Abstract
To standardise regenerative medicine using cultured cells, the use of serum-free, chemically defined media will be necessary. We have reported that IL-1α inhibits the growth of epithelial cells in culture and that recombinant IL-1 receptor antagonist (IL-1RA) significantly promotes epithelial cell growth in no feeder layer condition. In this study, we examined inhibitors of calpain, a cysteine proteinase that plays crucial roles in various cellular functions, including IL-1α maturation and secretion. The culturing of epithelial cells in serum-free media supplemented with a membrane-permeable calpain inhibitor significantly promoted growth while suppressing IL-1α maturation and secretion. By contrast, non-membrane-permeable calpain inhibitor treatment did not have these effects. Interestingly, immunoblotting analysis revealed that immature, untruncated, IL-1α expression was also downregulated by cell-permeable calpain inhibitor treatment, and the difference in IL-1α gene expression increased from day 2 to day 6. Although IL-1RA has been reported to promote epithelial cell growth, we detected no synergistic promotion of epithelial cell growth using a calpain inhibitor and IL-1RA. These findings indicate that calpain inhibitors promote epithelial cell proliferation by inhibiting IL-1α maturation at an early phase of epithelial cell culture and by suppressing the positive feedback-mediated amplification of IL-1α signalling.
Introduction
Following the establishment of the human epidermal keratinocyte culture method using foetal bovine serum (FBS) and a 3T3 feeder layer [1, 2], fabricated epidermal cell sheets have been used as epidermal grafts to treat skin defects, such as severe burns [3], ulcers [4] and giant congenital nevi [5]. This culture method has also been applied to oral mucosal epithelial cells [6] and used clinically to treat skin [7, 8] and oral defects [7, 9, 10]. We have also treated corneal defects with transplantable cell sheets fabricated from autologous oral mucosal epithelial cells cultured using FBS and a 3T3 feeder layer [11].
However, the possibility of pathogen transmission or infection from these xenogeneic materials cannot be eliminated. We have reported that cell culture inserts with micropores (0.4 μm) on the bottom promote the proliferation and stratification of canine oral mucosal epithelial cells, even in the absence of both a feeder layer and serum [12]. However, the proliferation of primary human oral mucosal epithelial cells was found to be poor and unstable under these culture conditions. Therefore, transplantable epithelial cell sheets that were fabricated from the autologous oral mucosal epithelium in media supplemented with autologous serum in the absence of a feeder layer have subsequently been used to treat oesophageal ulcers after endoscopic cancer resection [13].
If serum-free culture conditions could be used to fabricate transplantable human epithelial cell sheets, such an approach could yield benefits to patients by avoiding the stress of blood collection and the variance in serum quality between patients.
To develop serum-free culture conditions to fabricate transplantable epithelial cell sheets, factors that promote proliferation should be included as a serum alternative to support the stable culture of epithelial cells. Based on screens for cytokines with epithelial cell proliferation activity, we reported that IL-1α inhibits the growth of epithelial cells, whereas IL-1 receptor antagonist (IL-1RA) promotes growth [14]. This finding suggests that the regulation of endogenous IL-1α signalling might play an important role in epithelial cell proliferation and stem cell maintenance.
Calpain, a Ca2+-dependent neutral cysteine proteinase, is known to regulate various cellular functions in vitro and in vivo [15, 16]. The immature 33-kDa pro-form of IL-1α is converted to the mature 17-kDa form via cytoplasmic calpain activity in various cell types [17–19]; importantly, only the mature 17-kDa form of IL-1α is secreted [18].
Therefore, we hypothesised that calpain might play an important role in epithelial cell growth. Herein, we assessed the cell proliferative effects of various calpain inhibitors applied as a supplement to serum-free culture medium.
Materials and Methods
Isolation of oral mucosal epithelial cells
Experimental animals were treated in accordance with experimental procedures approved by the Committee for Animal Research of Tokyo Women’s Medical University in Tokyo, Japan. Totally 22 rats were used in this study. After the humane euthanasia with CO2, Lewis rats (8 weeks old, male, from Charles River, Wilmington, MA), the oral mucosal tissues were surgically excised from buccal mucosa, disinfected with povidone-iodine (Meiji Seika Pharma, Tokyo, Japan), and washed with Dulbecco’s Modified Eagle Medium (DMEM; Sigma-Aldrich, St Louis, MO) containing 100 IU/mL penicillin and 100 μg/mL streptomycin (Life Technologies, Carlsbad, CA, USA). The oral mucosal tissues were digested with 1000 PU of dispase (Godo Shusei, Tokyo, Japan) at 4°C overnight, and then, the epithelial tissue was “peeled off” using forceps. The epithelium was then torn using forceps and was dissociated using 1.25% trypsin-0.5% ethylenediaminetetraacetic acid (EDTA) in Dulbecco’s phosphate buffered saline (Sigma-Aldrich) at 37°C for 15 min to obtain epithelial cell suspensions. Disaggregated cells were filtered through 40-μm cell strainers (BD Biosciences, Franklin Lakes, NJ). Enzymatic treatment was stopped by adding a trypsin inhibitor (DS Pharma Biomedical, Osaka, Japan), and the cells were then cultured.
Cell culture
Keratinocyte culture medium (KCM) was composed of a basal mixture of 3 parts DMEM to 1 part nutrient mixture F-12 Ham (Sigma-Aldrich) supplemented with 2 nM triiodothyronine (Wako Pure Chemicals, Osaka, Japan), 0.5% insulin-transferrin-selenium reagent (Life Technologies), 5 μg/ml transferrin (Life Technologies), 10 ng/mL epidermal growth factor (Protein Express, Chiba, Japan), 0.4 μg/mL hydrocortisone (Kowa Pharmaceutical, Tokyo, Japan), 1 nM cholera toxin (List Biological Labs, Campbell, CA), 0.5 μg/mL amphotericin B (Fungizone; Bristol-Myers Squibb, Park Avenue, NY), and 40 μg/mL gentamicin (Gentacin; Schering-Plough, Kenilworth, NJ).
Epithelial cell culture in the presence of calpain inhibitors
Isolated primary rat oral mucosal epithelial cells were cultured on cell culture inserts (23-mm in diameter; BD Biosciences) at an initial density of 4.0 × 104 cells/cm2 in KCM without a 3T3 feeder layer at 37°C in a humidified atmosphere containing 5% CO2. Cell morphology and proliferation were monitored using a phase-contrast microscope (ECLIPSE TE2000-U, Nikon, Tokyo, Japan). We evaluated the effects of the following calpain inhibitors: calpeptin (50 nM; MW: 362.5, ENZO Life Sciences, Farmingdale, NY) calpain inhibitor III (4 nM; MW: 382.5, Calbiochem, San Diego, CA), calpastatin (50 nM; MW: 14000, Takara Bio, Shiga, Japan), B27-WT (1 μM; MW: 3136.6, AnaSpec, Fremont, CA), and cystatin C (15 nM; MW: 15 kDa, R&D Systems, Minneapolis, MN). For specific experiment, 1 μg/mL IL-1RA or 10 ng/mL IL-1α was exogenously added. The utilised concentrations of these inhibitors and cytokines were determined based on preliminary experiments and previous study [14].
Cell number quantification
Initial cell attachment at 48 h (Fig 1C) was quantified by counting the cells in randomly taken 15 images (n = 4). The images were taken after several time washing with warmed KCM. For quantification of cultured cells, the cells were harvested by using 1.25% trypsin-0.5% EDTA when the most rapid growth condition reached 90% confluence (8–11 days).
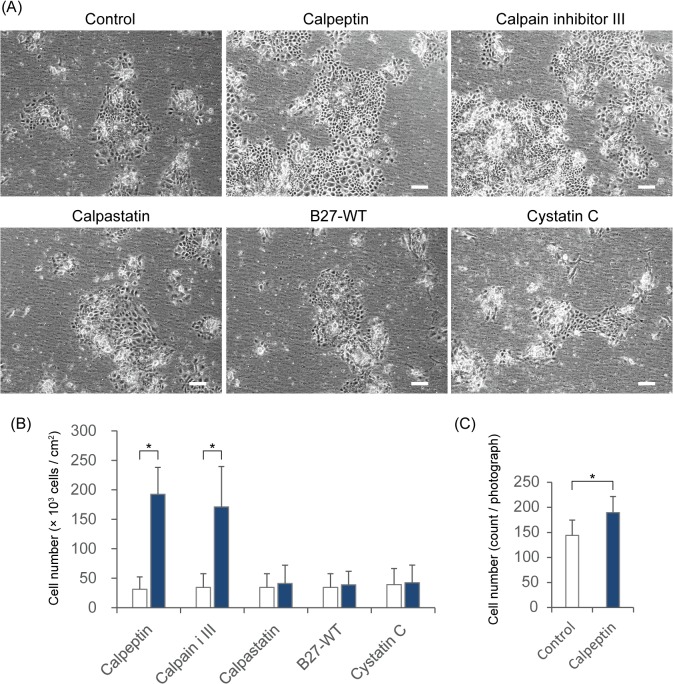
Fig 1. The proliferation and attachment of oral mucosal epithelial cells in the presence of calpain inhibitors.
(A) Phase contrast microscopic images of cultured rat oral mucosal epithelial cells. The bars indicate 200 μm. (B) The cells were collected and counted at 8–11 days after seeding (calpeptin, n = 4; calpain inhibitor III, n = 3; calpastatin, n = 3; B27-WT, n = 3; and cystatin C, n = 2). White blocks, controls; blue blocks, inhibitors. * P < 0.05 using Student's t-test. (C) The number of initially attached epithelial cells was determined 48 h after seeding (n = 4). The error bars indicate the SD. * P < 0.05 using Student's t-test.
Quantification of IL-1α concentrations in conditioned medium
Supernatants from the cells subjected to different culture conditions were individually collected and examined via enzyme-linked immunosorbent assay (ELISA). All supernatants that were evaluated for IL-1α secretion were from cultures that were incubated in KCM in the presence or absence of a calpain inhibitor. The media were changed once every few days, and the culture supernatants were collected at 8 or 9 days after seeding. The amounts of secreted IL-1α were quantified using a specific Quantikine ELISA kit (R&D Systems) according to the manufacturer’s protocol.
Immunoblotting
The cells were treated with 10% w/v trichloroacetic acid (Sigma-Aldrich) in saline on ice for 30 min at 9 days after seeding. Whole cell lysates were treated with NuPAGE LDS sample buffer (Life Technologies) and then subjected to electrophoresis using a gradient gel (Nu-PAGE 4–12% Bis-Tris Gel, Life Technologies). The resolved proteins in the gel were electrophoretically transferred to nitrocellulose membranes (iBlot Gel Transfer Stack, Life Technologies), which were then incubated at room temperature for 1 h in blocking buffer composed of 5% skim milk (Cell Signaling Technology, Danvers, MA) in 20 mM Tris-buffered saline (pH 7.5) containing 0.1% Tween-20 (TBS-T). After blocking, the membranes were treated with one of the following primary antibodies: goat polyclonal anti-IL-1α (1:10,000 dilution in 5% BSA containing TBS-T; PeproTech), rabbit monoclonal anti-calpain I (1:2000 dilution in 5% BSA containing TBS-T; Cell Signaling Technology), rabbit monoclonal anti-calpain II (1:2000 dilution in 5% BSA containing TBS-T; Cell Signaling Technology), or rabbit monoclonal anti-β-actin (1:2000 dilution in 5% BSA containing TBS-T; 13E5, Cell Signaling Technology) at 4°C. After incubation overnight, the membranes were washed with TBS-T and then incubated with an HRP-conjugated anti-mouse or anti-rabbit IgG secondary antibody (1:20,000 dilution in blocking buffer; Cell Signaling) at room temperature for 1 h. Immunoreactive bands corresponding to the IL-1α, calpain, and β-actin proteins were detected using ECL Prime Western Blotting Detection Reagent (GE Healthcare).
Gene expression analysis
Rat oral mucosal epithelial cells were seeded at a density of 4.0 × 104 cells/cm2 and were cultured for 2, 4, 6, 8, or 10 days. RNA was extracted from cultured cells using an RNeasy Plus Mini Kit (Qiagen, Hilden, Germany). Single-stranded cDNA was synthesised via a reverse transcription reaction using a PrimeScript RT reagent Kit (Takara Bio) in an iCycler Thermal Cycler (Bio-Rad Laboratories, Hercules, CA). The gene expression levels were quantified using a TaqMan Fast Universal PCR Master Mix and a StepOnePlus Real-Time PCR System (Life Technologies). Primer pairs and TaqMan MGB probes were designed for B2M, IL1α, IL1RA, TNFα, p63, Bmi1, IL1RI, IL1RII, calpain I, and calpain II for TaqMan Gene Expression Assays (Life Technologies). The mRNA expression levels were normalised to that of β2-microglobulin. The data obtained from measurements of three independent cultures were statistically analysed using Student's t-test.
Results
Calpain inhibitors promote epithelial cell growth
Calpain inhibitors of various molecular weights were added to cultures of primary rat oral mucosal epithelial cells, which were seeded at a density of 4.0 × 104 cells/cm2 on cell culture inserts in KCM without FBS or a 3T3 feeder layer. Treatment with a low molecular compound membrane-permeable calpain inhibitor (calpeptin or calpain inhibitor III) strongly promoted cell proliferation (Fig 1A and 1B). However, higher molecular weight peptide or protein (membrane-impermeable) calpain inhibitors (B27-WT, calpastatin, and cystatin C) had no effect on cell proliferation (Fig 1A and 1B). To determine whether calpain is involved in the early process of cell attachment, cells were seeded at a density of 4.0 × 104 cells/cm2, and the number of attached cells was counted prior to colony formation. Cell attachment was slightly promoted only in the presence of calpeptin (Fig 1C). Therefore, the calpain inhibitor-mediated promotion of epithelial cell growth is probably not mainly due to an early promotion of cell attachment.
Calpain inhibitors reduce IL-1α secretion
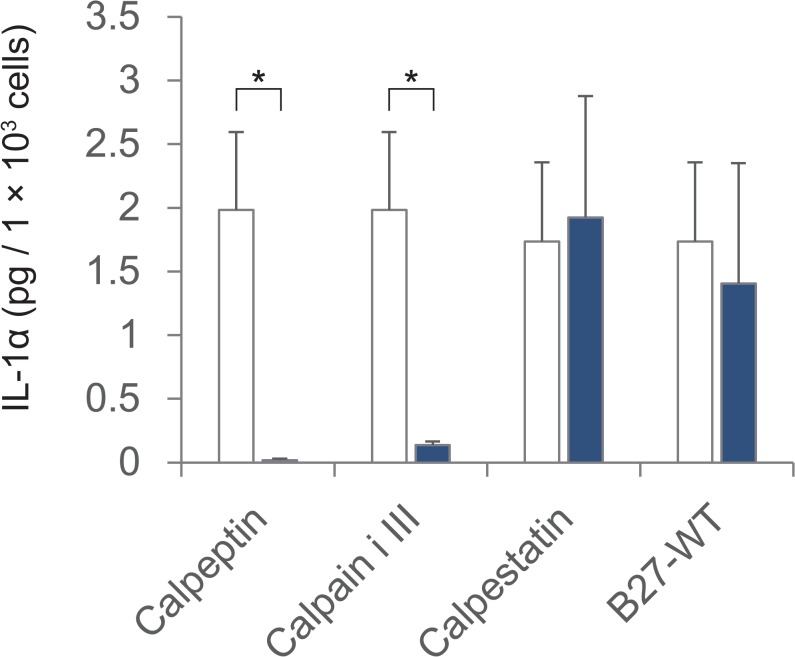
Cultured oral mucosal epithelial cells constitutively express IL-1α [20, 21]. Moreover, cytoplasmic calpain is known to cleave pro-IL-1α to mature IL-1α, which can be secreted [17, 18]. Furthermore, we recently reported that exogenous IL-1α treatment reduces epithelial cell growth [14]. Therefore, we firstly hypothesised that a cell culture environment containing membrane-permeable calpain inhibitors might promote epithelial cell growth by inhibiting the maturation and secretion of endogenous IL-1α. The concentration of IL-1α in the conditioned medium was measured via ELISA of the cell culture supernatants. The concentration of IL-1α in the conditioned medium was lower in the presence of membrane-permeable calpain inhibitors than in the other culture conditions. In cultures treated with a low-molecular-weight calpain inhibitor, such as calpeptin or calpain inhibitor III, the release of IL-1α was potently inhibited (Fig 2). By contrast, IL-1α release was not inhibited by the high-molecular-weight membrane-impermeable calpain inhibitors (Fig 2). These results indicate that the inhibition of cytoplasmic calpain proteolytic activity suppressed endogenous IL-1α maturation, reduced the secretion of mature IL-1α, and suppressed the IL-1α-mediated inhibition of epithelial cell growth in culture.
Fig 2. IL-1α levels in the supernatant of epithelial cell cultures.
IL-1α was measured in supernatants of rat oral mucosal epithelial cell cultures via ELISA. The amount of supernatant assessed on day 8 or 9 was adjusted based on the number of cells for each condition. Calpeptin, n = 3; calpain inhibitor III, n = 3; calpastatin, n = 2; and B27-WT, n = 2. White blocks, controls; blue blocks, inhibitors. The error bars indicate the SD. * P < 0.05 using Student's t-test.
Detection of cellular IL-1α expression via immunoblotting
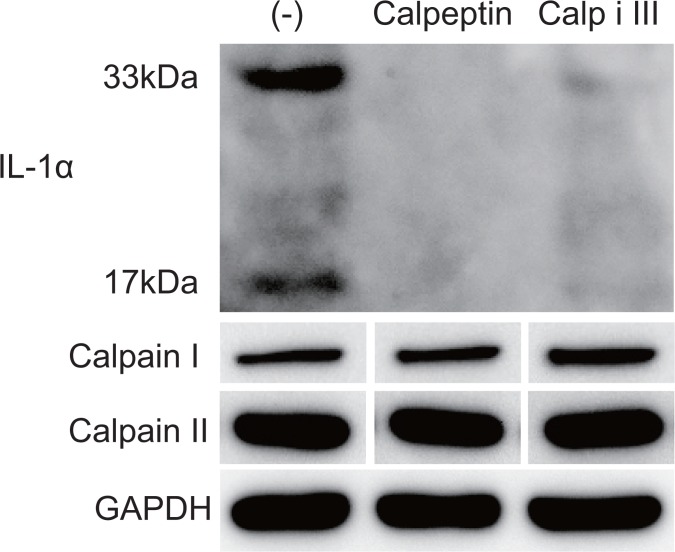
Immunoblotting of cell lysates using an anti-IL-1α antibody revealed that a small amount of mature IL-1α (17 kDa) accumulated in the cytoplasm in the presence of membrane-permeable calpain inhibitors (Fig 3 and S1–S4 Figs). Interestingly, the accumulation of the untruncated form of IL-1α (33 kDa) was also suppressed. By contrast, the expression levels of calpain I and II were not affected by calpain inhibitor treatment.
Fig 3. Expression levels of cellular IL-1α and calpains.
The expression levels of the 33-kDa pro-IL-1α, the 17-kDa mature IL-1α, calpain I, and calpain II were detected in cultured rat oral mucosal epithelial cells via immunoblotting. GAPDH expression was used as an internal control. The cultured epithelial cells were harvested on day 8.
Gene expression kinetics
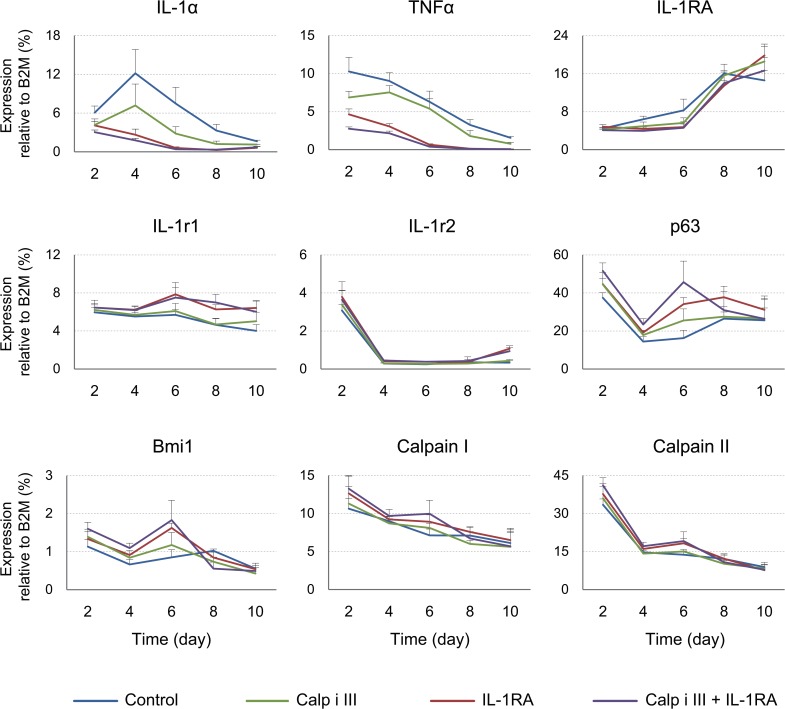
To investigate the periodical effects of the calpain inhibitors on IL-1α gene expression, time-course qPCR was performed on cultured oral mucosal epithelial cells. Also, recombinant IL-1RA was exogenously added as a binding blocker of IL-1α to the IL-1 receptor, inhibiting IL-1 signalling [22, 23]. The time-course of IL-1α gene expression is shown in Fig 4. Under control conditions in the absence of a membrane-permeable calpain inhibitor, the gene expression of IL-1α increased from day 2 to 4 and then decreased. TNF-α expression declined throughout the culture period, and IL-1RA expression increased throughout the culture period under all culture conditions. Additionally, calpain inhibitor III and IL-1RA significantly attenuated the expression of the IL-1α and TNF-α genes. The inhibitory effect of IL-1RA was stronger than that of calpain inhibitor III. Treatment with both calpain inhibitor III and IL-1RA resulted in a similar downregulatory effect to that of IL-1RA treatment alone. The expression of the IL-1 receptor II, calpain I, and calpain II genes was unchanged. The expression of IL-1 receptor I after treatment with IL-1RA was slightly but significantly increased. The expression of p63 and Bmi1, epithelial stem/progenitor markers, was modestly but significantly increased in cultures treated with calpain inhibitor III, IL-1RA, or both at days 2, 4, and 6.
Fig 4. Time-course of gene expression in cultured oral mucosal epithelial cells.
The gene expression levels of IL-1α, TNF-α, IL-1RA, calpain I, calpain II, IL-1 receptor 1 (IL-1r1), IL-1 receptor 2 (IL1r2), p63, and Bmi1 were quantified via qRT-PCR. Primary cultures of rat oral mucosal epithelial cells were harvested on day 2, 4, 6, 8, or 10 (n = 4). β-2-microglobulin gene expression was used as an internal control. The error bars indicate the SD.
IL-1RA and calpain inhibitors do not exert synergistic effects on epithelial cell growth
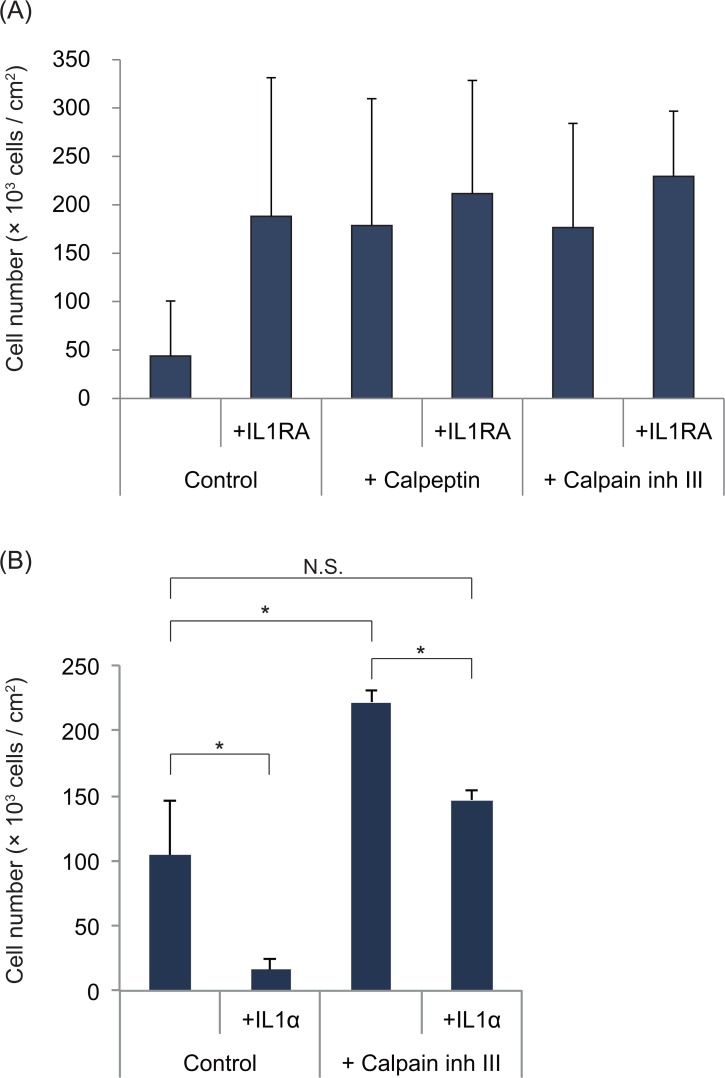
Based on the expression of the immature and mature protein and mRNA encoding IL-1α, we hypothesised that the cell growth promotion effect of calpain inhibitors is related to the regulation of IL-1α expression. Therefore, we added IL-1RA to cultures treated with a calpain inhibitor to confirm the proliferation-enhancing effect of the calpain inhibitors (Fig 5A). Calpain inhibitor and/or IL-1RA treatment significantly promoted cell proliferation compared to the control conditions. However, no significant difference was detected between treatment with either a calpain inhibitor or IL-1RA alone and treatment with both compounds.
Fig 5. Treatment of cell cultures with a calpain inhibitor (A) and/or IL-1RA (B) and/or IL-1α.
The reagents were applied upon seeding the cells, and the cells were collected and counted on (A) day 7 or 11 (n = 3) (B) day10 (n = 3). The error bars indicate the SD. (A) No significant differences were detected between any of the reagent-treated cultures. (B) * P < 0.05 using Student's t-test. N.S. indicates non-significant difference.
To clarify calpain inhibitor’s effect on IL-1α signaling, exogenous IL-1α was added to calpain inhibitor-containing condition (Fig 5B). Exogenous IL-1α dramatically inhibited epithelial cell growth as we have shown in previous study [14]. In calpain-inhibiting condition, exogenous IL-1α cancelled the growth promoting effect of calpain inhibitor, indicating that calpain inhibition acts by reducing IL-1α signaling.
Discussion
The roles of calpain in cellular signalling have been widely investigated. However, the effect of calpain inhibition on epithelial cell proliferation has not been reported. To make cell therapy using epithelial cells a viable option, a chemically defined serum-free culture system should be established, even though serum depletion sharply reduces cell growth in high Ca2+-containing media.
In this study, we found that inhibition of cytoplasmic calpain-mediated proteolytic activity using a membrane-permeable inhibitor significantly promoted epithelial cell proliferation, even under serum-free culture conditions (Fig 1). Calpeptin inhibits not only calpain I and II but also cathepsin K and L. Calpain inhibitor III, a specific inhibitor of calpain I and II, also exerted growth-promoting effects (Fig 1). However, a membrane-permeable cathepsin-specific inhibitor (Cathepsin K Inhibitor I, Merck, Darmstadt, Germany) had no growth-promoting effect (data not shown). Therefore, cytoplasmic calpain is most likely responsible for the negative regulation of epithelial cell growth via IL-1α maturation. Calpain I, referred to as μ-calpain, displays proteolytic enzyme activity when Ca2+ concentrations are in the micro molar range or higher. Calpain II, also known as m-calpain, is active when Ca2+ concentrations are in the mill molar range or higher. KCM contains 1.095 mM Ca2+. Thus, both calpains can be active in the extracellular environment, but cell-impermeable calpain inhibitors were unable to increase cell proliferation (Fig 1). Therefore, cytoplasmic calpain is most likely responsible for the promotion of epithelial cell proliferation. The cytoplasmic free [Ca2+] and molecular-bound [Ca2+] are approximately 100 nM and 10 μM, respectively, under physiological conditions [24]. However, these concentrations can be promoted by certain components in the medium. It is difficult to determine whether calpain I or II more effectively regulates the growth of cultured epithelial cells.
Calpain converts immature IL-1α into mature IL-1α, and exogenously added IL-1α inhibits epithelial cell growth. Therefore, we first hypothesised that this effect of calpain occurs through its effects on IL-1α maturation and secretion. However, surprisingly, the expression of both the mature (17 kDa) and immature (33-kDa) forms of IL-1α was inhibited by calpain inhibitor treatment for 8 days (Fig 3). This result appeared that calpain could promote the expression of immature IL-1α. Moreover, gene expression difference of IL-1α between the calpain inhibitor-treated condition and control was increased on days 4 and 6 compared to day 2 (Fig 4). Furthermore, intracellular IL-1α protein was initially not detectable because of its low abundance under all conditions. This result is possibly due to the inhibition of the unique “positive feedback” regulation of IL-1α signaling [25], as we inhibited calpain upon seeding the cells. Calpain inhibitors inhibit the initial step in IL-1α maturation, thus suppressing the accumulation of IL-1α. By contrast, more IL-1α was truncated and secreted of the cultures under control condition, which invokes further positive feedback of IL-1α.
TNF-α strongly inhibits the growth of human neonatal foreskin keratinocytes [26], and we also confirmed this inhibitory effect on rat oral mucosal epithelial cells (data not shown). In this study, TNF-α expression was significantly downregulated by calpain inhibitor treatment (Fig 4). Exogenous IL-1α slightly induces the expression of the TNF-α gene in human epidermal keratinocytes [14], whereas TNF-α induces IL-1α expression in human epidermal keratinocytes [27]. This finding indicates that IL-1α and TNF-α might amplify the expression of one another, thus strongly inhibiting epithelial cell proliferation in vitro. The mechanisms by which IL-1α and TNF-α inhibit cell growth have yet to be elucidated, but both cytokines have been reported to activate NF-κB, which arrests the growth of normal human skin epithelial cells [28]. Presumably, calpain inhibition might promote cell growth by suppressing NF-κB signalling via the inhibition of the expression of these cytokines. IL-1RA blocks the IL-1 receptor. IL-1RA expression increased over time under all culture conditions (Fig 4), but its expression occurred too late to suppress the positive feedback of IL-1α expression under the control conditions. The expression of the stem/progenitor markers p63 and Bmi1 was slightly but significantly enhanced at early time points (days 2–6; when the cells were not confluent) due to treatment with a calpain inhibitor and/or IL-1RA (Fig 4). These genes might be involved in the strong cell proliferative effects of these inhibitors at early growth stages. Moreover, p63 and Bmi1 expression were not abnormally increased (Fig 4), and the cells that reached confluence displayed contact inhibition (data not shown). Therefore, the cultured cells probably did not acquire malignant properties and may be useful for therapeutic use.
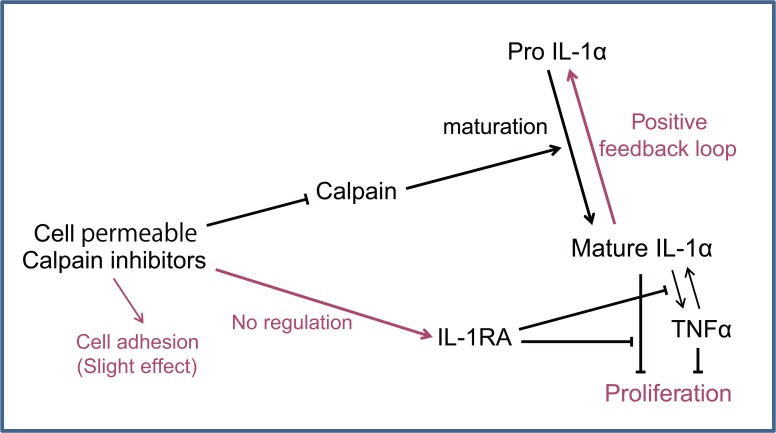
Furthermore, the combined application of IL-1RA and a calpain inhibitor did not significantly promote cell growth compared to application of a calpain inhibitor alone (Fig 5A) and exogenous IL-1α cancelled the calpain inhibitor’s cell growth promotional effect (Fig 5B). These results indicate that calpain inhibition affects cell proliferation by regulating IL-1α. Presumably, there is crosstalk between epithelial cell proliferation pathways and calpain activity, as shown in Fig 6.
Fig 6. Scheme of calpain and IL-1α signalling in regulating epithelial proliferation by cell permeable calpain inhibitors.
The cellular responses to medium components vary between cells from experimental animals and humans [29]. In addition, primary cultures of keratinocytes and commercially available keratinocytes display distinct responses to medium components, such as Ca2+ and serum [30], owing to differences in the isolation procedure that affect cell proliferation and differentiation properties. Therefore, oral mucosal epithelial cells from human volunteers and patients should be examined, even though their availability is limited. Further elucidation of the roles of the calpain super-family and of potential calpain inhibitors will be important for evaluating various approaches and will be useful for optimising the epithelial cell culture process for regenerative medicine.
Supporting Information
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
This study was supported by the Formation of Innovation Center for Fusion of Advanced Technologies in the Special Coordination Funds for Promoting Science and Technology; the Cell Sheet Tissue Engineering Center from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT); the Global COE program; The Multidisciplinary Education and Research Center for the Establishment of Regenerative Medicine from MEXT; and a JSPS research fellowship (DC1: 22–3791) from the Japanese Society for the Promotion of Science. The authors would like to thank Dr. Norio Ueno, Mr. Hiroaki Sugiyama, and Ms. Sumako Kameishi for their useful comments concerning this study.
Data Availability
All relevant files are available from the Figshare database (accession number 1472983).
Funding Statement
This work was supported by the Formation of Innovation Center for Fusion of Advanced Technologies in the Special Coordination Funds for Promoting Science and Technology, "The Cell Sheet Tissue Engineering Center" from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) (to TO); the Global COE program, "The Multidisciplinary Education and Research Center for the Establishment of Regenerative Medicine" (K08) from MEXT (to MY) (http://www.jsps.go.jp/j-globalcoe/05_kyoten_k.html); and JSPS research fellowship (DC1: 22-3791) from the Japanese Society for the Promotion of Science (to MK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6(3):331–43. . [DOI] [PubMed] [Google Scholar]
- 2. Green H, Kehinde O, Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(11):5665–8. Epub 1979/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gallico GG 3rd, O'Connor NE, Compton CC, Kehinde O, Green H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984;311(7):448–51. . [DOI] [PubMed] [Google Scholar]
- 4. Phillips TJ, Kehinde O, Green H, Gilchrest BA. Treatment of skin ulcers with cultured epidermal allografts. Journal of the American Academy of Dermatology. 1989;21(2 Pt 1):191–9. Epub 1989/08/01. . [DOI] [PubMed] [Google Scholar]
- 5. Gallico GG 3rd, O'Connor NE, Compton CC, Remensnyder JP, Kehinde O, Green H. Cultured epithelial autografts for giant congenital nevi. Plastic and reconstructive surgery. 1989;84(1):1–9. Epub 1989/07/01. . [DOI] [PubMed] [Google Scholar]
- 6. Muller-Glauser W, Preisig E. The effect of cholera toxin and epidermal growth factor on the in-vitro growth of human oral epithelial cells. Archives of oral biology. 1983;28(8):765–71. Epub 1983/01/01. . [DOI] [PubMed] [Google Scholar]
- 7. Hata K, Ueda M. Fabrication of cultured epithelium using oral mucosal cells and its clinical applications. Human cell. 1996;9(1):91–6. Epub 1996/03/01. . [PubMed] [Google Scholar]
- 8. Ueda M, Hata K, Horie K, Torii S. The potential of oral mucosal cells for cultured epithelium: a preliminary report. Annals of plastic surgery. 1995;35(5):498–504. Epub 1995/11/01. . [DOI] [PubMed] [Google Scholar]
- 9. de Luca M, Albanese E, Megna M, Cancedda R, Mangiante PE, Cadoni A, et al. Evidence that human oral epithelium reconstituted in vitro and transplanted onto patients with defects in the oral mucosa retains properties of the original donor site. Transplantation. 1990;50(3):454–9. Epub 1990/09/01. . [DOI] [PubMed] [Google Scholar]
- 10. Tsai CY, Ueda M, Hata K, Horie K, Hibino Y, Sugimura Y, et al. Clinical results of cultured epithelial cell grafting in the oral and maxillofacial region. Journal of cranio-maxillo-facial surgery: official publication of the European Association for Cranio-Maxillo-Facial Surgery. 1997;25(1):4–8. Epub 1997/02/01. . [DOI] [PubMed] [Google Scholar]
- 11. Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351(12):1187–96. . [DOI] [PubMed] [Google Scholar]
- 12. Murakami D, Yamato M, Nishida K, Ohki T, Takagi R, Yang J, et al. The effect of micropores in the surface of temperature-responsive culture inserts on the fabrication of transplantable canine oral mucosal epithelial cell sheets. Biomaterials. 2006;27(32):5518–23. 10.1016/j.biomaterials.2006.05.042 . [DOI] [PubMed] [Google Scholar]
- 13. Ohki T, Yamato M, Ota M, Takagi R, Murakami D, Kondo M, et al. Prevention of Esophageal Stricture After Endoscopic Submucosal Dissection Using Tissue-Engineered Cell Sheets. Gastroenterology. 2012. Epub 2012/05/09. 10.1053/j.gastro.2012.04.050 . [DOI] [PubMed] [Google Scholar]
- 14. Kondo M, Yamato M, Takagi R, Namiki H, Okano T. The regulation of epithelial cell proliferation and growth by IL-1 receptor antagonist. Biomaterials. 2013;34(1):121–9. 10.1016/j.biomaterials.2012.09.036 . [DOI] [PubMed] [Google Scholar]
- 15. Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiological reviews. 2003;83(3):731–801. Epub 2003/07/05. 10.1152/physrev.00029.2002 . [DOI] [PubMed] [Google Scholar]
- 16. Sorimachi H, Hata S, Ono Y. Calpain chronicle—an enzyme family under multidisciplinary characterization. Proceedings of the Japan Academy Series B, Physical and biological sciences. 2011;87(6):287–327. Epub 2011/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kobayashi Y, Yamamoto K, Saido T, Kawasaki H, Oppenheim JJ, Matsushima K. Identification of calcium-activated neutral protease as a processing enzyme of human interleukin 1 alpha. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(14):5548–52. Epub 1990/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Watanabe N, Kobayashi Y. Selective release of a processed form of interleukin 1 alpha. Cytokine. 1994;6(6):597–601. Epub 1994/11/01. . [DOI] [PubMed] [Google Scholar]
- 19. Sultana T, Wahab-Wahlgren A, Assmus M, Parvinen M, Weber G, Soder O. Expression and regulation of the prointerleukin-1alpha processing enzymes calpain I and II in the rat testis. International journal of andrology. 2003;26(1):37–45. Epub 2003/01/22. . [DOI] [PubMed] [Google Scholar]
- 20. Luger TA, Stadler BM, Katz SI, Oppenheim JJ. Epidermal cell (keratinocyte)-derived thymocyte-activating factor (ETAF). Journal of immunology. 1981;127(4):1493–8. . [PubMed] [Google Scholar]
- 21. Kupper TS, Ballard DW, Chua AO, McGuire JS, Flood PM, Horowitz MC, et al. Human keratinocytes contain mRNA indistinguishable from monocyte interleukin 1 alpha and beta mRNA. Keratinocyte epidermal cell-derived thymocyte-activating factor is identical to interleukin 1. The Journal of experimental medicine. 1986;164(6):2095–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eisenberg SP, Evans RJ, Arend WP, Verderber E, Brewer MT, Hannum CH, et al. Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature. 1990;343(6256):341–6. 10.1038/343341a0 . [DOI] [PubMed] [Google Scholar]
- 23. Carter DB, Deibel MR Jr, Dunn CJ, Tomich CS, Laborde AL, Slightom JL, et al. Purification, cloning, expression and biological characterization of an interleukin-1 receptor antagonist protein. Nature. 1990;344(6267):633–8. 10.1038/344633a0 . [DOI] [PubMed] [Google Scholar]
- 24. Bygrave FL, Benedetti A. What is the concentration of calcium ions in the endoplasmic reticulum? Cell calcium. 1996;19(6):547–51. . [DOI] [PubMed] [Google Scholar]
- 25. Dinarello CA, Ikejima T, Warner SJ, Orencole SF, Lonnemann G, Cannon JG, et al. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. Journal of immunology. 1987;139(6):1902–10. Epub 1987/09/15. . [PubMed] [Google Scholar]
- 26. Pillai S, Bikle DD, Eessalu TE, Aggarwal BB, Elias PM. Binding and biological effects of tumor necrosis factor alpha on cultured human neonatal foreskin keratinocytes. The Journal of clinical investigation. 1989;83(3):816–21. 10.1172/JCI113963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kutsch CL, Norris DA, Arend WP. Tumor necrosis factor-alpha induces interleukin-1 alpha and interleukin-1 receptor antagonist production by cultured human keratinocytes. The Journal of investigative dermatology. 1993;101(1):79–85. . [DOI] [PubMed] [Google Scholar]
- 28. Seitz CS, Deng H, Hinata K, Lin Q, Khavari PA. Nuclear factor kappaB subunits induce epithelial cell growth arrest. Cancer research. 2000;60(15):4085–92. . [PubMed] [Google Scholar]
- 29. Kondo M, Yamato M, Takagi R, Murakami D, Namiki H, Okano T. Significantly different proliferative potential of oral mucosal epithelial cells between six animal species. Journal of biomedical materials research Part A. 2013. 10.1002/jbm.a.34849 . [DOI] [PubMed] [Google Scholar]
- 30. Lamb R, Ambler CA. Keratinocytes propagated in serum-free, feeder-free culture conditions fail to form stratified epidermis in a reconstituted skin model. PloS one. 2013;8(1):e52494 10.1371/journal.pone.0052494 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant files are available from the Figshare database (accession number 1472983).