Supplemental Digital Content is available in the text
Keywords: HIV, immunogenicity, pneumococcal conjugate vaccine, pneumococcal disease, safety
Abstract
Objective:
Immunocompromised individuals are at an increased risk of pneumococcal disease. Vaccination is recommended as an important strategy to reduce risk of pneumococcal disease in HIV-infected individuals. This study evaluated the safety and immunogenicity of three 13-valent pneumococcal conjugate vaccine (PCV13) doses followed by one dose of 23-valent pneumococcal polysaccharide vaccine (PPSV23) at 1-month intervals in pneumococcal vaccine-naive, HIV-infected individuals.
Design:
This was a phase 3, open-label, single-arm study.
Methods:
Pneumococcal vaccine-naive, HIV-infected individuals at least 6 years of age with CD4+ T-cell count at least 200 cells/μl and viral load less than 50 000 copies/ml received three doses of PCV13 followed by one dose of PPSV23 at 1-month intervals. Serotype-specific antipneumococcal immune responses were assessed by IgG geometric mean concentrations (GMCs) and opsonophagocytic activity (OPA) assay geometric mean titres (GMTs) after each dose. Local reactions at the PCV13 injection site, systemic and other adverse events were collected.
Results:
Three hundred and one individuals were enrolled and vaccinated; 279 completed the study. Statistically significant increases in IgG GMCs and OPA GMTs were observed for all serotypes after dose 1 of PCV13 compared with prevaccine levels. GMCs and GMTs were comparable or only modestly increased for all serotypes after PCV13 doses 2 and 3 and after PPSV23. The majority of local reactions and systemic events were mild to moderate in severity.
Conclusion:
A three-dose regimen of PCV13 was well tolerated in pneumococcal vaccine-naive, HIV-infected individuals. Significant immune responses to all serotypes were observed following the first dose of PCV13, with only modest increases in antibody titres following subsequent PCV13 or PPSV23 administration.
Introduction
Immunologic deficits associated with HIV infection predispose individuals to an increased risk of pneumococcal disease compared with HIV-uninfected individuals [1]. Studies conducted in Africa and the United States found a nine-fold to 43-fold increase in invasive pneumococcal disease (IPD) among HIV-infected children compared with HIV-uninfected children before widespread use of HAART [1]. The rate of IPD is six to 324 times higher and recurrent disease was found to be five to nine times more common in HIV-infected adults than in uninfected adults [1]. When IPD occurred in adults infected by HIV, case fatality rates were as high as 33%; in adults with AIDS and bacteremic pneumococcal pneumonia, mortality rates reached 57% [1]. Although treatment of HIV-infected individuals with HAART has had a significant effect on the burden of IPD in children (51% reduction), and a more variable effect in adults, the risk of IPD remains 28 to 42-fold greater in HIV-infected individuals than in the healthy population [2–6].
Vaccination of HIV-infected individuals with the 23-valent pneumococcal polysaccharide vaccine (PPSV23) has been recommended in many countries worldwide [7,8]. However, a randomized, controlled trial of PPSV23 in HIV-infected patients in Uganda showed no efficacy [9]. Additional studies with PPSV23 have shown that this vaccine is often not immunogenic and poorly efficacious in HIV-infected children and adults who have or have not received HAART [10–13].
The seven-valent pneumococcal conjugate vaccine (PCV7) has been shown to be well tolerated and immunogenic in infants [14], children and adolescents [15], as well as adults [16]. In a study conducted in Malawi, the administration of two doses of PCV7 four weeks apart in HIV-infected adolescents and adults resulted in a vaccine efficacy of 74% against recurrent IPD caused by vaccine serotypes and 6A [17]. In addition, an investigational nine-valent pneumococcal conjugate vaccine was 65% efficacious for the prevention of IPD in HIV-infected infants [18]. The 13-valent pneumococcal conjugate vaccine (PCV13), which contains additional serotypes that are sometimes more prevalent in HIV-infected adults [19,20], may offer a greater breadth of protection than the earlier PCV7. Guidelines recommend that immunocompromised individuals (including those infected with HIV) who are PPSV23-naive should receive a single vaccination with PCV13 followed at least 8 weeks later with a single vaccination with PPSV23 [7]. Those who previously received PPSV23 should receive a single dose of PCV13 [7].
As suppression of immune responses in HIV-infected individuals is frequently observed [21,22], this study assessed the potential advantage of a three-dose series of PCV13 administered 1 month apart. As PPSV23 is generally recommended in HIV-infected individuals, one dose of PPSV23 was administered 1 month after completion of the three-dose series of PCV13.
Materials and methods
Study design
This phase 3, open-label, single-arm study assessed safety, tolerability and immunogenicity of three doses of PCV13 followed by PPSV23 administered at 1-month intervals in pneumococcal vaccine-naive, HIV-infected individuals. The study was performed at 12 sites (nine in South Africa, three in Romania) and was conducted in accordance with International Conference on Harmonisation Guideline for Good Clinical Practice and the ethical principles that have their origin in the Declaration of Helsinki. Written informed consent was obtained from all individuals/parents/legal guardians before enrolment in the study. Independent ethics committees reviewed and provided written approval for all relevant study documents. This study is registered on ClinicalTrials.gov with identifier NCT00962780.
Study participants
Eligible individuals were at least 6 years of age at the time of enrolment, were HIV-infected with a CD4+ T-cell count at least 200 cells/μl and a viral load less than 50 000 copies/ml obtained on the most recent two occasions within 6 months before first vaccination; received a stable dose of HAART for at least 6 weeks before first vaccination, or were not receiving any antiretroviral therapy; and were naive to any licensed or experimental pneumococcal vaccine. Individuals were ineligible if they had active AIDS-related illness, including opportunistic infection or malignancy, previous anaphylactic reaction to any vaccine or vaccine-related component, or a history of culture-proven invasive disease caused by Streptococcus pneumoniae within 12 months before enrolment.
Vaccines
PCV13 contains saccharides of serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F individually conjugated to nontoxic diphtheria toxin CRM197 (cross-reactive material). The vaccine is formulated to contain 2.2 μg of each saccharide, except for 4.4 μg of serotype 6B, in 5 mmol/l succinate buffer, 0.02% polysorbate 80, and 0.125 mg aluminium as aluminium phosphate per 0.5-ml dose. The PPSV23 vaccine contains serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F and 33F. The vaccine is composed of 25 μg of each polysaccharide in phenol, sodium chloride and water per 0.5-ml dose.
Study objectives
The objectives of the study were to assess the immunogenicity of PCV13 after each of three PCV13 doses administered to HIV-infected individuals and to compare the antibody responses after three doses of PCV13 to those after two doses. Endpoints included evaluation of serotype-specific IgG geometric mean concentrations (GMCs) and serotype-specific opsonophagocytic activity (OPA) geometric mean titres (GMTs) in the overall study population, as well as in children (6 to <18 years of age) and adults (≥18 years of age) separately. The safety of PCV13 was assessed by evaluating the incidence rates of local reactions, systemic events and adverse events.
Study assessments
Immunogenicity assessments
Blood samples were obtained at each vaccination visit (Visit 1–4) and at Visit 5, approximately 1 month after vaccination with PPSV23. Antipneumococcal immune responses were evaluated by measuring serotype-specific anticapsular polysaccharide and functional antipneumococcal opsonophagocytic antibodies at each time point. For each of the 13 pneumococcal serotypes, serum concentrations of anticapsular IgG were determined using an ELISA employing two adsorbents: a C polysaccharide containing a purified lysate containing pneumococcal cell wall polysaccharide and serotype 22F capsular polysaccharide. Functional antipneumococcal opsonophagocytic antibodies to all PCV13 serotypes were measured by a validated OPA assay [23]. Titres in OPA assays were defined as the interpolated reciprocal serum dilution that results in complement-mediated killing of 50% of the bacteria in each OPA assay.
Safety assessments
Local reactions (redness, swelling, pain, limitation of arm movement) at the PCV13 injection site and systemic events (fatigue, headache, vomiting, diarrhoea, muscle pain, joint pain), and oral temperature were monitored daily for 14 days after vaccination and recorded in an electronic diary by the individuals/parents/legal guardians. Acute reactions were assessed for at least 20 min after each vaccination. Adverse events were also collected from the signing of the informed consent document through the visit 1 month after PPSV23 vaccination. Newly diagnosed chronic conditions that occurred between the visit 1 month after the PPSV23 vaccination and the 6-month follow-up telephonic contact were recorded. Serious adverse events were collected through the end of the study.
Statistical analysis
Sample size was based on the precision of the two-sided 95% confidence interval (CI) for the IgG mean-fold rise. A sample of 200 evaluable individuals provided precision of at least 0.237 on the two-sided 95% CI mean-fold rise among the PCV13 serotypes. Allowing for a rate of approximately 33% for dropouts and major protocol violations, a total of 300 enrolled individuals provided at least 200 evaluable individuals for the study. The evaluable immunogenicity population included all eligible individuals who received at least two PCV13 doses and had valid immunogenicity results 1 month after each dose for at least one serotype, received no prohibited vaccines and had no major protocol violations.
Pneumococcal serotype-specific IgG GMCs and OPA GMTs were calculated using logarithmically transformed assay results. At each visit, two-sided 95% CIs were constructed by back transformation of the CI for the mean of the logarithmically transformed assay results computed using the Student's t-distribution. IgG and OPA GMTs and geometric mean-fold rises (GMFRs) were computed for individuals aged at least 6 years, as well as for individuals 6–17 years and those aged at least 18 years. Differences in GMTs/GMCs were considered statistically significantly increased or decreased when the lower or upper limit of the two-sided 95% CI for GMFR was more than 1 or less than 1, respectively.
The safety population included all participants who received at least one dose of investigational product. The local reactions and systemic events, including fever and use of antipyretics and pain medication, were summarized for each PCV13 vaccination, age group and the overall study population separately. In addition, for local reactions and systemic events, including fever, the duration of the event was summarized using descriptive statistics. Exact two-sided 95% CIs (Clopper and Pearson) were constructed based upon the observed proportion of individuals. Adverse events were categorized according to the Medical Dictionary for Regulatory Activities. The adverse events and serious adverse events (SAEs) were summarized by age group and the overall study population separately for each vaccine dose.
Results
Individual disposition and baseline characteristics
From March 2010 through April 2013, 303 individuals (6–17 years, n = 151; ≥18 years, n = 152) consented to participate in the study and were assigned to receive vaccine (see Figure, Supplemental Digital Content 1 which presents subject disposition). A total of 301 individuals [paediatric group (6–17 years), n = 150; adult group (≥18 years), n = 151] received at least one dose of PCV13 (the safety population), and 279 individuals received all vaccine doses, completed all blood draws and were contacted 6 months after the PPSV23 dose. Characteristics of the individuals are presented in Table 1. The majority of the individuals were female (54.5%) and black (84.7%), with a mean age at vaccination of 25.8 years. The mean ages for the paediatric and adult subgroups were 10.3 years and 41.2 years, respectively. HIV was acquired through sexual contact in 48.5% of all individuals, and in 94.7% of adults (≥18 years of age); perinatal transmission occurred in 47.5% of all individuals, all of whom (95.3%) were in the paediatric subgroup. Nearly all individuals (97.3%) were on HAART; 70.8% had viral loads 50 copies/ml or less; the mean (SD) CD4+ T-cell count was 717 ± 381 cells/μl. Demographic characteristics were generally similar between adult and paediatric individuals, unless otherwise noted.
Table 1.
Demographic characteristics: safety population.
| Age 6–17 years Na = 150 | Age ≥18 years Na = 151 | Age ≥6 years Na = 301 | ||
| Sex, n (%) | Female | 76 (50.7) | 88 (58.3) | 164 (54.5) |
| Race, n (%) | Black | 136 (90.7) | 119 (78.8) | 255 (84.7) |
| White | 7 (4.7) | 32 (21.2) | 39 (13.0) | |
| Other | 7 (4.7) | 0 | 7 (2.3) | |
| Age | Years, mean (SD) | 10.3 (3.0) | 41.2 (8.5) | 25.8 (16.7) |
| Baseline HIV profile | Receiving HAART, n (%) | 147 (98.0) | 146 (96.7) | 293 (97.3) |
| CD4+ cell count,b cells/μl, mean (SD) | 897.9 (381.0) | 537.3 (283.8) | 717.0 (380.8) | |
| CD4+ cell count <200 cells/μl (%) | 0 | 1.3 | 0.7 | |
| CD4+ cell count 200 to <350 cells/μl (%) | 4.7 | 25.2 | 15.0 | |
| CD4+ cell count 350–500 cells/μl (%) | 10.0 | 27.2 | 18.6 | |
| CD4+ cell count >500 copies/ml (%) | 85.3 | 46.4 | 65.8 | |
| Viral load,b copies/ml, (SD) | 1704.8 (5503.7) | 2472.6 (13 139.0) | 2090.0 (10 075.2) | |
| Viral load ≤50 copies/ml (%) | 70.7 | 70.9 | 70.8 | |
| Viral load 51 to ≤200 copies/ml (%) | 9.3 | 11.9 | 10.6 | |
| Viral load 201 to <1000 copies/ml (%) | 3.3 | 7.3 | 5.3 | |
| Viral load 1000 to <10 000 copies/ml (%) | 10.0 | 4.0 | 7.0 | |
| Viral load 10 000–50 000 copies/ml (%) | 6.7 | 5.3 | 6.0 | |
| Viral load >50 000 copies/ml (%) | 0 | 0.7 | 0.3 | |
| Mode of HIV transmission, % | Sexual | 2.0 | 94.7 | 48.5 |
| Perinatal | 95.3 | 0 | 47.5 | |
| Other | 2.7 | 5.3 | 4.0 |
aN = number of individuals in the total population or age group.
bFirst assessment.
Immunogenicity
The evaluable immunogenicity population consisted of 270 individuals for PCV13 dose 1, 266 individuals for PCV13 dose 2, 259 individuals for PCV13 dose 3 and 263 individuals for the PPSV23 dose. The most frequent reasons for exclusion from the dose 1 evaluable immunogenicity population were study eligibility (7.6%), most recent CD4+ T-cell window more than 180 days (5.0%) or receipt of less than two doses of PCV13 in the sequence assigned (4.0%). Individuals excluded for these reasons were also excluded from the analysis at all subsequent doses.
There was an increase in IgG GMCs for all serotypes after dose 1 of PCV13 in the overall population with statistically significant increases in GMCs observed for all serotypes after dose 1 compared with before dose 1 (Table 2 and Fig. 1a). IgG GMCs were similar or increased for all serotypes after doses 2 and 3 of PCV13 compared with the previous doses and after the single dose of PPSV23 compared with PCV13 dose 3; however, increases were modest in contrast to those occurring from before to after dose 1 of PCV13. Statistically significant increases in IgG GMCs were observed for six of the 13 serotypes after dose 3 compared with after dose 2. In addition, IgG GMCs were only modestly increased after dose 3 compared with after dose 1 (see Table, Supplemental Digital Content 2). Generally, results for the 6- to 17-year and the at least 18-year age groups were numerically similar to each other and to the overall evaluable immunogenicity population (see Table, Supplemental Digital Content 3). In both age groups, statistically significant increases in IgG GMCs were observed for all serotypes after dose 1 compared with before dose 1. IgG GMCs were comparable or modestly increased in both age groups for most serotypes after doses 2 and 3 of PCV13 compared with after the previous dose and after the single dose of PPSV23 compared with after PCV13 dose 3. Statistically significant increases in IgG GMCs were observed in five and three of the 13 serotypes in the 6 to 17-years and at least 18-years age groups, respectively, after dose 3 compared with after dose 2. A posthoc analysis of IgG GMCs according to sex found similar immune responses to those that occurred in the overall population (data not shown).
Table 2.
IgG geometric mean concentrations (μg/ml) at each time point and geometric mean-fold rises between selected timepoints – evaluable immunogenicity population at least 6 years of age.
| IgG GMC at specified time pointsa,b (n = 202–270)c | GMFR between indicated time pointsd,e (n = 200−270)f | ||||||||
| Serotype | Before dose 1 | After dose 1 | After dose 2 | After dose 3 | After PPSV23 | After dose 1/before dose 1 (95% CI) | After dose 2/after dose 1 (95% CI) | After dose 3/after dose 2 (95% CI) | After PPSV23/after dose 3 (95% CI) |
| 1 | 0.88 | 3.60 | 3.90 | 3.97 | 4.12 | 4.37 (3.63–5.25) | 1.08 (1.02–1.14) | 1.03 (0.99–1.07) | 1.05 (1.00–1.10) |
| 3 | 0.61 | 0.99 | 1.23 | 1.25 | 1.28 | 1.57 (1.42–1.74) | 1.24 (1.17–1.31) | 1.05 (1.00–1.10) | 1.02 (0.97–1.08) |
| 4 | 0.26 | 2.73 | 3.06 | 3.25 | 3.15 | 10.38 (8.64–12.47) | 1.14 (1.07–1.21) | 1.09 (1.03–1.14) | 0.97 (0.94–1.01) |
| 5 | 3.74 | 5.04 | 5.16 | 5.11 | 5.61 | 1.38 (1.28–1.50) | 1.02 (0.97–1.06) | 1.01 (0.97–1.05) | 1.09 (1.05–1.14) |
| 6A | 3.81 | 7.16 | 7.54 | 7.53 | 7.38 | 1.83 (1.60–2.10) | 1.06 (1.00–1.11) | 1.02 (0.97–1.07) | 0.96 (0.93–1.00) |
| 6B | 4.17 | 8.63 | 9.77 | 10.02 | 9.59 | 2.05 (1.80–2.33) | 1.14 (1.08–1.19) | 1.04 (1.01–1.08) | 0.95 (0.92–0.98) |
| 7F | 1.00 | 4.69 | 5.04 | 5.46 | 5.52 | 4.99 (4.24–5.88) | 1.08 (1.03–1.13) | 1.10 (1.05–1.16) | 1.01 (0.98–1.05) |
| 9V | 1.80 | 4.87 | 5.06 | 5.23 | 5.54 | 2.76 (2.45–3.10) | 1.04 (1.00–1.08) | 1.05 (1.01–1.09) | 1.06 (1.02–1.09) |
| 14 | 1.44 | 13.93 | 14.23 | 13.83 | 14.50 | 9.91 (8.11–12.11) | 1.04 (0.98–1.09) | 1.04 (1.00–1.09) | 1.02 (0.97–1.07) |
| 18C | 0.75 | 4.55 | 4.48 | 4.46 | 4.12 | 6.23 (5.29–7.35) | 0.99 (0.95–1.04) | 0.99 (0.96–1.02) | 0.92 (0.90–0.95) |
| 19A | 5.97 | 13.15 | 13.72 | 13.67 | 13.22 | 2.20 (1.98–2.45) | 1.04 (1.00–1.09) | 0.99 (0.96–1.03) | 0.97 (0.94–1.00) |
| 19F | 1.35 | 4.81 | 5.64 | 5.82 | 6.30 | 3.54 (3.04–4.13) | 1.19 (1.12–1.27) | 1.06 (1.01–1.11) | 1.07 (1.02–1.11) |
| 23F | 1.90 | 5.30 | 6.38 | 6.90 | 6.49 | 2.87 (2.49–3.31) | 1.21 (1.14–1.28) | 1.09 (1.03–1.15) | 0.93 (0.89–0.96) |
CI, confidence interval; GMC, geometric mean concentration; GMFR, geometric mean-fold rise; Ig, immunoglobulin; SAP, statistical analysis plan.
aSAP-specified timing for blood samples: just prior to vaccination and 1 month after each vaccination.
bGMCs were calculated using all individuals with available data for the specified blood draw.
cn, Number of individuals with valid and determinate assay results for the specified serotype at the given visit.
dGMFRs were calculated using all individuals with available data from both the specified blood draws.
eCIs are back transformations of a confidence interval on the basis of the Student's t distribution for the mean logarithm of the mean-fold rise.
fn, Number of individuals with valid and determinate assay results for the specified serotype at both specified blood draws.
Fig. 1.
Antipneumococcal responses after PCV13 vaccination.
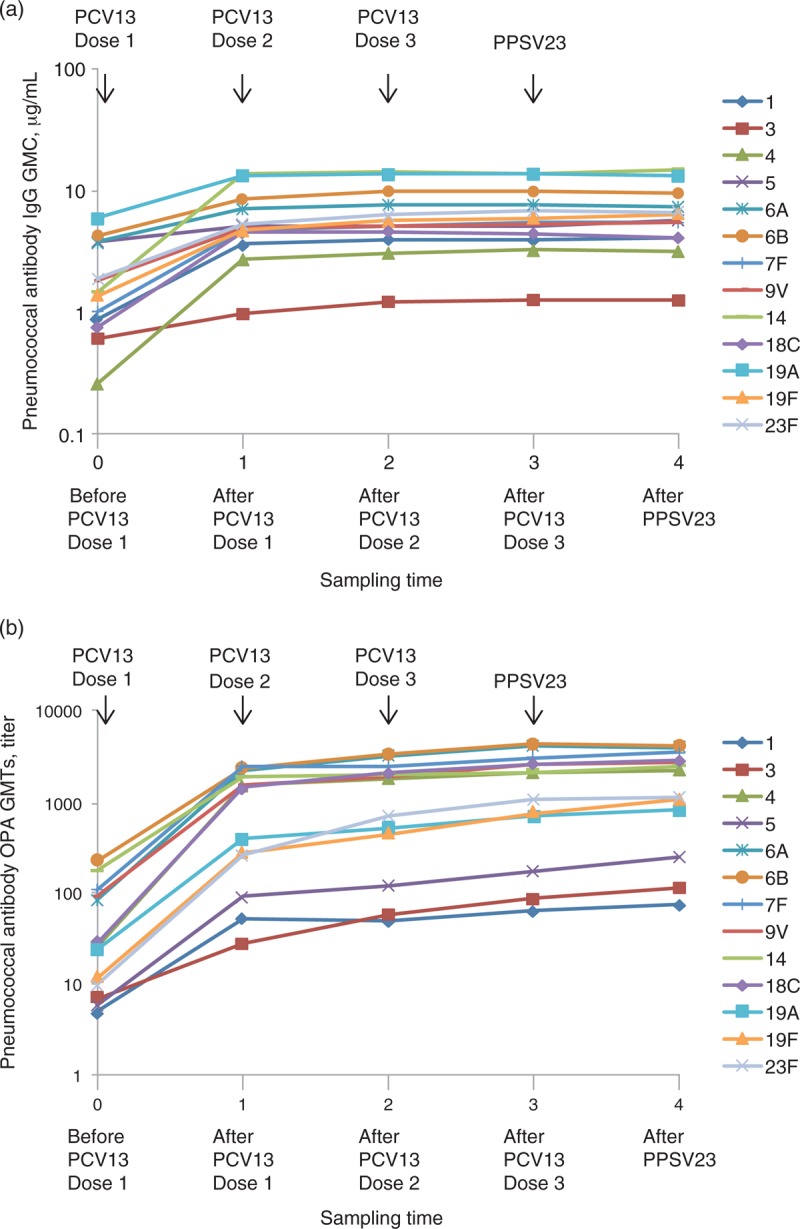
(a) Anticapsular polysaccharide IgG GMCs before the first PCV13 dose and 1 month after the 3 PCV13 doses and PPSV23 dose. (b) OPA GMTs before the first PCV13 dose and 1 month after the 3 PCV13 doses and PPSV23 dose. GMC, geometric mean concentration; GMT, geometric mean titre; IgG, immunoglobulin G; OPA, opsonophagocytic activity; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
OPA GMTs increased for all serotypes after dose 1 of PCV13 in the overall population with statistically significant increases in OPA GMTs observed for all serotypes after dose 1 compared with before dose 1 (Table 3 and Fig. 1b). OPA GMTs were increased for most serotypes after doses 2 and 3 of PCV13 compared with after the previous dose and after the single dose of PPSV23 compared with after PCV13 dose 3; however, increases were modest compared with those occurring from before to after dose 1 of PCV13. In addition, OPA GMTs were only modestly increased after dose 3 compared with after dose 1 (see Table, Supplemental Digital Content, 2). Statistically significant increases in OPA GMTs were observed for all serotypes except serotype 14 after dose 3 compared with after dose 2. In both age groups, OPA GMTs increased for all serotypes after dose 1 of PCV13 (see Table, Supplemental Digital Content 4). In general, after dose 1 compared with before dose 1, GMFRs were numerically similar between age groups, with a few exceptions; GMFRs for serotypes 4, 18C, 19F and 23F were numerically higher in the 6 to 17-year age group than in the at least 18-year age group, whereas the GMFR for serotype 7F was numerically lower in the 6 to 17-year age group than in the at least 18-year age group. In both age groups, OPA GMTs for all serotypes were comparable or modestly increased after dose 2 compared with after dose 1 and after PPSV23 compared with after PCV13 dose 3, whereas statistically significant increases were observed for all serotypes except serotype 14 after dose 3 compared with after dose 2. A posthoc analysis of OPA GMTs according to sex found similar immune responses as those seen for the overall population (data not shown).
Table 3.
Opsonophagocytic activity geometric mean titres at each time point and geometric mean-fold rise between selected time points – evaluable immunogenicity population at least 6 years of age.
| OPA GMT at specified time pointsa,b (n = 205–265)c | GMFR between indicated time pointsd,e (n = 197−260)f | ||||||||
| Serotype | Before dose 1 | After dose 1 | After dose 2 | After dose 3 | After PPSV23 | After dose 1/before dose 1 (95% CI) | After dose 2/after dose 1 (95% CI) | After dose 3/after dose 2 (95% CI) | After PPSV23/after dose 3 (95% CI) |
| 1 | 5 | 55 | 54 | 69 | 82 | 10.9 (8.65–13.81) | 1.0 (0.86–1.09) | 1.3 (1.16–1.48) | 1.2 (1.08–1.31) |
| 3 | 7 | 30 | 62 | 95 | 124 | 4.5 (3.73–5.35) | 2.1 (1.84–2.39) | 1.5 (1.40–1.65) | 1.3 (1.20–1.44) |
| 4 | 27 | 1780 | 2054 | 2458 | 2527 | 66.8 (46.17–96.75) | 1.2 (1.02–1.32) | 1.2 (1.13–1.37) | 1.0 (0.97–1.10) |
| 5 | 6 | 98 | 132 | 194 | 271 | 17.6 (13.25–23.44) | 1.4 (1.19–1.64) | 1.5 (1.33–1.72) | 1.4 (1.20–1.53) |
| 6A | 91 | 2494 | 3799 | 4856 | 4500 | 23.6 (15.98–34.94) | 1.4 (1.24–1.62) | 1.4 (1.23–1.51) | 0.9 (0.87–0.95) |
| 6B | 243 | 2738 | 3923 | 5133 | 4764 | 11.0 (7.87–15.37) | 1.4 (1.24–1.67) | 1.3 (1.23–1.46) | 0.9 (0.86–0.99) |
| 7F | 117 | 2815 | 2804 | 3575 | 4018 | 22.0 (15.42–31.42) | 1.1 (0.97–1.16) | 1.3 (1.14–1.40) | 1.1 (1.02–1.19) |
| 9V | 100 | 1746 | 2150 | 3026 | 3228 | 17.2 (11.87–25.04) | 1.3 (1.12–1.42) | 1.4 (1.24–1.60) | 1.1 (0.97–1.21) |
| 14 | 194 | 2141 | 2283 | 2357 | 2813 | 10.6 (7.76–14.44) | 1.0 (0.94–1.12) | 1.1 (1.00–1.18) | 1.2 (1.08–1.23) |
| 18C | 29 | 1699 | 2412 | 3026 | 3372 | 51.6 (34.27–77.78) | 1.3 (1.10–1.48) | 1.3 (1.13–1.42) | 1.1 (1.03–1.27) |
| 19A | 25 | 449 | 574 | 795 | 942 | 17.4 (13.51–22.38) | 1.3 (1.20–1.48) | 1.4 (1.27–1.54) | 1.2 (1.10–1.27) |
| 19F | 12 | 304 | 492 | 832 | 1194 | 24.6 (17.69–34.25) | 1.5 (1.28–1.86) | 1.7 (1.48–2.06) | 1.4 (1.26–1.62) |
| 23F | 10 | 284 | 802 | 1229 | 1262 | 28.6 (20.34–40.10) | 2.8 (2.24–3.46) | 1.6 (1.40–1.85) | 1.0 (0.91–1.09) |
CI, confidence interval; GMFR, geometric mean-fold rise; GMT, geometric mean titre; OPA, opsonophagocytic activity; SAP, statistical analysis plan.
aSAP-specified timing for blood samples: just prior to vaccination and 1 month after each vaccination.
bGMTs were calculated using all individuals with available data for the specified blood draw.
cn, Number of individuals with valid and determinate assay results for the specified serotype at the given visit.
dGMFRs were calculated using all individuals with available data from both the specified blood draws.
eCIs are back transformations of a confidence interval based on the Student's t distribution for the mean logarithm of the mean-fold rise.
fn, Number of individuals with valid and determinate assay results for the specified serotype at both specified blood draws.
Safety
The safety population included 301 vaccinated individuals. Local reactions and systemic events within 14 days of vaccination of each PCV13 dose in individuals at least 6 years of age are summarized in Table 4. The majority of local reactions were mild to moderate in severity. Injection site pain was the most frequent local reaction. The mean duration of local reactions was similar after each of the doses and did not exceed 2.7 days. The rate of local reactions was similar among paediatric and adult individuals (see Table, Supplemental Digital Content 5). Generally, the percentage of individuals experiencing systemic events (mostly mild or moderate) decreased with each subsequent dose. The most frequently occurring systemic events were muscle pain, fatigue and headache. The mean durations of systemic events were numerically similar after each of the three doses and did not exceed 5.1 days. Fever at least 40°C was reported in only one individual (after PCV13 dose 3). The frequency of systemic reactions was similar for paediatric and adult individuals (see Table, Supplemental Digital Content 5).
Table 4.
Percentage of individuals reporting local reactions and systemic events within 14 days of each PCV13 dose – safety population at least 6 years of age.
| Dose 1 | Dose 2 | Dose 3 | |
| %a | %a | %a | |
| Local reactions | |||
| Redness | |||
| Any (N = 154–187) | 11.7 | 8.6 | 4.5 |
| Severeb (N = 150–178) | 0.0 | 0.6 | 0.0 |
| Swelling | |||
| Any (N = 160–191) | 19.9 | 17.3 | 11.9 |
| Severeb (N = 150–178) | 0.6 | 0.0 | 0.0 |
| Pain at the injection site | |||
| Any (N = 213–238) | 65.5 | 70.3 | 61.5 |
| Severec (N = 156–179) | 5.1 | 4.5 | 5.1 |
| Most common systemic events | |||
| Fever | |||
| ≥38°C (N = 155–188) | 13.5 | 13.3 | 9.0 |
| >40°C (N = 150–178) | 0 | 0 | 0.7 |
| Fatigue | |||
| Any (N = 183–230) | 53.5 | 40.8 | 36.1 |
| Severed (N = 156–183) | 9.0 | 6.6 | 6.4 |
| Headache | |||
| Any (N = 177–220) | 50.5 | 39.1 | 32.2 |
| Severed (N = 154–182) | 8.0 | 2.7 | 5.2 |
| Vomiting | |||
| Any (N = 159–184) | 12.3 | 9.8 | 8.8 |
| Severee (N = 150–176) | 0 | 0 | 0 |
| Diarrhoea | |||
| Any (N = 162–199) | 30.2 | 18.4 | 16.7 |
| Severef (N = 154–179) | 1.2 | 2.8 | 2.6 |
| Muscle pain | |||
| Any (N = 191–226) | 55.8 | 44.9 | 42.4 |
| Severed (N = 156–180) | 5.6 | 3.9 | 7.1 |
| Joint pain | |||
| Any (N = 174–205) | 38.5 | 30.3 | 28.2 |
| Severed (N = 152–179) | 4.6 | 3.4 | 2.6 |
PCV13, 13-valent pneumococcal vaccine.
aNumerator is the number of individuals reporting the given characteristic; denominator (N) is the number of individuals with known values (individuals reporting a reaction on ≥1 day, or who reported no reaction for all 14 days, in the eDiary).
bFor ages 6 to <12 years, severe is >7.0 cm. For ages ≥12 years, severe is >10.0 cm.
cSevere prevents daily activity.
dSevere prevents routine daily activity.
eSevere requires intravenous hydration.
fSevere 6 or more loose stools in 24 h.
Among individuals at least 6 years of age, the most frequently occurring types (system organ class) of adverse events after any PCV13 dose were infections and infestations (15.9% of individuals; 6–17 years, 18.0%; ≥18 years, 13.9%). The most frequently reported individual adverse events after any PCV13 dose were influenza, upper respiratory tract infection and cough. Infections and infestations were also the most frequently occurring types (system organ class) of adverse events among individuals at least 6 years of age after any investigational vaccine dose (PCV13 and PPSV23; 17.9% of individuals; 6–17 years, 19.3%; ≥18 years, 16.6%), and at the 6-month follow-up contact after the PPSV23 dose (0.7% of individuals for each age group). Eight individuals reported nine SAEs during the study, with similar incidence among adult and paediatric individuals; the most common were infections and infestations, experienced by three individuals; none of the SAEs were considered related to PCV13 as assessed by the investigator. One death (traffic accident) occurred during the study, which was deemed by the investigator as not related to PCV13.
Discussion
The conjugation of polysaccharides to carrier proteins enables the elicitation of antipolysaccharide T-cell dependent immune responses [24]. This is of particular importance to immunocompromised individuals such as those infected with HIV. Pneumococcal conjugate vaccines, including PCV7 and the nine-valent PCV, are immunogenic [16–18,25] and have been demonstrated to reduce the risk of pneumococcal disease in HIV-infected individuals [17,18]. Vaccination with PCV13 provides wider coverage against commonly occurring serotypes than the earlier PCVs. As observed in healthy individuals [26,27], recent studies in HIV-infected individuals suggest that vaccination with PPSV23 may blunt immune responses to subsequent pneumococcal vaccine administration [28,29]. Pneumococcal conjugate vaccines have demonstrated efficacy in HIV-infected adults [17,18], whereas pneumococcal polysaccharide vaccines have failed to do so [9]. To avoid the potential risk for impaired responses by prior PPSV23 vaccination, the current study enrolled HIV-infected individuals naive to any prior pneumococcal vaccination and was conducted to describe the safety and immunogenicity of a three-dose series of PCV13 followed by one dose of PPSV23 administered 1 month apart.
There were significant increases in serotype-specific IgG GMCs and OPA GMTs after dose 1 of PCV13 compared with before the first vaccination. Further increases occurred after doses 2 and 3 of PCV13, but these were more modest compared with those seen after dose 1. Serotype-specific GMFRs after dose 1 compared with before dose 1 were greater than the GMFRs after dose 2 than after dose 1, and after dose 3 than after dose 2. Although IgG GMFRs and OPA GMFRs tended to be somewhat higher in the younger age group, immune responses were generally similar for paediatric and adult individuals.
Local reactions, systemic events and adverse events were generally mild, did not increase with the number of PCV13 doses administered and did not largely differ between the age groups.
Previous studies involving multidose pneumococcal vaccine regimens in HIV-infected older children and adults have not consistently demonstrated enhanced antipneumococcal responses compared with a single dose [16,28,30–34]. Some studies suggest that enhancement of the antipneumococcal response does occur following a second PCV injection [28,32], although this was not always seen [16]. This may be due, at least in part, to variability in the study size, immune and disease status of study participants (CD4+ cell counts and viral load), receipt and type of HAART, pneumococcal immunization history and the particular endpoint(s) assessed.
Similar to the current study in PPSV23-naive, HIV-infected adults, a recent study evaluated three doses of PCV13 administered at 6-month intervals to HIV-infected adults previously vaccinated with PPSV23. IgG GMCs and OPA GMTs increased for all serotypes after the initial PCV13 dose with modest further increases after the second and third PCV13 doses [35]. Thus, second and third vaccine doses may provide little advantage for increasing circulating antipneumococcal antibody in this population. These data are consistent with findings from earlier studies, which also indicated that pneumococcal conjugate vaccines induce functional antibody and establish immunologic memory [36] with a single vaccine dose [37].
The immunocompromised status of HIV-infected individuals may affect functional responses to some vaccines [22,38]. In the current study, even after the third PCV13 dose, the OPA GMT levels in the HIV-infected, PPSV23-naive study population 6−17 years of age were lower than after a single dose of PCV13 in healthy children of similar age regardless of prior PCV7 vaccination [39]. Among individuals aged at least 18 years, responses were lower than those in healthy adults up to age 60 years and similar to those elicited in healthy adults 60−64 years of age after PCV13 vaccination [27].
PPSV23 is recommended in many countries for HIV-infected individuals; therefore, study participants received one dose of PPSV23 1 month after the third PCV13 dose. Immune responses after the single dose of PPSV23 showed that responses were generally maintained compared with responses after the last dose of PCV13. As there was no control group receiving PPSV23 alone, it is not known whether the prior PCV13 doses enhanced the response to serotypes common to both vaccines.
In summary, PCV13 appeared well tolerated and immunogenic in pneumococcal vaccine-naive, HIV-infected individuals at least 6 years of age. A single dose of PCV13 elicited significant increases in IgG and OPA antibody responses from before to 1 month after vaccination, although two subsequent PCV13 doses given at 1-month intervals resulted in only moderate further increases of antibody levels.
On the basis of the potential benefit of PCV13, several countries have implemented recommendations for one dose of PCV13 vaccine in the vaccination regimen of HIV-infected adults [7,40]. The safety and immunogenicity data from this study support PCV13 vaccination and current ACIP recommendations in this population.
Acknowledgements
Medical writing support was provided by Daniel E. McCallus, PhD, at CHC Inc. and was funded by Pfizer Inc. The authors would like to thank Drs Anthonet Koen and Lisa Jose of the Respiratory and Meningeal Pathogens Research Unit; Vaccine Preventable Diseases, University of Witwatersrand, South Africa, for assisting in data collection and subject management.
F.L., A.G., D.A.S., E.A.E., W.C.G. and B.S.-T. were involved in study conception or design; A.B., S.A.M., A.G., K.U.J. and D.A.S. were involved in acquisition of data; V.S. and D.A.S. were involved in statistical analysis; S.A.M., F.L., V.S., A.G., K.U.J., D.A.S., W.C.G. and B.S.-T. were involved in analysis and interpretation of data; all authors participated in revising the manuscript critically for important intellectual content and approved the final version for publication.
This work was supported by Pfizer Inc. (ClinicalTrials.gov identifier NCT00962780).
These results were previously presented at the 9th International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD); 9–13 March 2014; Hyderabad, India: Bhorat A, et al. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine in HIV-infected children and adults not previously immunized with pneumococcal vaccine.
Conflicts of interest
S.A.M. has received grant support from Pfizer and has received honoraria for serving on Pfizer speaker bureaus and advisory boards. F.L., A.G., K.U.J., D.A.S., E.A.E., W.C.G. and B.S.-T. are employees of Pfizer Inc and may hold stock or stock options. V.S. is an employee of a company contracted by Pfizer Inc. A.E.B. declares no conflict of interest.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (http://www.AIDSonline.com).
References
- 1.Bliss SJ, O’Brien KL, Janoff EN, Cotton MF, Musoke P, Coovadia H, Levine OS. The evidence for using conjugate vaccines to protect HIV-infected children against pneumococcal disease. Lancet Infect Dis 2008; 8:67–80. [DOI] [PubMed] [Google Scholar]
- 2.Nunes MC, von Gottberg A, de Gouveia L, Cohen C, Kuwanda L, Karstaedt AS, et al. Persistent high burden of invasive pneumococcal disease in South African HIV-infected adults in the era of an antiretroviral treatment program. PLoS One 2011; 6:e27929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nunes MC, von Gottberg A, de Gouveia L, Cohen C, Moore DP, Klugman KP, Madhi SA. The impact of antiretroviral treatment on the burden of invasive pneumococcal disease in South African children: a time series analysis. AIDS 2011; 25:453–462. [DOI] [PubMed] [Google Scholar]
- 4.Everett DB, Mukaka M, Denis B, Gordon SB, Carrol ED, van Oosterhout JJ, et al. Ten years of surveillance for invasive Streptococcus pneumoniae during the era of antiretroviral scale-up and cotrimoxazole prophylaxis in Malawi. PLoS One 2011; 6:e17765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heffernan RT, Barrett NL, Gallagher KM, Hadler JL, Harrison LH, Reingold AL, et al. Declining incidence of invasive Streptococcus pneumoniae infections among persons with AIDS in an era of highly active antiretroviral therapy, 1995-2000. J Infect Dis 2005; 191:2038–2045. [DOI] [PubMed] [Google Scholar]
- 6.Grau I, Pallares R, Tubau F, Schulze MH, Llopis F, Podzamczer D, et al. Epidemiologic changes in bacteremic pneumococcal disease in patients with human immunodeficiency virus in the era of highly active antiretroviral therapy. Arch Intern Med 2005; 165:1533–1540. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–819. [PubMed] [Google Scholar]
- 8.Eramova I, Matic S, Munz M. HIV/AIDS treatment and care. Clinical protocols for the WHO European region. Copenhagen, Denmark: World Health Organization Europe; 2007. [Google Scholar]
- 9.French N, Nakiyingi J, Carpenter LM, Lugada E, Watera C, Moi K, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet 2000; 355:2106–2111. [DOI] [PubMed] [Google Scholar]
- 10.Arpadi SM, Back S, O’Brien J, Janoff EN. Antibodies to pneumococcal capsular polysaccharides in children with human immunodeficiency virus infection given polyvalent pneumococcal vaccine. J Pediatr 1994; 125:77–79. [DOI] [PubMed] [Google Scholar]
- 11.Kroon FP, van Dissel JT, Ravensbergen E, Nibbering PH, van Furth R. Antibodies against pneumococcal polysaccharides after vaccination in HIV-infected individuals: 5-year follow-up of antibody concentrations. Vaccine 1999; 18:524–530. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Palomo C, Martin-Zamorano M, Benitez E, Fernandez-Gutierrez C, Guerrero F, Rodriguez-Iglesias M, Giron-Gonzalez JA. Pneumonia in HIV-infected patients in the HAART era: incidence, risk, and impact of the pneumococcal vaccination. J Med Virol 2004; 72:517–524. [DOI] [PubMed] [Google Scholar]
- 13.Teshale EH, Hanson D, Flannery B, Phares C, Wolfe M, Schuchat A, Sullivan P. Effectiveness of 23-valent polysaccharide pneumococcal vaccine on pneumonia in HIV-infected adults in the United States, 1998–2003. Vaccine 2008; 26:5830–5834. [DOI] [PubMed] [Google Scholar]
- 14.Nachman S, Kim S, King J, Abrams EJ, Margolis D, Petru A, et al. Safety and immunogenicity of a heptavalent pneumococcal conjugate vaccine in infants with human immunodeficiency virus type 1 infection. Pediatrics 2003; 112:66–73. [DOI] [PubMed] [Google Scholar]
- 15.Tarrago D, Casal J, Ruiz-Contreras J, Ramos JT, Rojo P, Snippe H, Jansen WT. Assessment of antibody response elicited by a 7-valent pneumococcal conjugate vaccine in pediatric human immunodeficiency virus infection. Clin Diagn Lab Immunol 2005; 12:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feikin DR, Elie CM, Goetz MB, Lennox JL, Carlone GM, Romero-Steiner S, et al. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine 2001; 20:545–553. [DOI] [PubMed] [Google Scholar]
- 17.French N, Gordon SB, Mwalukomo T, White SA, Mwafulirwa G, Longwe H, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med 2010; 362:812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med 2003; 349:1341–1348. [DOI] [PubMed] [Google Scholar]
- 19.Cornick JE, Everett DB, Broughton C, Denis BB, Banda DL, Carrol ED, Parry CM. Invasive Streptococcus pneumoniae in children, Malawi, 2004-2006. Emerg Infect Dis 2011; 17:1107–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Gottberg A, Cohen C, de Gouveia L, Meiring S, Quan V, Whitelaw A, et al. Epidemiology of invasive pneumococcal disease in the preconjugate vaccine era: South Africa, 2003–2008. Vaccine 2013; 31:4200–4208. [DOI] [PubMed] [Google Scholar]
- 21.de Vries-Sluijs TE, Hansen BE, van Doornum GJ, Springeling T, Evertsz NM, de Man RA, van der Ende ME. A prospective open study of the efficacy of high-dose recombinant hepatitis B rechallenge vaccination in HIV-infected patients. J Infect Dis 2008; 197:292–294. [DOI] [PubMed] [Google Scholar]
- 22.Powis JE, Raboud J, Ostrowski M, Loutfy MR, Kovacs C, Walmsley SL. The recombinant hepatitis B surface antigen vaccine in persons with HIV: is seroconversion sufficient for long-term protection?. J Infect Dis 2012; 205:1534–1538. [DOI] [PubMed] [Google Scholar]
- 23.Cooper D, Yu X, Sidhu M, Nahm MH, Fernsten P, Jansen KU. The 13-valent pneumococcal conjugate vaccine (PCV13) elicits cross-functional opsonophagocytic killing responses in humans to Streptococcus pneumoniae serotypes 6C and 7A. Vaccine 2011; 29:7207–7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rabian C, Tschope I, Lesprit P, Katlama C, Molina JM, Meynard JL, et al. Cellular CD4 T cell responses to the diphtheria-derived carrier protein of conjugated pneumococcal vaccine and antibody response to pneumococcal vaccination in HIV-infected adults. Clin Infect Dis 2010; 50:1174–1183. [DOI] [PubMed] [Google Scholar]
- 25.Lesprit P, Pedrono G, Molina JM, Goujard C, Girard PM, Sarrazin N, et al. Immunological efficacy of a prime-boost pneumococcal vaccination in HIV-infected adults. AIDS 2007; 21:2425–2434. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg RN, Gurtman A, Frenck RW, Strout C, Jansen KU, Trammel J, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults 60-64 years of age. Vaccine 2014; 32:2364–2374. [DOI] [PubMed] [Google Scholar]
- 27.Jackson LA, Gurtman A, van Cleeff M, Frenck RW, Treanor J, Jansen KU, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on antipneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine 2013; 31:3594–3602. [DOI] [PubMed] [Google Scholar]
- 28.Lu CL, Chang SY, Chuang YC, Liu WC, Su CT, Su YC, et al. Revaccination with 7-valent pneumococcal conjugate vaccine elicits better serologic response than 23-valent pneumococcal polysaccharide vaccine in HIV-infected adult patients who have undergone primary vaccination with 23-valent pneumococcal polysaccharide vaccine in the era of combination antiretroviral therapy. Vaccine 2014; 32:1031–1035. [DOI] [PubMed] [Google Scholar]
- 29.Tasker SA, Wallace MR, Rubins JB, Paxton WB, O’Brien J, Janoff EN. Reimmunization with 23-valent pneumococcal vaccine for patients infected with human immunodeficiency virus type 1: clinical, immunologic, and virologic responses. Clin Infect Dis 2002; 34:813–821. [DOI] [PubMed] [Google Scholar]
- 30.Chen M, Ssali F, Mulungi M, Awio P, Yoshimine H, Kuroki R, et al. Induction of opsonophagocytic killing activity with pneumococcal conjugate vaccine in human immunodeficiency virus-infected Ugandan adults. Vaccine 2008; 26:4962–4968. [DOI] [PubMed] [Google Scholar]
- 31.Crum-Cianflone NF, Huppler Hullsiek K, Roediger M, Ganesan A, Patel S, Landrum ML, et al. A randomized clinical trial comparing revaccination with pneumococcal conjugate vaccine to polysaccharide vaccine among HIV-infected adults. J Infect Dis 2010; 202:1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miiro G, Kayhty H, Watera C, Tolmie H, Whitworth JA, Gilks CF, French N. Conjugate pneumococcal vaccine in HIV-infected Ugandans and the effect of past receipt of polysaccharide vaccine. J Infect Dis 2005; 192:1801–1805. [DOI] [PubMed] [Google Scholar]
- 33.Nunes MC, Madhi SA. Safety, immunogenicity and efficacy of pneumococcal conjugate vaccine in HIV-infected individuals. Hum Vaccin Immunother 2012; 8:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sogaard OS, Lohse N, Harboe ZB, Offersen R, Bukh AR, Davis HL, et al. Improving the immunogenicity of pneumococcal conjugate vaccine in HIV-infected adults with a toll-like receptor 9 agonist adjuvant: a randomized, controlled trial. Clin Infect Dis 2010; 51:42–50. [DOI] [PubMed] [Google Scholar]
- 35.Glesby MJ, Watson W, Brinson C, Greenberg RN, Lalezari JP, Skiest D, et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in HIV-infected adults previously vaccinated with pneumococcal polysaccharide vaccine. J Infect Dis 2014; doi: 10.1093/infdis/jiu631. [DOI] [PubMed] [Google Scholar]
- 36.de Roux A, Schmole-Thoma B, Siber GR, Hackell JG, Kuhnke A, Ahlers N, et al. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin Infect Dis 2008; 46:1015–1023. [DOI] [PubMed] [Google Scholar]
- 37.Clutterbuck EA, Lazarus R, Yu LM, Bowman J, Bateman EA, Diggle L, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis 2012; 205:1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nair N, Moss WJ, Scott S, Mugala N, Ndhlovu ZM, Lilo K, et al. HIV-1 infection in Zambian children impairs the development and avidity maturation of measles virus-specific immunoglobulin G after vaccination and infection. J Infect Dis 2009; 200:1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frenck R, Jr, Thompson A, Senders S, Harris-Ford L, Sperling M, Patterson S, et al. 13-Valent pneumococcal conjugate vaccine in older children and adolescents either previously immunized with or naive to 7-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2014; 33:183–189. [DOI] [PubMed] [Google Scholar]
- 40.National Health and Medical Research Council. The Australian Immunisation Handbook 10th edition 2013. Commonwealth of Australia. Canberra: (updated January 2014). 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


