Abstract
The neural networks that control escape from predators often show very clear relationships between defined sensory inputs and stereotyped motor outputs. This feature provides unique opportunities for researchers, but it also provides novel opportunities for neuroscience educators. Here we introduce new teaching modules using adult Drosophila that have been engineered to express csChrimson, a red-light sensitive channelrhodopsin, in specific sets of neurons and muscles mediating visually guided escape behaviors. This lab module consists of both behavior and electrophysiology experiments that explore the neural basis of flight escape. Three preparations are described that demonstrate photo-activation of the giant fiber circuit and how to quantify these behaviors. One of the preparations is then used to acquire intracellular electrophysiology recordings from different flight muscles. The diversity of action potential waveforms and firing frequencies observed in the flight muscles make this a rich preparation to study the ionic basic of cellular excitability. By activating different cells within the giant fiber pathway we also demonstrate principles of synaptic transmission and neural circuits. Beyond conveying core neurobiological concepts it is also expected that using these cutting edge techniques will enhance student motivation and attitudes towards biological research. Data collected from students and educators who have been involved in development of the module are presented to support this notion.
Keywords: optogenetics, Drosophila, giant fiber escape, flight muscle, electrophysiology, neuroethology
INTRODUCTION
Many animals produce fast, reliable movements that facilitate escape from predators. The neural circuits that underlie such behaviors have proven to be powerful systems for understanding principles of neuroscience. Escape circuits have provided key insights into the ionic basis of the action potential (Hodgkin and Huxley, 1952), functional and anatomical properties of central electrical (Furshpan and Potter, 1957) and chemical synapses (Faber and Korn, 1982; Korn et al., 1982), and mechanisms of neuromodulator action (Edwards et al., 2002). Study of escape circuits has also provided key insights into the neural basis of animal behavior, primarily because sensory evoked circuit activity is directly coupled to highly reproducible motor output (Edwards et al., 1999; Card, 2012; Pirri and Alkema, 2012).
The same features that make escape circuits excellent research tools also make them attractive for neuroscience teaching laboratories. In studying such networks, students potentially have an opportunity to examine at the anatomical, electrophysiological and behavioral level how sensory inputs lead to adaptive motor outputs. While there is an array of research articles outlining how to study escape circuits, particularly those in invertebrates, to date, there are very few resources focused on helping educators use escape circuits as teaching tools, except for specific, limited goals (see for example, Kladt et al., 2010).
The giant fiber (GF) escape circuit in Drosophila is one particularly attractive system for use in teaching laboratories. GF interneurons project from the fly visual system to the flight motor system. When activated, they trigger jumping and escape flights in response to looming visual stimuli (Card, 2012; von Reyn et al., 2014). The GF system provides a host of advantages for teachers: 1) The circuit has been relatively well characterized electrophysiologically and anatomically (Allen and Godenschwege, 2010), 2) fly lines that allow targeting of transgenes to GF neurons and flight muscles are freely available, 3) methods for recording flight muscle activity and behavioral responses to GF activation have been established. Despite the many advantages of the GF system for teachers, its use in education has to date, been limited. This has been due in part to a lack of simple inexpensive methodologies for selectively stimulating the GF pathway in behaving animals and a lack of inexpensive approaches for recording activity within the flight motor system.
In recent years, new optogenetic technologies have emerged that allow educators to overcome the difficulties associated with using the GF system in teaching. Optogenetic technologies allow researchers to remotely manipulate the activity of excitable cells with light and have become important components of neuroscience research. Most optogenetics work to date has centered on the use of the blue light sensitive ion channel channelrhodopsin-2 (ChR2). ChR2 has been used extensively in experiments involving largely transparent Drosophila larvae (Hwang et al., 2007; Pulver et al., 2009; Crisp et al., 2011; Su et al., 2012) and the methods have been exported to educational circles (Pulver et al., 2011). In contrast, ChR2 has not been widely used in adult fly experiments. Blue light evokes strong visual responses in adults and does not penetrate insect cuticle well (Zhang et al., 2007; Inagaki et al., 2014), this often makes it difficult to parse the behavioral effects of remotely activating neuronal populations in flies. More recent research has led to the development of a new generation of optogenetic activation tools centered on the use of ChR2 channels sensitive to red light (Inagaki et al., 2014; Klapoetke et al., 2014). One primary advantage of such ‘red-shifted’ ChR2s is that they can be activated at wavelengths that are largely invisible to flies and penetrate insect cuticle. These advantages have been highlighted in recent research where a newly available red shifted ChR2 was used to remotely activate GF neurons and trigger escape behavior in adult flies (von Reyn et al., 2014).
Here we present a teaching module that describes how to optogenetically activate components of the GF escape circuit using csChrimson, a newly developed, red-shifted ChR2 (Klapoetke et al., 2014). The experiments incorporate both behavioral and electrophysiological studies and provide platforms for student driven exploration. Feedback from both students and educators at multiple institutions suggest that the module helps promote teaching of integrative neuroscience.
MATERIALS AND METHODS
Fly strains and husbandry
The fly strains used in these experiments can be obtained from the Bloomington Stock Center http://flystocks.bio.indiana.edu/ or by request. Details on setting up crosses have been described elsewhere (Hornstein et al., 2009; Pulver and Berni, 2012). Ultimately the flies will need to have the UAS-csChrimson transgene (BL stock no. 55135) and a Gal4 transgene. The Gal4 lines used in this paper include OK371-Gal4 (motor neuron driver; BL stock no. 26160), A307-Gal4 (giant fiber interneuron driver, BL stock no. 6488, also expresses in downstream interneurons and motor neurons [Allen et al., 2007]), MHC-82-Gal4 (muscle driver; BL stock no. 55132). Heterozygous progeny from Gal4>csChrimson parents were transferred to fly food containing all trans-retinal (ATR) for two days prior to experiments. Details on preparing food with this cofactor have been described previously (Hornstein et al., 2009).
Optical activation of escape behavior
Initially, students can observe fly escape behavior by simply describing the effect of red light on control and transgenic adult flies in a vial. For more detailed fly escape behavior observations, adult flies were anesthetized on CO2 or ice. Under a dissecting microscope, the flies were either decapitated (Figure 1A) or positioned dorsal side up with pins placed in the center of the abdomen and through the center of the head (Figure 1B). Care was taken not to smash or stretch the specimen. For optical stimulation we used single side-emitting LEDs (Luxeon Star 627 nm; 106 lm @700mA; LED Supply, Randolph, VT). An inexpensive system to control the LEDs has been described previously (Pulver et al., 2011). These systems make use of the digital output feature on analog/digital conversion boards, though any digital output signal that delivers 0–5V pulses can be used to control the LED. Here we have controlled it using the Arduino Uno, a USB-powered microcontroller board (costs less than $25). The Uno was programmed to deliver ten 0.5s pulses with 3.0s between each pulse (programming script available at: https://github.com/jstitlow/Arduino-Optogenetics).
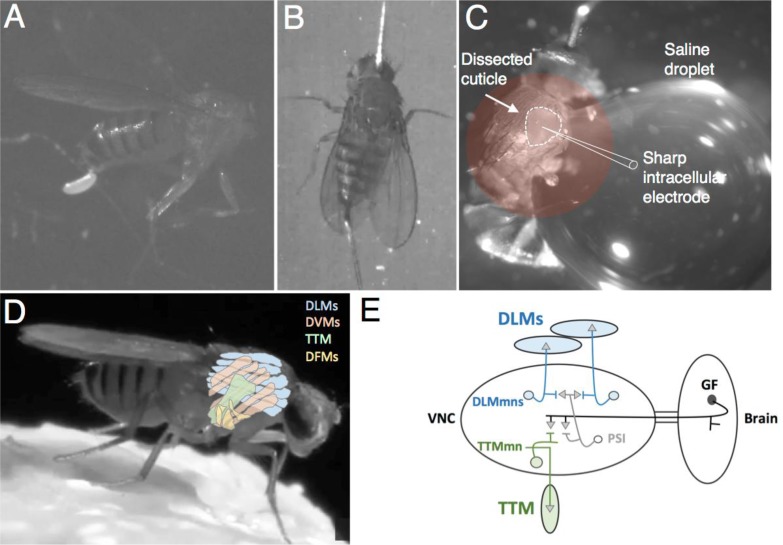
Figure 1.
Drosophila flight muscle preparations for combining optogenetic stimulation with behavioral experiments (A and B) or intracellular recording (C). (D) Note the position of different muscle groups in the thorax: DLM- dorsal longitudinal flight muscle (indirect flight muscle); DVM- dorsal ventral muscle (indirect flight muscle); TTM- tergotrochanteral muscle (jump muscle); and DFM- direct flight muscle. (E) Neural circuit diagram of a single giant fiber circuit. VNC- ventral nerve cord; DLMmn- dorsal longitudinal muscle motor neuron; TTMmn- tergotrochanteral muscle motor neuron; PSI- peripheral synapsing interneuron; GF- giant fiber. Drosophila photo in (D) taken by Tony Allen and Ilan Davis.
Recording optically-evoked action potentials in flight muscles
Flies were anesthetized and pinned to an elastomer-lined dish as above with the following modifications: 1) specimen was placed on its side with pins placed laterally through the center of the abdomen and head, 2) wings and legs were removed to reduce movement during the recordings, 3) a small window was cut into the lateral thorax using a sharp insect pin, 4) a large droplet of physiological saline was placed next to the specimen. It is important that the droplet cover the window but that it does not submerge the specimen, as doing so drastically reduces viability of the preparation. The droplet should also extend far enough away from the preparation to accommodate an electrode ground wire (Figure 1C). A video of the dissection is available online: (https://drive.google.com/open?id=0Bz3zc5KT9B4HRTU3RXRXTkZ3Wm8&authuser=1).
Sharp glass electrodes (5–30 MΩ) filled with 3M KCL were used for intracellular recordings. Signals were amplified with a model 2400 patch clamp amplifier (A–M Systems, Sequim, WA, USA) or Neuroprobe Model 1600 intracellular amplifier (A–M Systems). Signals were digitized at 10KHz with a Powerlab 8/30 data acquisition system (ADinstruments, Colorado Springs, CO, USA) and recorded and analyzed in LabChart 8 (ADInstruments). The saline used for these recordings consisted of (in mM): 1.0 CaCl2 ·2H2O, 70 NaCl, 20 mM MgCl2, 5 KCl, 10 NaHCO3, 5 trehalose, 115 sucrose.
RESULTS AND DISCUSSION
Behavioral responses to optogenetic stimulation
The Drosophila Gal4/UAS system (Brand and Perrimon, 1993) provides genetic access to specific sets of neurons in the escape circuit. When the UAS-csChrimson transgene is combined with Gal4 drivers for motor neurons, flight circuit neurons, or the giant fiber neuron specifically (OK371-Gal4, A307-Gal4, MHC-Gal4 respectively) the flies become extremely sensitive to red light.
The first exercise for examining a fly’s sensitivity to light can be to simply focus a red LED on the flies as they are freely moving in the culture vial. After tapping flies to the bottom of the vial they reflexively crawl up the side and hover near the top of the vial. Placing a red light beam in the middle of the vial interrupts this behavior. Red light sensitive fly responses can be compared to those of wild type flies. A second approach to investigate optogenetic stimulation of escape behavior is to decapitate the flies and observe them under a dissecting microscope. Headless flies stay alive and respond to stimuli for several hours, making it easy to manipulate them. Using the A307-Gal4 x UAS-csChrimson genotype, the flight escape response is still intact in decapitated flies. With this line students can observe and quantify high frequency wing beat behavior and prominent mesothoracic leg extension immediately after red light activation (Figure 2A).
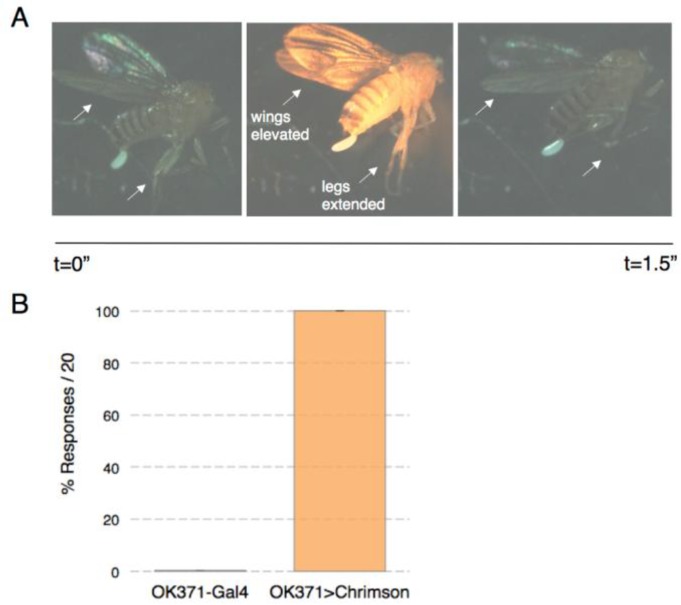
Figure 2.
Optogenetic activation of escape flight behavior. A) Time series of a typical response to red light in flies that express csChrimson in the giant fiber pathway. Wings elevate and legs extend (arrows) for the duration of the stimulus (0.5s) and relax when the stimulus is removed. B) Quantification of wing responses to stimuli in the motor neuron driver line and in a line expressing csChrimson (n= 10 and 16 animals respectively).
In the OK371-Gal4 x UAS-csChrimson genotype high frequency wing beats were less common. Instead we observed a mixture of prominent wing elevation, lateral movement of the wings towards the midline, and tilting movements along the rostral-caudal axis. Though the wing movements were less coordinated in the OK371>csChrimson line, the response probability was still high. Each of the 16 animals that were analyzed responded to 20/20 light pulses (0.5s pulses x 3s duty cycle). No responses were observed in the OK371-Gal4 driver line without the csChrimson transgene (Figure 2B). A third behavioral preparation we have tested is the pinned down approach that is also used for electrophysiology recording. The fly is placed on its side and pinned to a Sylgard-lined dish through its abdomen and through its head (Figure 1C). In this preparation we also observe robust escape behavior in response to light stimulation (see Supplementary video: https://drive.google.com/open?id=0Bz3zc5KT9B4HRTU3RXRXTkZ3Wm8&authuser=1).
Intracellular responses to optogenetic stimulation
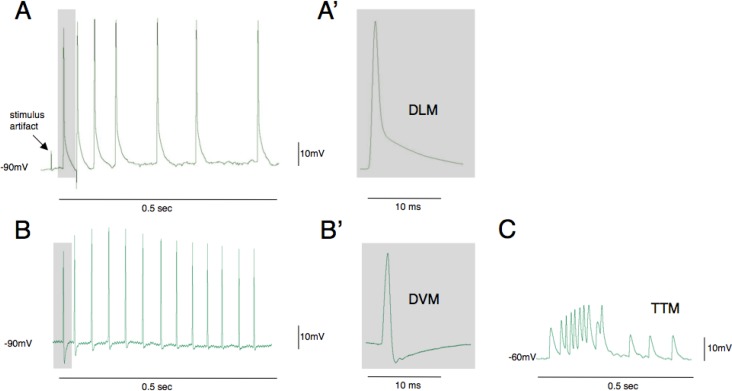
Most of the fruit fly thoracic segment is filled with muscles, the largest of which are involved in flight and jumping (Figure 1D). This makes it relatively easy for students to impale cells having a variety of action potential waveforms and electrophysiological signatures. Light-activated voltage-time traces from three of the most common cells recorded in the A307>csChrimson line are shown in Figure 3. The large dorsal longitudinal flight muscles (DLMs) have resting potentials close to −90 mV and are slow to repolarize after the action potential peaks (Figure 3A). Dorsal ventral muscles (DVMs) also have resting membrane potentials close to −90 mV but are recognizable by their large afterhyperpolarization (Figure 3B). More subtle signatures of these muscles are a slower rise time and a longer latency after activation of the GF neuron relative to DLMs (Tanouye and Wyman, 1980). Direct flight muscles also exhibit a large afterhyperpolarization but these muscles are relatively small and difficult to record from in this preparation (Nachtigall and Wilson, 1967). The tergotrochanteral muscle cells (jump muscle) have a much more positive resting potential (∼60 mV) and action potential amplitudes are much smaller (Figure 3C). In addition to these differences in action potential waveform, there are also differences in spike frequency recorded from these muscles when the giant fiber pathway is activated.
Figure 3.
Action potential waveforms evoked by optogenetic activation of the escape circuit. The most conspicuous muscle fibers in the thorax are dorsal longitudinal muscles (DLMs) and these are most frequently impaled when electrodes are inserted using this preparation (A, A′). The cells have highly negative resting potentials (−90mV) and are easiest to maintain recording as contractions are evoked. Dorsal ventral muscles (DVMs) are also frequently recorded from and are identified by their large after hyperpolarization potential (B, B′). The tergotrochanteral (TTM) jump muscles have much more positive resting potentials and lower amplitude action potentials (C). Traces in B and C are from student data.
Analysis of the different action potential waveforms can lead to a rich discussion of the mechanism that causes them. Membrane currents in the DLMs have been cleverly dissected using ion channel mutants (Elkins and Ganetzky, 1988) and looking at different stages of development (Salkoff and Wyman, 1981; Salkoff and Wyman, 1983). In addition to the large glutamate-evoked synaptic current, the DLMs have four distinguishable membrane currents. A voltage-gated calcium current, ICa, begins activating at −40 mV. A transient potassium current also activates at similar voltages and is mediated by both voltage-dependent inactivating potassium channels (IA) and calcium dependent potassium channels (IKCa). A voltage dependent, non-inactivating delayed rectifier (IKV) potassium channel is also present. IA and IKv arise during pupae development whereas ICa and IKCa emerge several hours after eclosion. Resting potential and membrane currents have not been investigated at this level of detail in the other muscle groups, which leaves the door open for student research projects.
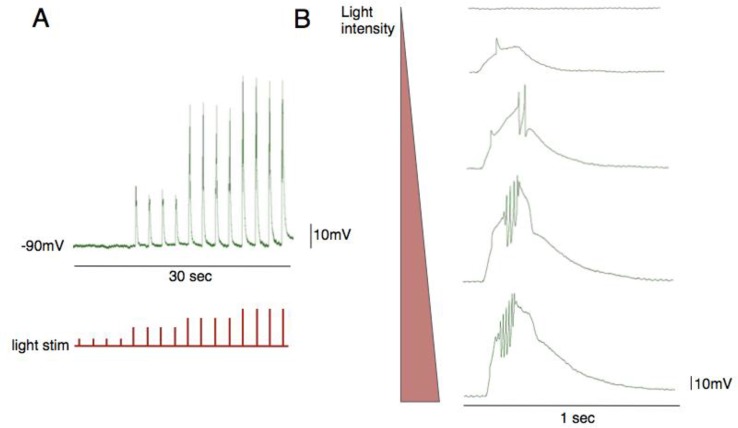
We found that it is possible to depolarize the muscles directly by driving expression of csChrimson in muscle fibers (MHC-Gal4) and activating them with red light. Traces from this experiment resembled the voltage response that is evoked by injecting direct current into the muscle with an electrode (Figure 4). Increased light intensity resulted in larger “depolarizing steps” that caused the cell to spike. With this technique students could directly compare the excitable properties of different muscle groups. By combining this technique with developmental staging and classic ion channel mutants, students could contribute real scientific discoveries.
Figure 4.
Direct muscle photo-activation and intracellular recording from a flight muscle. The LED instensity can be controlled by adjusting the input voltage signal. As the light intensity is increased the cell becomes more depolarized (A) and reaches threshold to induce action potentials (B).
Data generated by students
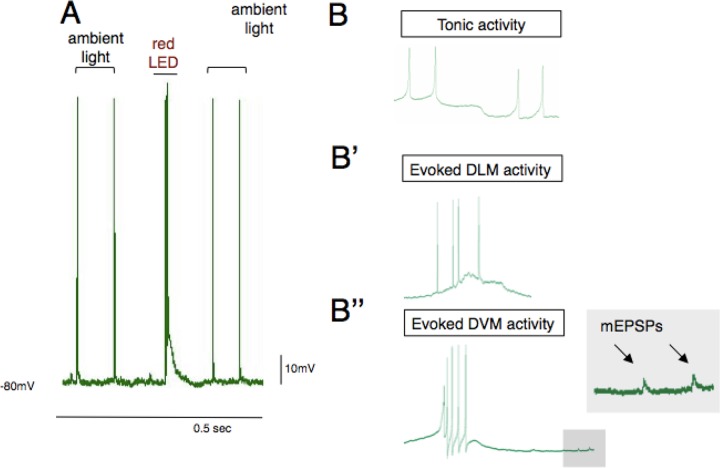
Our first cohort of students attempted this experiment with an earlier version of the channelrhodopsin transgene, ChR2. While many students were able to impale cells and observe tonic activity, none were able to induce activity with light pulses. Research grade LEDs and mercury lamp light sources are powerful enough to evoke activity but the small side-emitting LEDs that we describe here and in previous work are not strong enough to reliably activate ChR2. The small red LEDs are however, strong enough to activate the csChrimson transgene, as this transgene has a higher photosensitivity than ChR2 and red light passes through insect cuticle more efficiently. The csChrimson variant is so sensitive that students observed single periodic spikes under ambient room light in the absence of red light stimulus (Figure 5A). This activity is clearly different from tonic activity that was observed in deeper muscle groups (Figure 5B). Tonic activity likely arises from the pleurosternal muscle or direct flight muscles, both of which have been shown to exhibit spontaneous bursts of activity in other dipteran species (Nachtigall and Wilson, 1967). These muscles are less hyperpolarized than flight muscles (∼70 mV) and exhibit tonic activity that may be due to a hyperpolarization-activated current (Ih) observed in post-inhibitory rebound. The slow periodic spiking in Figure 5A′ was from ambient light by switching on and off room lights Lastly, the signal-to-noise ratio was high enough using teaching laboratory amplifiers to observe quantal transmission in flight muscles (miniature EPSPs; Figure 5B″ (Ikeda and Koenig (1988), which further increases the breadth of neurophysiological investigations that can be pursued with this preparation.
Figure 5.
Sources of spontaneous activity in flies expressing csChrimson. A) The csChrimson channelrhodopsin is extremely sensitive to visible light. Low frequency periodic activity in DLMs can arise from room lighting (also see Figure 6). B) Tonic activity that arises from endogenous pattern generators is clearly distinguishable from evoked activity in DLMs (B′) and DVMs (B″). Miniature EPSPs were also observed in low noise recordings. Traces in B are from student data.
Student and instructor feedback
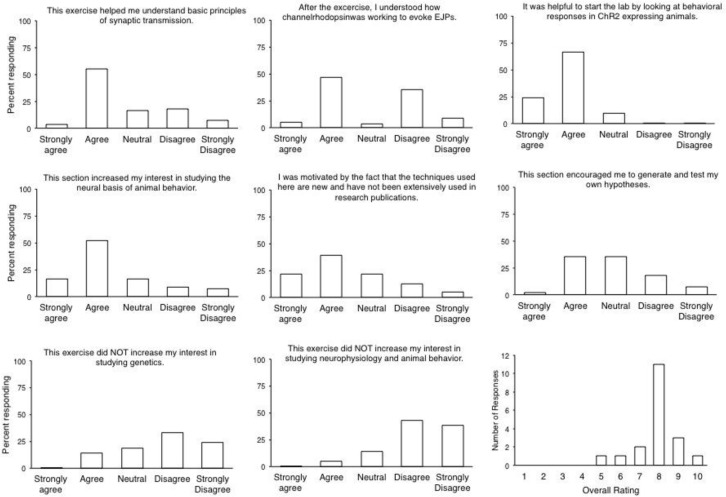
We conducted self-report surveys after working through the lab in two separate junior/senior level neuroscience elective courses, animal physiology (University of Kentucky) and neurophysiology (Cornell University). Questions on the survey were based on an earlier study that addressed pedagogical and motivational impressions from the experience (Pulver et al., 2011). The majority of students agreed that the lab module enhanced their interest in studying neurophysiology, genetics, behavior and its neural mechanisms, and thought the introduction to synaptic potentials helped them understand synaptic transmission better (Figure 6). They found the behavioral exercises helpful to set the stage for the physiology, and they felt they understood the optogenetic techniques. These data show that the lab module succeeds in its most fundamental goal, which is to let students understand the neurophysiological mechanisms of behavior. We also find that the relative novelty of the technique makes it exciting for the students, as the majority of them were “motivated by the fact that optogenetics is relatively novel and hasn’t been used frequently in publications”, and they felt inspired to hypothesize and plan more experiments.
Figure 6.
Student feedback. Students were given several days to voluntarily answer questions about the lab. N=56 students for questions, N=21 students for overall rating.
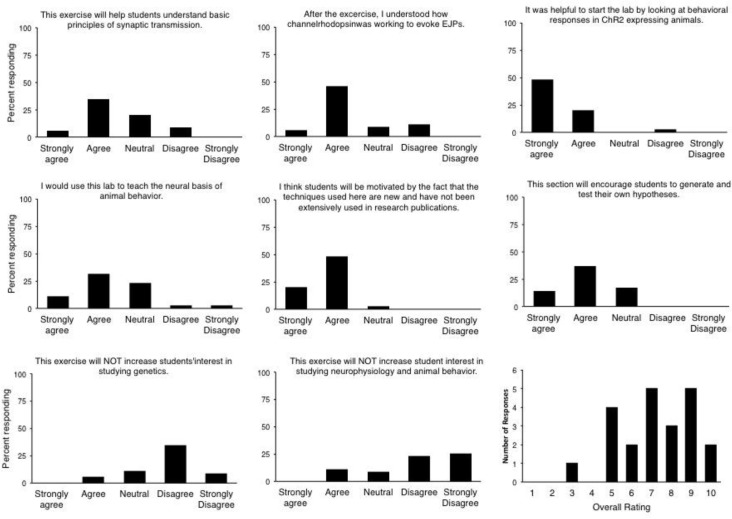
The lab module was also demonstrated at the August 2014 Faculty for Undergraduate Neuroscience Workshop at Ithaca College attended by many of the leading faculty who teach undergraduate neuroscience in the US. After working through the protocols presented here we surveyed their impressions of the lab and its pedagogical/motivational potential. Faculty feedback correlated well with the student assessments and gave strong support to the notion that the lab will help students understand the principles of synaptic transmission and that the novelty of the experiments would motivate students (Figure 7). Nearly half of the instructors said that they would use the lab in their own course, and the group of FUN faculty gave the module an overall score of 7.2/10.
Figure 7.
Instructor feedback. Self-report evaluations were given to 22 instructors attending the Faculty for Undergraduate Neuroscience workshop in August 2014 at Ithaca College.
The biggest technical challenge with this preparation is pinning the fly and dissecting s small window into the cuticle. Students often reported that the dissection was too difficult and that it would be helpful to provide pre-dissected animals. As the goal of the exercise is to experiment with stimulation and recordings, we suggest that the instructor/teaching assistant provide students with dissected preparations. One of the instructors mentioned that her previous lab secured flies in petroleum jelly rather than pinning them to an elastomer-lined dish. This could simplify the dissection. Another point raised by workshop attendees was that students might be interested in studying other behaviors. The Drosophila field has discovered numerous behaviors that can be induced by optical activation of specific groups of neurons and these tools are ideal for inquiry-based student projects. Students can observe activation of most other appendages using the motor neuron Gal4 line described here, e.g., proboscis extension or abdomen flexion. Several other behaviors are accessible to heat activation in select neurons (Bath et al., 2014), such behaviors should also be accessible with light stimulation by expressing csChrimson in those sets of neurons. More ambitious projects could investigate the cognitive effects of optically disrupting activity in mushroom body neurons, which are involved in associative learning.
Conclusion
We have developed an optogenetics lab module that can be used to directly experiment with escape behavior and its underlying physiology in adult Drosophila. Students and instructors who evaluated the exercise found it to be effective and interesting. Our module successfully combines a traditional, well-known escape circuit with modern research advances in optogenetics. In doing so, this work provides a blueprint for marrying traditional approaches in invertebrate neuroethology with state-of-the-art technology in ways that promote student driven exploration.
Supplementary Material
Videos from this article can be downloaded at the following web address: https://drive.google.com/open?id=0Bz3zc5KT9B4HRTU3RXRXTkZ3Wm8&authuser=1.
Acknowledgments
We are grateful to the students of BIO 350 (University of Kentucky) and BioNB/BME/ECE 4910 (Cornell University) as well as participants at the 2014 Faculty for Undergraduate Neuroscience workshop for their assistance in evaluating these exercises. We would also like to thank Dr. Robin Cooper for his help in developing the protocol and allowing it to be beta-tested in his course.
Footnotes
This work was supported by the Howard Hughes Medical Institute (Janelia Research Campus Junior Fellowship to SRP), the Department of Neurobiology and Behavior at Cornell University (BRJ), and a dissertation enhancement award from the University of Kentucky (JST). Initial module design was carried out at the 2013 Neurobiology of Drosophila summer course at Cold Spring Harbor Laboratories. The 2013 course was supported by Cold Spring Harbor laboratories, the National Science Foundation (NSF grant IOS-1238832), and the National Institutes of Drug Abuse (NIDA grant 1R13DA034437-03).
REFERENCES
- Allen MJ, Godenschwege TA. Electrophysiological recordings from the Drosophila giant fiber system. In: Zhang, et al., editors. Drosophila neurobiology. Cold Spring Harbor, NY: CSHL Press; 2010. pp. 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MJ, Drummond JA, Sweetman DJ, Moffat KG. Analysis of two P-element enhancer-trap insertion lines that show expression in the giant fibre neuron of Drosophila melanogaster. Genes Brain Behav. 2007;6:347–358. doi: 10.1111/j.1601-183X.2006.00263.x. [DOI] [PubMed] [Google Scholar]
- Bath DE, Stowers JR, Hormann D, Poehlmann A, Dickson BJ, Straw AD. FlyMAD: rapid thermogenetic control of neuronal activity in freely walking Drosophila. Nat Methods. 2014;11:756–762. doi: 10.1038/nmeth.2973. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Card GM. Escape behaviors in insects. Current Opin Neurobiol. 2012;22:180–186. doi: 10.1016/j.conb.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Crisp SJ, Evers JF, Bate M. Endogenous patterns of activity are required for the maturation of a motor network. The J Neurosci. 2011;31:10445–10450. doi: 10.1523/JNEUROSCI.0346-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DH, Heitler WJ, Krasne FB. Fifty years of a command neuron: the neurobiology of escape behavior in the crayfish. Trends Neurosci. 1999;22:153–161. doi: 10.1016/s0166-2236(98)01340-x. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Yeh SR, Musolf BE, Antonsen BL, Krasne FB. Metamodulation of the crayfish escape circuit. Brain Behavior Evol. 2002;60:360–369. doi: 10.1159/000067789. [DOI] [PubMed] [Google Scholar]
- Elkins T, Ganetzky B. The roles of potassium currents in Drosophila flight muscles. J Neurosci. 1988;8:428–434. doi: 10.1523/JNEUROSCI.08-02-00428.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber DS, Korn H. Transmission at a central inhibitory synapse. I. Magnitude of unitary postsynaptic conductance change and kinetics of channel activation. J Neurophysiol. 1982;48:654–678. doi: 10.1152/jn.1982.48.3.654. [DOI] [PubMed] [Google Scholar]
- Furshpan EJ, Potter DD. Mechanism of nerve-impulse transmission at a crayfish synapse. Nature. 1957;180:342–343. doi: 10.1038/180342a0. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornstein NJ, Pulver SR, Griffith LC. Channelrhodopsin2 mediated stimulation of synaptic potentials at Drosophila neuromuscular junctions. J Viz Exp. 2009 Mar 16;(25):1133. doi: 10.3791/1133. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang RY, Zhong L, Xu Y, Johnson T, Zhang F, Deisseroth K, Tracey WD. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol. 2007;17:2105–2116. doi: 10.1016/j.cub.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Koenig JH. Spontaneous release of multiquantal miniature excitatory junction potentials induced by a Drosophila mutant. J Physiol. 1988;406:215–223. doi: 10.1113/jphysiol.1988.sp017377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki HK, Jung Y, Hoopfer ED, Wong AM, Mishra N, Lin JY, Tsien RY, Anderson DJ. Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nat Methods. 2014;11:325–332. doi: 10.1038/nmeth.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldt N, Hanslik U, Heinzel HG. Teaching basic neurophysiology using intact earthworms. J Undergrad Neurosci Educ. 2010;9:A20–A35. [PMC free article] [PubMed] [Google Scholar]
- Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, Wang J, Xie Y, Yan Z, Zhang Y, Chow BY, Surek B, Melkonian M, Jayaraman V, Constantine-Paton M, Wong GK, Boyden ES. Independent optical excitation of distinct neural populations. Nat Methods. 2014;11:338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H, Mallet A, Triller A, Faber DS. Transmission at a central inhibitory synapse. II. Quantal description of release, with a physical correlate for binomial n. J Neurophysiol. 1982;48:679–707. doi: 10.1152/jn.1982.48.3.679. [DOI] [PubMed] [Google Scholar]
- Nachtigall W, Wilson DM. Neuro-muscular control of dipteran flight. J Exp Biol. 1967;47:77–97. doi: 10.1242/jeb.47.1.77. [DOI] [PubMed] [Google Scholar]
- Pirri JK, Alkema MJ. The neuroethology of C. elegans escape. Curr Opin Neurobiol. 2012;22:187–193. doi: 10.1016/j.conb.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver SR, Berni J. The fundamentals of flying: simple and inexpensive strategies for employing Drosophila genetics in neuroscience teaching laboratories. J Undergrad Neurosci Educ. 2012;11:A139–A148. [PMC free article] [PubMed] [Google Scholar]
- Pulver SR, Hornstein NJ, Land BL, Johnson BR. Optogenetics in the teaching laboratory: using channelrhodopsin-2 to study the neural basis of behavior and synaptic physiology in Drosophila. Adv Physiol Educ. 2011;35:82–91. doi: 10.1152/advan.00125.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver SR, Pashkovski SL, Hornstein NJ, Garrity PA, Griffith LC. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J Neurophysiol. 2009;101:3075–3088. doi: 10.1152/jn.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L, Wyman R. Outward currents in developing Drosophila flight muscle. Science. 1981;212:461–463. doi: 10.1126/science.6259736. [DOI] [PubMed] [Google Scholar]
- Salkoff LB, Wyman RJ. Ion currents in Drosophila flight muscles. J Physiol. 1983;337:687–709. doi: 10.1113/jphysiol.1983.sp014649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CY, Menuz K, Reisert J, Carlson JR. Non-synaptic inhibition between grouped neurons in an olfactory circuit. Nature. 2012;492:66–71. doi: 10.1038/nature11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanouye MA, Wyman RJ. Motor outputs of giant nerve fiber in Drosophila. J Neurophysiol. 1980;44:405–421. doi: 10.1152/jn.1980.44.2.405. [DOI] [PubMed] [Google Scholar]
- von Reyn CR, Breads P, Peek MY, Zheng GZ, Williamson WR, Yee AL, Leonardo A, Card GM. A spike-timing mechanism for action selection. Nature Neurosci. 2014;17:962–970. doi: 10.1038/nn.3741. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ge W, Wang Z. A toolbox for light control of Drosophila behaviors through Channelrhodopsin 2-mediated photoactivation of targeted neurons. Eur J Neurosci. 2007;26:2405–2416. doi: 10.1111/j.1460-9568.2007.05862.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Videos from this article can be downloaded at the following web address: https://drive.google.com/open?id=0Bz3zc5KT9B4HRTU3RXRXTkZ3Wm8&authuser=1.