Abstract
Myosin heavy chain (MHC) is the motor protein of muscle thick filaments. Most organisms produce many muscle MHC isoforms with temporally and spatially regulated expression patterns. This suggests that isoforms of MHC have different characteristics necessary for defining specific muscle properties. The single Drosophila muscle Mhc gene yields various isoforms as a result of alternative RNA splicing. To determine whether this multiplicity of MHC isoforms is critical to myofibril assembly and function, we introduced a gene encoding only an embryonic MHC into Drosophila melanogaster. The embryonic transgene acts in a dominant antimorphic manner to disrupt flight muscle function. The transgene was genetically crossed into an MHC null background. Unexpectedly, transformed flies expressing only the embryonic isoform are viable. Adult muscles containing embryonic MHC assemble normally, indicating that the isoform of MHC does not determine the dramatic ultrastructural variation among different muscle types. However, transformed flies are flightless and show reduced jumping and mating ability. Their indirect flight muscle myofibrils progressively deteriorate. Our data show that the proper MHC isoform is critical for specialized muscle function and myofibril stability.
Full text
PDF
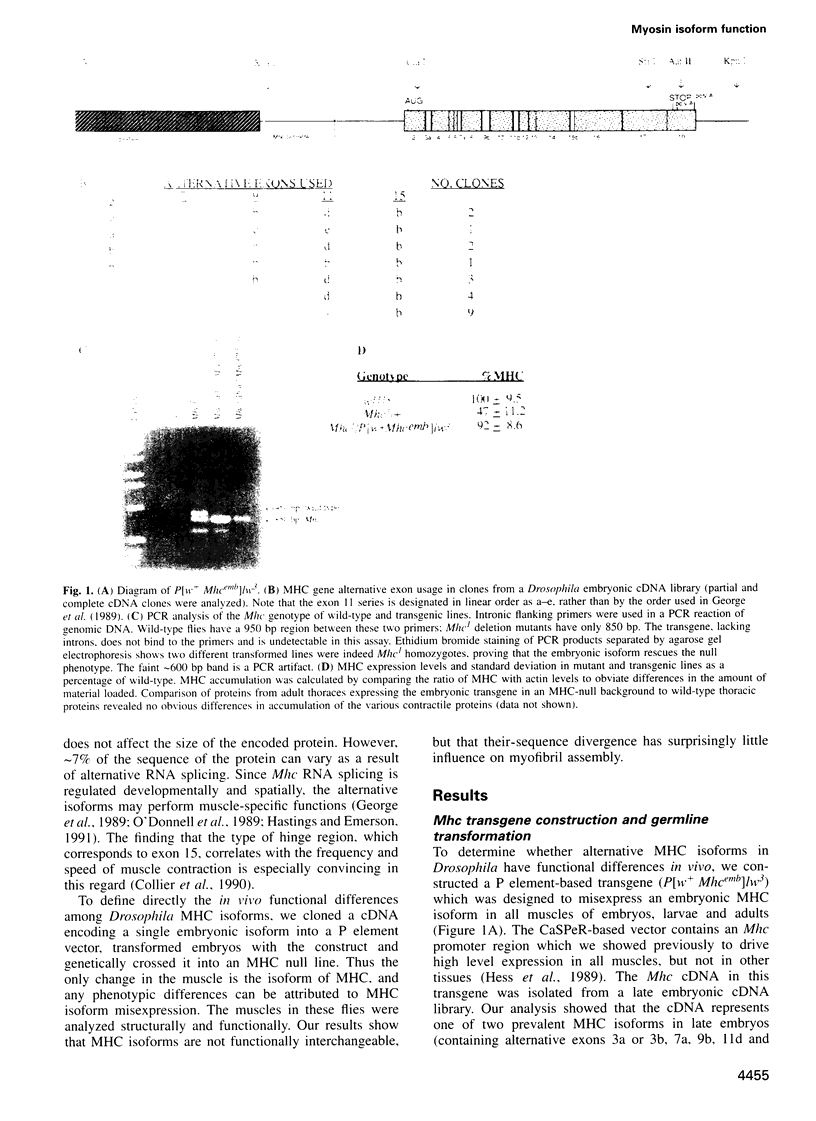

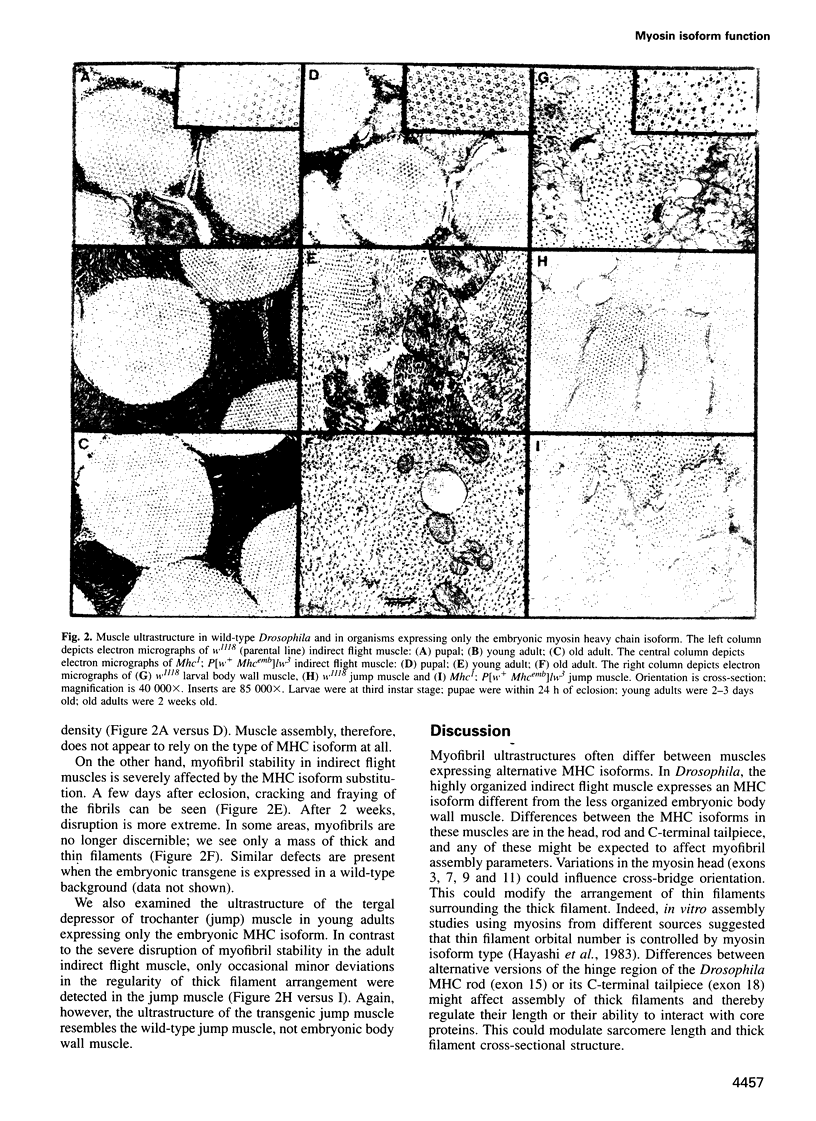


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beall C. J., Fyrberg E. Muscle abnormalities in Drosophila melanogaster heldup mutants are caused by missing or aberrant troponin-I isoforms. J Cell Biol. 1991 Sep;114(5):941–951. doi: 10.1083/jcb.114.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall C. J., Sepanski M. A., Fyrberg E. A. Genetic dissection of Drosophila myofibril formation: effects of actin and myosin heavy chain null alleles. Genes Dev. 1989 Feb;3(2):131–140. doi: 10.1101/gad.3.2.131. [DOI] [PubMed] [Google Scholar]
- Becker K. D., O'Donnell P. T., Heitz J. M., Vito M., Bernstein S. I. Analysis of Drosophila paramyosin: identification of a novel isoform which is restricted to a subset of adult muscles. J Cell Biol. 1992 Feb;116(3):669–681. doi: 10.1083/jcb.116.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein S. I., Mogami K., Donady J. J., Emerson C. P., Jr Drosophila muscle myosin heavy chain encoded by a single gene in a cluster of muscle mutations. 1983 Mar 31-Apr 6Nature. 302(5907):393–397. doi: 10.1038/302393a0. [DOI] [PubMed] [Google Scholar]
- Bernstein S. I., O'Donnell P. T., Cripps R. M. Molecular genetic analysis of muscle development, structure, and function in Drosophila. Int Rev Cytol. 1993;143:63–152. doi: 10.1016/s0074-7696(08)61874-4. [DOI] [PubMed] [Google Scholar]
- Brown N. H., Kafatos F. C. Functional cDNA libraries from Drosophila embryos. J Mol Biol. 1988 Sep 20;203(2):425–437. doi: 10.1016/0022-2836(88)90010-1. [DOI] [PubMed] [Google Scholar]
- Caiozzo V. J., Baker M. J., Herrick R. E., Tao M., Baldwin K. M. Effect of spaceflight on skeletal muscle: mechanical properties and myosin isoform content of a slow muscle. J Appl Physiol (1985) 1994 Apr;76(4):1764–1773. doi: 10.1152/jappl.1994.76.4.1764. [DOI] [PubMed] [Google Scholar]
- Collier V. L., Kronert W. A., O'Donnell P. T., Edwards K. A., Bernstein S. I. Alternative myosin hinge regions are utilized in a tissue-specific fashion that correlates with muscle contraction speed. Genes Dev. 1990 Jun;4(6):885–895. doi: 10.1101/gad.4.6.885. [DOI] [PubMed] [Google Scholar]
- Cooke R. The mechanism of muscle contraction. CRC Crit Rev Biochem. 1986;21(1):53–118. doi: 10.3109/10409238609113609. [DOI] [PubMed] [Google Scholar]
- Deitiker P. R., Epstein H. F. Thick filament substructures in Caenorhabditis elegans: evidence for two populations of paramyosin. J Cell Biol. 1993 Oct;123(2):303–311. doi: 10.1083/jcb.123.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson C. P., Jr, Bernstein S. I. Molecular genetics of myosin. Annu Rev Biochem. 1987;56:695–726. doi: 10.1146/annurev.bi.56.070187.003403. [DOI] [PubMed] [Google Scholar]
- Epstein H. F., Berliner G. C., Casey D. L., Ortiz I. Purified thick filaments from the nematode Caenorhabditis elegans: evidence for multiple proteins associated with core structures. J Cell Biol. 1988 Jun;106(6):1985–1995. doi: 10.1083/jcb.106.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein H. F., Waterston R. H., Brenner S. A mutant affecting the heavy chain of myosin in Caenorhabditis elegans. J Mol Biol. 1974 Dec 5;90(2):291–300. doi: 10.1016/0022-2836(74)90374-x. [DOI] [PubMed] [Google Scholar]
- Fyrberg E., Beall C. Genetic approaches to myofibril form and function in Drosophila. Trends Genet. 1990 Apr;6(4):126–131. doi: 10.1016/0168-9525(90)90127-r. [DOI] [PubMed] [Google Scholar]
- Fyrberg E., Fyrberg C. C., Beall C., Saville D. L. Drosophila melanogaster troponin-T mutations engender three distinct syndromes of myofibrillar abnormalities. J Mol Biol. 1990 Dec 5;216(3):657–675. doi: 10.1016/0022-2836(90)90390-8. [DOI] [PubMed] [Google Scholar]
- Hastings G. A., Emerson C. P., Jr Myosin functional domains encoded by alternative exons are expressed in specific thoracic muscles of Drosophila. J Cell Biol. 1991 Jul;114(2):263–276. doi: 10.1083/jcb.114.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Wozniak P. M., Cayer M. L., Smith D. S. Actin-myosin interaction: the role of myosin in determining the actin pattern in self-assembled 'hybrid' contractile units. Tissue Cell. 1983;15(6):955–963. doi: 10.1016/0040-8166(83)90060-5. [DOI] [PubMed] [Google Scholar]
- Hess N. K., Bernstein S. I. Developmentally regulated alternative splicing of Drosophila myosin heavy chain transcripts: in vivo analysis of an unusual 3' splice site. Dev Biol. 1991 Aug;146(2):339–344. doi: 10.1016/0012-1606(91)90235-u. [DOI] [PubMed] [Google Scholar]
- Kazzaz J. A., Rozek C. E. Tissue-specific expression of the alternately processed Drosophila myosin heavy-chain messenger RNAs. Dev Biol. 1989 Jun;133(2):550–561. doi: 10.1016/0012-1606(89)90057-2. [DOI] [PubMed] [Google Scholar]
- Kronert W. A., Edwards K. A., Roche E. S., Wells L., Bernstein S. I. Muscle-specific accumulation of Drosophila myosin heavy chains: a splicing mutation in an alternative exon results in an isoform substitution. EMBO J. 1991 Sep;10(9):2479–2488. doi: 10.1002/j.1460-2075.1991.tb07787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labeit S., Kolmerer B. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science. 1995 Oct 13;270(5234):293–296. doi: 10.1126/science.270.5234.293. [DOI] [PubMed] [Google Scholar]
- McCormick K. M., Baldwin K. M., Schachat F. Coordinate changes in C protein and myosin expression during skeletal muscle hypertrophy. Am J Physiol. 1994 Aug;267(2 Pt 1):C443–C449. doi: 10.1152/ajpcell.1994.267.2.C443. [DOI] [PubMed] [Google Scholar]
- Miedema K., Harhangi H., Mentzel S., Wilbrink M., Akhmanova A., Hooiveld M., Bindels P., Hennig W. Interspecific sequence comparison of the muscle-myosin heavy-chain genes from Drosophila hydei and Drosophila melanogaster. J Mol Evol. 1994 Oct;39(4):357–368. doi: 10.1007/BF00160268. [DOI] [PubMed] [Google Scholar]
- Mogami K., O'Donnell P. T., Bernstein S. I., Wright T. R., Emerson C. P., Jr Mutations of the Drosophila myosin heavy-chain gene: effects on transcription, myosin accumulation, and muscle function. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1393–1397. doi: 10.1073/pnas.83.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa A., Hayashi H. Isoform transition of contractile proteins related to muscle remodeling with an axial gradient during metamorphosis in Xenopus laevis. Dev Biol. 1994 Sep;165(1):86–94. doi: 10.1006/dbio.1994.1236. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. T., Bernstein S. I. Molecular and ultrastructural defects in a Drosophila myosin heavy chain mutant: differential effects on muscle function produced by similar thick filament abnormalities. J Cell Biol. 1988 Dec;107(6 Pt 2):2601–2612. doi: 10.1083/jcb.107.6.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P. T., Collier V. L., Mogami K., Bernstein S. I. Ultrastructural and molecular analyses of homozygous-viable Drosophila melanogaster muscle mutants indicate there is a complex pattern of myosin heavy-chain isoform distribution. Genes Dev. 1989 Aug;3(8):1233–1246. doi: 10.1101/gad.3.8.1233. [DOI] [PubMed] [Google Scholar]
- Rayment I., Holden H. M. The three-dimensional structure of a molecular motor. Trends Biochem Sci. 1994 Mar;19(3):129–134. doi: 10.1016/0968-0004(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Rozek C. E., Davidson N. Drosophila has one myosin heavy-chain gene with three developmentally regulated transcripts. Cell. 1983 Jan;32(1):23–34. doi: 10.1016/0092-8674(83)90493-2. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Sutherland C. J., Esser K. A., Elsom V. L., Gordon M. L., Hardeman E. C. Identification of a program of contractile protein gene expression initiated upon skeletal muscle differentiation. Dev Dyn. 1993 Jan;196(1):25–36. doi: 10.1002/aja.1001960104. [DOI] [PubMed] [Google Scholar]
- Waterston R. H. The minor myosin heavy chain, mhcA, of Caenorhabditis elegans is necessary for the initiation of thick filament assembly. EMBO J. 1989 Nov;8(11):3429–3436. doi: 10.1002/j.1460-2075.1989.tb08507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]





