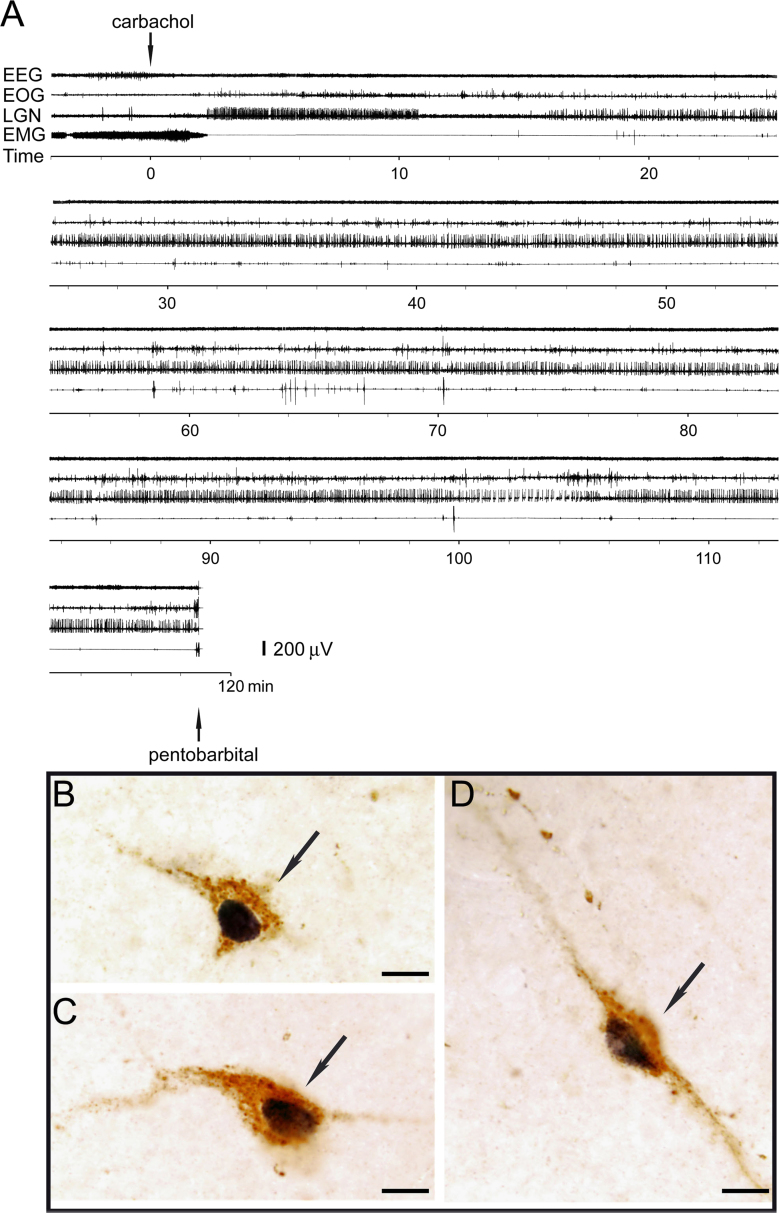
Studies in the cat, strongly suggest that there is hypocretinergic neuronal activity during REM sleep, probably during the phasic events of this state. An increase in the number of Hcrt+Fos+neurons was observed during REM sleep induced by carbachol microinjections into the NPO [48]. In this study, REM sleep was induced by carbachol microinjections in order to generate a state of sufficiently long duration to allow Fos protein to be synthesized in high concentration. Fig. 3A illustrates a representative carbachol-induced period of REM sleep, which has the same characteristics as natural REM sleep. During this state, 34% of the hypocretinergic neurons were activated according to their Fos-expression; representative active “Hcrt+Fos+” neurons are shown in Fig. 3B. The distribution of the active hypocretinergic neurons of a representative experiment is shown in Fig. 4. This result is in agreement with the findings of Kiyashchenko et al. [59]. Utilizing microdialysis in freely moving cats they found an increase in Hcrt-1 release during REM sleep, both in the hypothalamus and basal forebrain. Hence, this study also indicates that hypocretinergic neurons are active during REM sleep, probably in conjunction with phasic events.
Fig. 3.
(A) Polygraphic recording of an episode of REM sleep induced by carbachol microinjection into the nucleus pontis oralis (NPO) in the cat. Arrows signal the beginning of the microinjection of carbachol into the NPO as well as pentobarbital injection for euthanasia. EEG, electroencephalogram; EOG, electro-oculogram; LGN, lateral geniculate nucleus electrogram (PGO waves); EMG, electromyogram. (B–D) Photomicrographs illustrate Fos immunoreactive hypocretinergic neurons (arrows) from the postero-lateral area of the hypothalamus during REM sleep induced by carbachol. Hypocretin immunoreaction is stained in brown. Fos immunoreactivity, which is shown in black, is restricted to the nuclei. Sections were processed employing the ABC method and the DAB–H2O2 reaction to detect peroxidase activity. This reaction was enhanced with nickel to label the Fos protein. Calibration bars: (B–D) 10 μm. Modified from [48]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
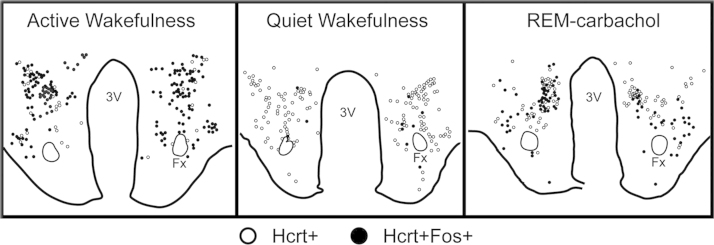
Fig. 4.
Distribution of Fos and hypocretinergic neurons during active wakefulness with motor-exploratory activity, quiet wakefulness and REM sleep induced by the microinjection of carbachol into the nucleus pontis oralis. The camera lucida drawing shows the distribution of Hcrt+Fos+neurons. Each mark indicates one labeled neuron. The percentage of Hcrt+Fos+neurons from the total number of hypocretinergic neurons was on average 79% for active wakefulness, 34% for REM-carbachol and 2% for quiet wakefulness [48]. 3V, third ventricle; Fx, fornix.
Hypocretinergic activation during REM sleep is likely related to the activation that occur during certain patterns of somatomotor activity [50]. Although motor output is inhibited at the motoneuron level during REM sleep, supraspinal motor systems are very active during this state [60–62]. In fact, in conjunction with PGO waves, a phasic event that occurs during REM sleep, there is a potentiation of the REM sleep-related hyperpolarization of motoneurons [3].
Microinjections studies also suggest that Hcrt may contribute with some aspects of REM sleep. Hcrt-1 applied into the gigantocellular nucleus and dorsal paragigantocellular nucleus of the medullary reticular formation, which are sites that have been previously identified that generate atonia by electrical stimulation, produced bilateral hindlimb muscle atonia, similar to which occurs during REM sleep atonia and cataplexy [63].
We demonstrated previously the presence of hypocretinergic projections to the NPO, the REM sleep executive area in the pons [45]. Microinjections of Hcrt-1 or Hcrt-2 into the NPO of the cat increase the time spent in REM sleep and result in a decrease in the latency to the generation of this state [64]. Furthermore, the juxtacellular application of Hcrt-1 results in an increase in the excitability of NPO neurons, which is associated with the induction of REM sleep [65]. In addition, Hcrt-1 increases acetylcholine release in the NPO of the rat [66,67]. In this regard, it is known that acetylcholine levels within this region increase during REM sleep [68]. However, a decrease in REM sleep when Hcrt-1 is microinjected into the NPO of the cat was also described [69]. Furthermore, the iontophoretic application of Hcrt-1 into the NPO of the rat produces an inhibition of NPO neurons, which can be blocked by previous iontophoretic application of bicuculline, a GABAA receptors antagonist [70]. In fact, it has been shown that Hcrt increases GABA levels in the NPO of the rat, and hypocretin and GABA interact within this nucleus to promote wakefulness [71,72]. The presence of Hcrt-2 receptors on GABAergic neurons within the NPO may be the cellular basis for this effect [73].
The paradoxical or contradictory findings involving the REM sleep and wakefulness promoting actions of Hcrt within the NPO were reconciled by Xi and Chase [74]. They demonstrated that the microinjections of Hcrt-1 within the NPO generate REM sleep when applied during NREM sleep, but promote wakefulness when applied during this behavioral state. Thus, the behavioral state of the animal at the time of the application of Hcrt determines whether REM sleep or wakefulness occurs.
It is attractive to note that hypocretinergic cells in the hypothalamus may be involved in the control of both active wakefulness and REM sleep, and that within both patterns of behavioral state control changes in motor activity play a predominant role. This pattern of duality of behavioral state control with opposite motor responses is reminiscent of the phenomenon of Reticular Response-Reversal in the NPO [75,76]. This phenomenon involves mechanisms that result in the facilitation of wakefulness and somatomotor activation during wakefulness, as well as the generation of REM sleep and its accompanying pattern of motor inhibition during this sleep state [75,76]. Reticular Response-Reversal determines, for example, that auditory stimulation promotes somatomotor activation during wakefulness, and also increases the hyperpolarization of motoneurons and produces atonia during REM sleep [3].
It is well known that in the “twitches” that occur during REM sleep, there is an increase in the frequency of motoneuron postsynaptic inhibitory potentials (IPSPs) that generate atonia compared with the tonic periods of REM sleep. Paradoxically, there is also an important increase in excitatory drives (excitatory postsynaptic potentials, EPSPs) that also impinge on motoneurons during “phasic” REM sleep. Hence, there is a competition between these opposing forces during phasic REM sleep; the winner determines whether motoneurons discharge or remain silent [3]. Hypocretins are likely related to this phenomenon; these peptides may facilitate motor activity during wakefulness and the phasic components of REM sleep by direct actions onto motoneurons (see below). Furthermore, throughout REM sleep, hypocretinergic activation of the NPO generates motor inhibition due to processes involving Reticular Response-Reversal process [3,52].
In narcolepsy–cataplexy, there is an increase in the incidence of REM sleep behavioral disorder (RBD) [77]. In RBD the mechanisms that produce atonia are significantly reduced, which supports the hypothesis that the dysfunction of the hypocretinergic system produces instability of motor regulatory systems during REM sleep.
9. Working hypothesis 1: hypocretinergic neurons are active during “phasic” REM sleep
EEG activation, theta activity in the hippocampus and muscle atonia are the classic biomarkers for the identification of REM sleep. Accompanying these “tonic” signs are rapid eye movements, muscle twitches, PGO waves, breathing irregularities as well as heart rate and blood pressure increases that constitute the phasic events of REM sleep. Other signs such as acceleration of the theta rhythm also correlate with these phasic events [78].
Experimental evidences suggest that while hypocretinergic neurons turn off during “tonic” REM sleep, at least a subset of these neurons discharge in burst during phasic REM sleep [53–55]. Furthermore, during REM sleep induced by carbachol, there is an activation of 34% of hypocretinergic neurons [48]. In addition, Hcrt-1 synaptic release increases during REM sleep [59]. These data suggest that hypocretinergic neurons are active during the “phasic” component of REM sleep (Fig. 5).
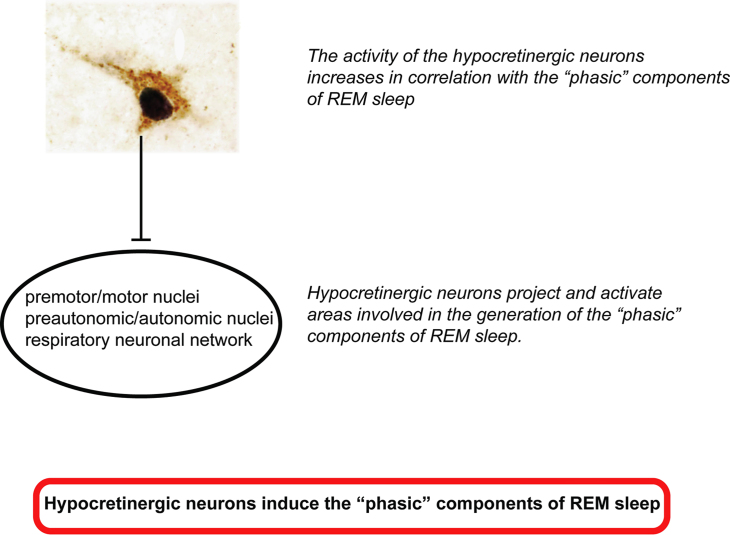
Fig. 5.
Schema of the working hypotheses 1 and 2. Hypocretinergic neurons not only are correlated with the “phasic” components of REM sleep but also promote its generation.
10. Working hypothesis 2: hypocretinergic neurons are involved in the generation of the “phasic” phenomena of REM sleep
If hypocretinergic neurons are active during “phasic” REM sleep, they are likely to promote the phasic events of REM sleep. Hypocretins directly activate motor nuclei, breathing neuronal networks and sympathetic output that control the cardiovascular system [40,79–81], as well as the medial septum where the pacemaker for the hypocamppal theta rhythm is located [82]. By these means, hypocretinergic neurons promote the “phasic” components of REM sleep (Fig. 5).
Yamuy et al. [40] demonstrated in lumbar motoneurons that the juxtacellular administration of hypocretin-1 and electrical activation of hypocretinergic neurons activate motoneurons. Therefore, it is likely that the “phasic” discharge of hypocretinergic neurons during REM facilitates motoneuron activity and muscle twitches. The intracerebroventricular injection of either hypocretin 1 and 2 increases heart rate, mean arterial pressure and renal sympathetic activity in conscious rats [79]. Hypocretins stimulate breathing and knocking-out the preprohypocretin gene in mice reduces CO2-induced increases in breathing by 50% and increases the frequency of spontaneous sleep apneas [81]. Lesions of medial septum neurons by hypocretin-2 conjugated with the neurotoxin saporin abolishes theta rhythm of the hippocampus during both active wakefulness and REM sleep, suggesting that hypocretin facilitates theta rhythm generation [82]. All these effects induced by hypocretin involve ergotropic or energy-expending behaviors [52].
Although disturbances in the phasic components of REM sleep occurs in narcolepsy–cataplexy and in hypocretin-deficient mice [83–85], new experimental approaches are needed in order to confirm the relationship between hypocretins and “phasic” REM sleep.
11. Working hypothesis 3: there are complementary but opposite functions of hypocretin and MCH in the control of REM sleep
Due to the importance of the MCHergic system in sleep physiology [86,87], it is relevant to examine the interactions between the MCHergic and the hypocretinergic systems. A strong anatomical relationship exists between hypocretinergic and MCHergic neurons in the hypothalamus. As it is shown in Fig. 1, MCHergic neurons are intermingled with Hcrt-containing neurons in the postero-lateral hypothalamus, mainly at the tuberal and tuberomammillar levels [9]. MCHergic fibers are in close relationship with hypocretinergic neurons and vice versa, which suggest the existence of reciprocal synaptic contacts between both types of cells [9,88]. This fact as well as the presence of hypocretinergic receptors on MCHergic neurons indicates the existence of an important functional interaction between both systems [89]. In this regard, Hcrt increases MCH mRNA expression in hypothalamic neurons, directly excites MCHergic neurons and increases glutamate release onto them [90,91]. On the other hand, MCH modulates Hcrt-mediated effects on behavioral state and synaptic transmission in the lateral hypothalamus [92]. In addition, the efficacy of glutamatergic synapses on hypocretinergic neurons is enhanced in MCHR1 knockout mice, and Hcrt-1-induced firing is facilitated. On the contrary, in wild-type mice, MCH significantly attenuates Hcrt-1 induced enhancement of spike frequency in hypocretinergic neurons, but not its basal activity. Furthermore, in these neurons, MCH attenuates Hcrt-1-induced enhancement of the frequency of miniature excitatory postsynaptic currents. These effects imply that MCH exerts a unique inhibitory influence on hypocretinergic signaling as a way to fine-tune the output of these neurons.
Interestingly, hypocretinergic and MCHergic neurons respond in a different way to most homeostatic signals such as glucose [93] or to waking-related neurotransmitters such as noradrenaline [31]. It is of note that while hypocretinergic neurons of the rat mainly express α1 adrenergic receptors, MCHergic neurons express the α2 adrenergic receptors, which are related to activation or inhibition of their targets, respectively [94].
We have shown that both hypocretinergic and MCHergic neurons project to the NPO [45,95]. In addition, fibers and terminals of both systems are highly intermingled, which suggests the presence of important interactions between these systems within their mesopontine targets, similar to the anatomical and functional interactions that have been described within the hypothalamus (see above).
Several studies suggest that MCHergic neurons are involved in the generation of sleep, especially REM sleep [86,87]. These neurons discharge in a reciprocal manner to hypocretinergic neurons across the sleep–wake cycle. MCHergic neurons have a high firing rate during tonic REM sleep, but do not increase their firing level during “phasic” REM sleep [96]. When MCH is microinjected in the area where REM-carbachol is generated, it produces an increase in time that the animals spend in REM sleep together with a decrease in the latency to this behavioral state [95]. MCH also exerts its REM sleep promoting functions acting through the DRN [97,98], where it exerts an inhibitory role on serotonergic neurons [99,100].
Interestingly, MCH blunts the central regulation of sympathetic tone and adaptive sympathetic reflexes, and decreases metabolism [101–103]. These are trophotropic or energy-conserving effects, which are opposite to the effects produced by the hypocretinergic system (see above).
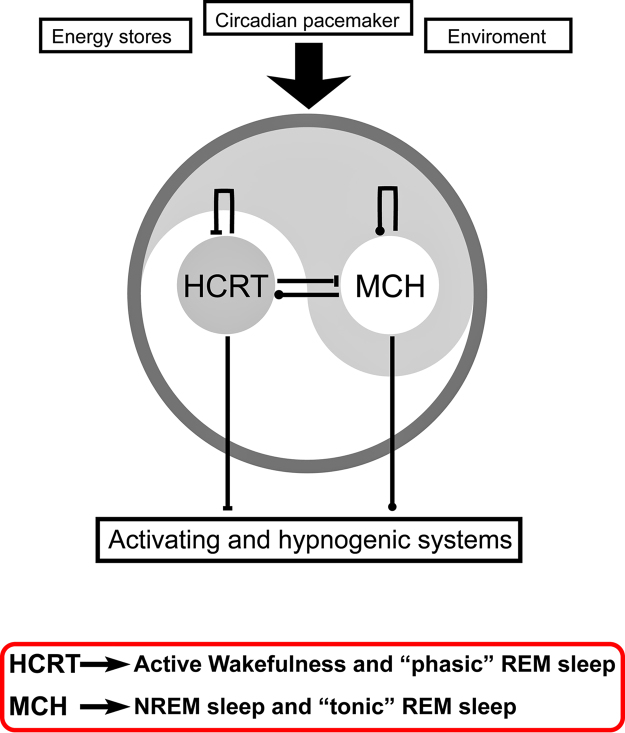
These experimental data are the basis for the hypothesis that while MCHergic neurons are active during tonic REM sleep and promote this “quiescent” behavioral state, hypocretinergic neurons are active during “phasic” REM sleep and induce (at least partially) phasic episodes of this state (see Fig. 6).
Fig. 6.
The yin and yang is the Taoist symbol used to describe how polar or seemingly contrary forces are interconnected and interdependent in the natural world, and how they give rise to each other in turn. This concept could be applied to the opposite but complementary role of hypocretin and melanin-concentrating hormone (MCH) in the control of wakefulness and sleep. Hypocretin (HCRT) an excitatory neuropeptide, and MCH an inhibitory neuropeptide, interact both at the lateral hypothalamic level as well as in the activating and hypnogenic neuronal areas, in order to regulate the generation of the sleep and wakefulness cycle.
12. Research agenda
In order to confirm the relationship between the hypocretins and the “phasic” episodes of REM sleep, additional experimental data are needed. Electrophysiological recording studies would be important to conduct in different species in order to correlate hypocretinergic discharge during REM sleep and the different aspects of the “phasic” components of REM sleep. In addition, studies of the effect on “phasic” REM sleep of activating or blocking the hypocretinergic system during REM sleep, for example by means of optogenetic stimulation [104] or by the administration of hypocretin agonists or antagonists, would be important to confirm our hypotheses. Similar approaches are also necessary to demonstrate that MCH plays a proactive role in “tonic” REM sleep but not in “phasic” REM sleep.
13. Conclusions
In the present report we reviewed the role of the hypocretinergic neurons in REM sleep generation. Experimental evidences suggest that these neurons are active during the “phasic” episodes of REM sleep and we hypothesize that they may be involved in the induction of these episodes. In addition, we consider that, by modulation of mesopontine networks such as those involving cholinergic neurons of the LDT–PPT, the monoaminergic neurons of the DRN and LC, and especially through the NPO, the executive area for REM sleep generation, MCHergic and hypocretinergic neurons induces “tonic” (trophotropic) and “phasic” (ergotropic) REM sleep states, respectively.
Acknowledgments
This work was partially supported by the “Programa de Desarrollo de Ciencias Básicas, PEDECIBA” and by the ANII-FCE-2–2011-1-7030 grant from Uruguay.
Footnotes
Peer review under responsibility of Brazilian Association of Sleep
References
- 1.Mignot E. Narcolepsy: pathophysiology and genetic predisposition. In: Krieger M.H., Roth T., Dement W., editors. Principles and practices of sleep medicine. Saunders; Philadelphia: 2011. pp. 938–956. [Google Scholar]
- 2.De la Herran-Arita A.K., Kornum B.R., Mahlios J., Jiang W., Lin L., Hou T. CD4+ T cell autoimmunity to hypocretin/orexin and cross-reactivity to a 2009 H1N1 influenza A epitope in narcolepsy. Sci Transl Med. 2013;5:216ra176. doi: 10.1126/scitranslmed.3007762. [DOI] [PubMed] [Google Scholar]
- 3.Chase M.H. Motor control during sleep and wakefulness: clarifying controversies and resolving paradoxes. Sleep Med Rev. 2013;17:299–312. doi: 10.1016/j.smrv.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Luppi P.H., Clement O., Fort P. Paradoxical (REM) sleep genesis by the brainstem is under hypothalamic control. Curr Opin Neurobiol. 2013;23:786–792. doi: 10.1016/j.conb.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Siegel J.M. REM sleep. In: Kryger M.H., Roth T., Dement W.C., editors. Principles and practices of sleep medicine. Elsevier-Saunders; Philadelphia: 2005. pp. 120–135. [Google Scholar]
- 6.de Lecea L., Kilduff T.S., Peyron C., Gao X., Foye P.E., Danielson P.E. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R.M., Tanaka H. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 8.Li J., Hu Z., de Lecea L. The hypocretins/orexins: integrators of multiple physiological functions. Br J Pharmacol. 2013 doi: 10.1111/bph.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torterolo P., Sampogna S., Morales F.R., Chase M.H. MCH-containing neurons in the hypothalamus of the cat: searching for a role in the control of sleep and wakefulness. Brain Res. 2006;1119:101–114. doi: 10.1016/j.brainres.2006.08.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernardis L.L., Bellinger L.L. The lateral hypothalamic area revisited: ingestive behavior. Neurosci Biobehav Rev. 1996;20:189–287. doi: 10.1016/0149-7634(95)00015-1. [DOI] [PubMed] [Google Scholar]
- 11.Anand B.K., Brobeck J.R. Localization of a feeding center in the hypothalamus of the rat. Proc Soc Exp Biol Med. 1951;77:323–324. doi: 10.3181/00379727-77-18766. [DOI] [PubMed] [Google Scholar]
- 12.Gregg T.R., Siegel A. Brain structures and neurotransmitters regulating aggression in cats: implications for human aggression. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:91–140. doi: 10.1016/s0278-5846(00)00150-0. [DOI] [PubMed] [Google Scholar]
- 13.Clemente C.D., Chase M.H. Neurological substrates of aggressive behavior. Annu Rev Physiol. 1973;35:329–356. doi: 10.1146/annurev.ph.35.030173.001553. [DOI] [PubMed] [Google Scholar]
- 14.Hess W.R. Grune and Stratton; New York: 1954. The diencephalon. [Google Scholar]
- 15.Swanson L.W. The hypothalamus. In: Bjorklund A., Hokfelt T., Swanson L.W., editors. vol. 5. Elsevier; 1987. pp. 1–124. (Handbook of chemical neuroanatomy). [Google Scholar]
- 16.Lin J.S., Sakai K., Vanni-Mercier G., Jouvet M. A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res. 1989;479:225–240. doi: 10.1016/0006-8993(89)91623-5. [DOI] [PubMed] [Google Scholar]
- 17.Gladfelter W.E., Brobeck J.R. Decreased spontaneous locomotor activity in the rat induced by hypothalamic lesions. Am J Physiol. 1962;203:811–817. doi: 10.1152/ajplegacy.1962.203.5.811. [DOI] [PubMed] [Google Scholar]
- 18.Blake D.J., Gladfelter W.E. Wheel-running activity after kainic acid injection into lateral hypothalamus of rats. Physiol Behav. 1986;36:1009–1016. doi: 10.1016/0031-9384(86)90472-5. [DOI] [PubMed] [Google Scholar]
- 19.Wardas J., Ossowska K., Wolfarth S. Evidence for the independent role of GABA synapses of the zona incerta-lateral hypothalamic region in haloperidol-induced catalepsy. Brain Res. 1988;462:378–382. doi: 10.1016/0006-8993(88)90569-0. [DOI] [PubMed] [Google Scholar]
- 20.Marciello M., Sinnamon H.M. Locomotor stepping initiated by glutamate injections into the hypothalamus of the anesthetized rat. Behav Neurosci. 1990;104:980–990. doi: 10.1037//0735-7044.104.6.980. [DOI] [PubMed] [Google Scholar]
- 21.Sinnamon H.M., Lee S.H., Adams D.B., Stopford C.K. Locomotor stepping elicited by electrical stimulation of the lateral hypothalamus requires an ipsilateral descending pathway. Physiol Behav. 1984;33:209–215. doi: 10.1016/0031-9384(84)90101-x. [DOI] [PubMed] [Google Scholar]
- 22.Shekhar A., DiMicco J.A. Defense reaction elicited by injection of GABA antagonists and synthesis inhibitors into the posterior hypothalamus in rats. Neuropharmacology. 1987;26:407–417. doi: 10.1016/0028-3908(87)90020-7. [DOI] [PubMed] [Google Scholar]
- 23.Olds J. Self-stimulation of the brain; its use to study local effects of hunger, sex, and drugs. Science. 1958;127:315–324. doi: 10.1126/science.127.3294.315. [DOI] [PubMed] [Google Scholar]
- 24.Margules D.L., Olds J. Identical feeding and rewarding systems in the lateral hypothalamus of rats. Science. 1962;135:374–375. doi: 10.1126/science.135.3501.374. [DOI] [PubMed] [Google Scholar]
- 25.Wyrwicka W., Clemente C.D., Chase M.H. Hypothalamic substrates of self-stimulation in cats. Exp Neurol. 1986;93:557–564. doi: 10.1016/0014-4886(86)90175-5. [DOI] [PubMed] [Google Scholar]
- 26.Peyron C., Tighe D.K., van den Pol A.N., de Lecea L., Heller H.C., Sutcliffe J.G. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J.H., Sampogna S., Morales F.R., Chase M.H. Orexin (hypocretin)-like immunoreactivity in the cat hypothalamus: a light and electron microscopic study. Sleep. 2001;24:1–10. doi: 10.1093/sleep/24.1.67. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida K., McCormack S., Espana R.A., Crocker A., Scammell T.E. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamanaka A. Afferent system of orexin neurons. In: Nishino S., Sakurai T., editors. The orexin/hypocretin system. Physiology and pathophysiology. Humana Press; Totowa (New Jersey): 2006. pp. 61–70. [Google Scholar]
- 30.Torterolo P., Vanini G. New concepts in relation to generating and maintaining arousal. Rev Neurol. 2010;50:747–758. [PubMed] [Google Scholar]
- 31.Bayer L., Eggermann E., Serafin M., Grivel J., Machard D., Muhlethaler M. Opposite effects of noradrenaline and acetylcholine upon hypocretin/orexin versus melanin concentrating hormone neurons in rat hypothalamic slices. Neuroscience. 2005;130:807–811. doi: 10.1016/j.neuroscience.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 32.Alberto C.O., Trask R.B., Quinlan M.E., Hirasawa M. Bidirectional dopaminergic modulation of excitatory synaptic transmission in orexin neurons. J Neurosci. 2006;26:10043–10050. doi: 10.1523/JNEUROSCI.1819-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Voisin T., Rouet-Benzineb P., Reuter N., Laburthe M. Orexins and their receptors: structural aspects and role in peripheral tissues. Cell Mol Life Sci. 2003;60:72–87. doi: 10.1007/s000180300005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerman I.A. Organization of brain somatomotor-sympathetic circuits. Exp Brain Res. 2008;187:1–16. doi: 10.1007/s00221-008-1337-5. [DOI] [PubMed] [Google Scholar]
- 35.Oldfield B.J., Allen A.M., Davern P., Giles M.E., Owens N.C. Lateral hypothalamic ‘command neurons’ with axonal projections to regions involved in both feeding and thermogenesis. Eur J Neurosci. 2007;25:2404–2412. doi: 10.1111/j.1460-9568.2007.05429.x. [DOI] [PubMed] [Google Scholar]
- 36.Kupfermann I., Weiss K. The command neuron concept. Behav Brain Sci. 1978;1:3–39. [Google Scholar]
- 37.McGregor R., Damian A., Fabbiani G., Torterolo P., Pose I., Chase M. Direct hypothalamic innervation of the trigeminal motor nucleus: a retrograde tracer study. Neuroscience. 2005;136:1073–1081. doi: 10.1016/j.neuroscience.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 38.Torterolo P., Vanini G., Zhang J., Sampogna S., Chase M. Hypocretinergic fibers and receptors in the inferior colliculus. Sleep. 2007;30:A15. [Google Scholar]
- 39.Fung S.J., Yamuy J., Sampogna S., Morales F.R., Chase M.H. Hypocretin (orexin) input to trigeminal and hypoglossal motoneurons in the cat: a double-labeling immunohistochemical study. Brain Res. 2001;903:257–262. doi: 10.1016/s0006-8993(01)02318-6. [DOI] [PubMed] [Google Scholar]
- 40.Yamuy J., Fung S.J., Xi M., Chase M.H. Hypocretinergic control of spinal cord motoneurons. J Neurosci. 2004;24:5336–5345. doi: 10.1523/JNEUROSCI.4812-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eriksson K.S., Sergeeva O., Brown R.E., Haas H.L. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21:9273–9279. doi: 10.1523/JNEUROSCI.21-23-09273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chemelli R.M., Willie J.T., Sinton C.M., Elmquist J.K., Scammell T., Lee C. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 43.Date Y., Ueta Y., Yamashita H., Yamaguchi H., Matsukura S., Kangawa K. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nambu T., Sakurai T., Mizukami K., Hosoya Y., Yanagisawa M., Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- 45.Torterolo P., Sampogna S., Chase M.H. Hypocretinergic and non-hypocretinergic projections from the hypothalamus to the REM sleep executive area of the pons. Brain Res. 2013;1491:68–77. doi: 10.1016/j.brainres.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hagan J.J., Leslie R.A., Patel S., Evans M.L., Wattam T.A., Holmes S. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piper D.C., Upton N., Smith M.I., Hunter A.J. The novel brain neuropeptide, orexin-A, modulates the sleep–wake cycle of rats. Eur J Neurosci. 2000;12:726–730. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- 48.Torterolo P., Yamuy J., Sampogna S., Morales F.R., Chase M.H. Hypothalamic neurons that contain hypocretin (orexin) express c-fos during active wakefulness and carbachol-induced active sleep. Sleep Res Online. 2001;4:25–32. 〈http://www.sro.org/2001/Torterolo/25〉 [Google Scholar]
- 49.Torterolo P., Yamuy J., Sampogna S., Morales F.R., Chase M.H. Hypocretinergic neurons are primarily involved in activation of the somatomotor system. Sleep. 2003;1:25–28. [PubMed] [Google Scholar]
- 50.Torterolo P., Ramos O.V., Sampogna S., Chase M.H. Hypocretinergic neurons are activated in conjunction with goal-oriented survival-related motor behaviors. Physiol Behav. 2011;104:823–830. doi: 10.1016/j.physbeh.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 51.McGregor R., Wu M.F., Barber G., Ramanathan L., Siegel J.M. Highly specific role of hypocretin (orexin) neurons: differential activation as a function of diurnal phase, operant reinforcement versus operant avoidance and light level. J Neurosci. 2011;31:15455–15467. doi: 10.1523/JNEUROSCI.4017-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chase M.H. A unified survival theory of the functioning of the hypocretinergic system. J Appl Physiol. 2013 doi: 10.1152/japplphysiol.00700.2012. [DOI] [PubMed] [Google Scholar]
- 53.Mileykovskiy B.Y., Kiyashchenko L.I., Siegel J.M. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee M.G., Hassani O.K., Jones B.E. Discharge of identified orexin/hypocretin neurons across the sleep–waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takahashi K., Lin J.S., Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake–sleep states in the mouse. Neuroscience. 2008;153:860–870. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 56.Amici R., Domeniconi R., Jones C.A., Morales-Cobas G., Perez E., Tavernese L. Changes in REM sleep occurrence due to rhythmical auditory stimulation in the rat. Brain Res. 2000;868:241–250. doi: 10.1016/s0006-8993(00)02337-4. [DOI] [PubMed] [Google Scholar]
- 57.Amici R., Morales-Cobas G., Jones C.A., Perez E., Torterolo P., Zamboni G. REM sleep enhancement due to rhythmical auditory stimulation in the rat. Behav Brain Res. 2001;123:155–163. doi: 10.1016/s0166-4328(01)00203-0. [DOI] [PubMed] [Google Scholar]
- 58.Ursin R., Sterman M. BIS/BRI, University of California; Los Angeles: 1981. Manual for standardized scoring of sleep and waking states in adult cats. [Google Scholar]
- 59.Kiyashchenko L.I., Mileykovskiy B.Y., Maidment N., Lam H.A., Wu M.F., John J. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fung S.J., Yamuy J., Sampogna S., Morales F.R., Chase M.H. Orexin (hypocretin) input to trigeminal and hypoglossal motoneurons in the cat. Soc Neurosci. 2000;26:693. [Google Scholar]
- 61.Chase M.H., Morales F.R. Subthreshold excitatory activity and motoneuron discharge during REM periods of active sleep. Science. 1983;221:1195–1198. doi: 10.1126/science.6310749. [DOI] [PubMed] [Google Scholar]
- 62.Nakamura Y., Goldberg L.J., Chandler S.H., Chase M.H. Intracellular analysis of trigeminal motoneuron activity during sleep in the cat. Science. 1978;199:204–207. doi: 10.1126/science.202025. [DOI] [PubMed] [Google Scholar]
- 63.Mileykovskiy B.Y., Kiyashchenko L.I., Siegel J.M. Muscle tone facilitation and inhibition after orexin-a (hypocretin-1) microinjections into the medial medulla. J Neurophysiol. 2002;87:2480–2489. doi: 10.1152/jn.2002.87.5.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xi M.C., Fung S.J., Yamuy J., Morales F.R., Chase M.H. Induction of active (REM) sleep and motor inhibition by hypocretin in the nucleus pontis oralis of the cat. J Neurophysiol. 2002;87:2880–2888. doi: 10.1152/jn.2002.87.6.2880. [DOI] [PubMed] [Google Scholar]
- 65.Xi M.C., Fung S.J., Yamuy J., Morales F.R., Chase M.H. Hypocretinergic facilitation of synaptic activity of neurons in the nucleus pontis oralis of the cat. Brain Res. 2003;976:253–258. doi: 10.1016/s0006-8993(03)02566-6. [DOI] [PubMed] [Google Scholar]
- 66.Bernard R., Lydic R., Baghdoyan H.A. Hypocretin-1 causes G protein activation and increases ACh release in rat pons. Eur J Neurosci. 2003;18:1775–1785. doi: 10.1046/j.1460-9568.2003.02905.x. [DOI] [PubMed] [Google Scholar]
- 67.Bernard R., Lydic R., Baghdoyan H.A. Hypocretin (orexin) receptor subtypes differentially enhance acetylcholine release and activate g protein subtypes in rat pontine reticular formation. J Pharmacol Exp Ther. 2006;317:163–171. doi: 10.1124/jpet.105.097071. [DOI] [PubMed] [Google Scholar]
- 68.Kodama T., Takahashi Y., Honda Y. Enhancement of acetylcholine release during paradoxical sleep in the dorsal tegmental field of the cat brain stem. Neurosci Lett. 1990;114:277–282. doi: 10.1016/0304-3940(90)90576-u. [DOI] [PubMed] [Google Scholar]
- 69.Moreno-Balandran E., Garzon M., Bodalo C., Reinoso-Suarez F., de Andres I. Sleep-wakefulness effects after microinjections of hypocretin 1 (orexin A) in cholinoceptive areas of the cat oral pontine tegmentum. Eur J Neurosci. 2008;28:331–341. doi: 10.1111/j.1460-9568.2008.06334.x. [DOI] [PubMed] [Google Scholar]
- 70.Nunez A., Moreno-Balandran M.E., Rodrigo-Angulo M.L., Garzon M., De Andres I. Relationship between the perifornical hypothalamic area and oral pontine reticular nucleus in the rat. Possible implication of the hypocretinergic projection in the control of rapid eye movement sleep. Eur J Neurosci. 2006;24:2834–2842. doi: 10.1111/j.1460-9568.2006.05159.x. [DOI] [PubMed] [Google Scholar]
- 71.Watson C.J., Soto-Calderon H., Lydic R., Baghdoyan H.A. Pontine reticular formation (PnO) administration of hypocretin-1 increases PnO GABA levels and wakefulness. Sleep. 2008;31:453–464. doi: 10.1093/sleep/31.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brevig H.N., Watson C.J., Lydic R., Baghdoyan H.A. Hypocretin and GABA interact in the pontine reticular formation to increase wakefulness. Sleep. 2011;33:1285–1293. doi: 10.1093/sleep/33.10.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brischoux F., Mainville L., Jones B.E. Muscarinic-2 and orexin-2 receptors on GABAergic and other neurons in the rat mesopontine tegmentum and their potential role in sleep–wake state control. J Comp Neurol. 2008;510:607–630. doi: 10.1002/cne.21803. [DOI] [PubMed] [Google Scholar]
- 74.Xi M., Chase M.H. The injection of hypocretin-1 into the nucleus pontis oralis induces either active sleep or wakefulness depending on the behavioral state when it is administered. Sleep. 2010;33:1236–1243. doi: 10.1093/sleep/33.9.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chase M.H., Babb M. Masseteric reflex response to reticular stimulation reverses during active sleep compared with wakefulness or quiet sleep. Brain Res. 1973;59:421–426. doi: 10.1016/0006-8993(73)90284-9. [DOI] [PubMed] [Google Scholar]
- 76.Chase M.H., Morales F.R. The atonia and myoclonia of active (REM) sleep. Annu Rev Psychol. 1990;41:557–584. doi: 10.1146/annurev.ps.41.020190.003013. [DOI] [PubMed] [Google Scholar]
- 77.Knudsen S., Gammeltoft S., Jennum P.J. Rapid eye movement sleep behaviour disorder in patients with narcolepsy is associated with hypocretin-1 deficiency. Brain. 2010;133:568–579. doi: 10.1093/brain/awp320. [DOI] [PubMed] [Google Scholar]
- 78.Rowe K., Moreno R., Lau T.R., Wallooppillai U., Nearing B.D., Kocsis B. Heart rate surges during REM sleep are associated with theta rhythm and PGO activity in cats. Am J Physiol. 1999;277:R843–R849. doi: 10.1152/ajpregu.1999.277.3.R843. [DOI] [PubMed] [Google Scholar]
- 79.Shirasaka T., Kunitake T., Takasaki M., Kannan H. Neuronal effects of orexins: relevant to sympathetic and cardiovascular functions. Regul Pept. 2002;104:91–95. doi: 10.1016/s0167-0115(01)00352-4. [DOI] [PubMed] [Google Scholar]
- 80.Zhang W., Fukuda Y., Kuwaki T. Respiratory and cardiovascular actions of orexin-A in mice. Neurosci Lett. 2005;385:131–136. doi: 10.1016/j.neulet.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 81.Williams R.H., Burdakov D. Hypothalamic orexins/hypocretins as regulators of breathing. Expert Rev Mol Med. 2008;10:e28. doi: 10.1017/S1462399408000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gerashchenko D., Salin-Pascual R., Shiromani P.J. Effects of hypocretin-saporin injections into the medial septum on sleep and hippocampal theta. Brain Res. 2001;913:106–115. doi: 10.1016/s0006-8993(01)02792-5. [DOI] [PubMed] [Google Scholar]
- 83.Vankova J., Nevsimalova S., Sonka K., Spackova N., Svejdova-Blazejova K. Increased REM density in narcolepsy–cataplexy and the polysymptomatic form of idiopathic hypersomnia. Sleep. 2001;24:707–711. doi: 10.1093/sleep/24.6.707. [DOI] [PubMed] [Google Scholar]
- 84.Geisler P., Meier-Ewert K., Matsubayshi K. Rapid eye movements, muscle twitches and sawtooth waves in the sleep of narcoleptic patients and controls. Electroencephalogr Clin Neurophysiol. 1987;67:499–507. doi: 10.1016/0013-4694(87)90051-4. [DOI] [PubMed] [Google Scholar]
- 85.Silvani A., Bastianini S., Berteotti C., Lo Martire V., Zoccoli G. Control of cardiovascular variability during undisturbed wake–sleep behavior in hypocretin-deficient mice. Am J Physiol: Regul Integr Comp Physiol. 2012;302:R958–R964. doi: 10.1152/ajpregu.00668.2011. [DOI] [PubMed] [Google Scholar]
- 86.Torterolo P., Lagos P., Monti J.M. Melanin-concentrating hormone (MCH): a new sleep factor? Front Neurol. 2011;2:1–12. doi: 10.3389/fneur.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Monti J.M., Torterolo P., Lagos P. Melanin-concentrating hormone control of sleep–wake behavior. Sleep Med Rev. 2013;17:293–298. doi: 10.1016/j.smrv.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 88.Guan J.L., Uehara K., Lu S., Wang Q.P., Funahashi H., Sakurai T. Reciprocal synaptic relationship between orexin-and melanin-concentrating hormone-containing neurons in the rat lateral hypothalamus: a novel circuit implicated in feeding regulation. Int J Obes Relat Metab Disord. 2002;26:1523–1532. doi: 10.1038/sj.ijo.0802155. [DOI] [PubMed] [Google Scholar]
- 89.Backberg M., Hervieu G., Wilson S., Meister B. Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur J Neurosci. 2002;15:315–328. doi: 10.1046/j.0953-816x.2001.01859.x. [DOI] [PubMed] [Google Scholar]
- 90.Bayer L., Mairet-Coello G., Risold P.Y., Griffond B. Orexin/hypocretin neurons: chemical phenotype and possible interactions with melanin-concentrating hormone neurons. Regul Pept. 2002;104:33–39. doi: 10.1016/s0167-0115(01)00320-2. [DOI] [PubMed] [Google Scholar]
- 91.van den Pol A.N., Acuna-Goycolea C., Clark K.R., Ghosh P.K. Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron. 2004;42:635–652. doi: 10.1016/s0896-6273(04)00251-x. [DOI] [PubMed] [Google Scholar]
- 92.Rao Y., Lu M., Ge F., Marsh D.J., Qian S., Wang A.H. Regulation of synaptic efficacy in hypocretin/orexin-containing neurons by melanin concentrating hormone in the lateral hypothalamus. J Neurosci. 2008;28:9101–9110. doi: 10.1523/JNEUROSCI.1766-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burdakov D., Gerasimenko O., Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci. 2005;25:2429–2433. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Modirrousta M., Mainville L., Jones B.E. Orexin and MCH neurons express c-Fos differently after sleep deprivation vs. recovery and bear different adrenergic receptors. Eur J Neurosci. 2005;21:2807–2816. doi: 10.1111/j.1460-9568.2005.04104.x. [DOI] [PubMed] [Google Scholar]
- 95.Torterolo P., Sampogna S., Chase M.H. MCHergic projections to the nucleus pontis oralis participate in the control of active (REM) sleep. Brain Res. 2009;1268:76–87. doi: 10.1016/j.brainres.2009.02.055. [DOI] [PubMed] [Google Scholar]
- 96.Hassani O.K., Lee M.G., Jones B.E. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep–wake cycle. Proc Natl Acad Sci USA. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lagos P., Torterolo P., Jantos H., Chase M.H., Monti J.M. Effects on sleep of melanin-concentrating hormone microinjections into the dorsal raphe nucleus. Brain Res. 2009;1265:103–110. doi: 10.1016/j.brainres.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 98.Lagos P., Torterolo P., Jantos H., Chase M.H., Monti J.M. Immunoneutralization of melanin-concentrating hormone (MCH) in the dorsal raphe nucleus: effects on sleep and wakefulness. Brain Res. 2011;1369:112–118. doi: 10.1016/j.brainres.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 99.Urbanavicius J., Lagos P., Torterolo P., Scorza C. Role of melanin concentrating hormone (MCH) on the dorsal raphe nucleus (DRN): its relevance for depression. J Neurochem. 2013;125:265. [Google Scholar]
- 100.Pascovich C, Devera A, Lagos P, Costa A, Falconi A, Torterolo P. Melanin-concentrating hormone (MCH) decreases presumed serotonergic neuronal activity in the dorsal and median raphe nucleus. Proceedings of the congreso de la sociedad Argentina de neurociencias. Huerta Grande (Argentina); 2011.
- 101.Saito Y., Nagasaki H. The melanin-concentrating hormone system and its physiological functions. Results Probl Cell Differ. 2008;46:159–179. doi: 10.1007/400_2007_052. [DOI] [PubMed] [Google Scholar]
- 102.Messina M.M., Overton J.M. Cardiovascular effects of melanin-concentrating hormone. Regul Pept. 2007;139:23–30. doi: 10.1016/j.regpep.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 103.Egwuenu E.J., Fong A.Y., Pilowsky P.M. Intrathecal melanin-concentrating hormone reduces sympathetic tone and blocks cardiovascular reflexes. Am J Physiol: Regul Integr Comp Physiol. 2005;303:R624–R632. doi: 10.1152/ajpregu.00215.2012. [DOI] [PubMed] [Google Scholar]
- 104.Adamantidis A.R., Zhang F., Aravanis A.M., Deisseroth K., de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Berman A.L., Jones E.G. University of Wisconsin; Madison: 1982. The thalamus and basal telencephalum of the cat. A citoarchitectonic atlas with stereotaxic coordinates. [Google Scholar]
- 106.Bleier R. The John Hopkins Press; Baltimore (MD): 1961. The hypothamus of the cat. [Google Scholar]