Abstract
Spemann's organizer has potent neural inducing and mesoderm dorsalizing activities in the Xenopus gastrula. A third activity, the organizer's ability to induce a secondary gut, has been difficult to analyze experimentally due to the lack of early gene markers. Here we introduce endodermin, a pan-endodermal gene marker, and use it to demonstrate that chordin (Chd), a protein secreted by the organizer region, is able to induce endodermal differentiation in Xenopus. The ability of chd, as well as that of noggin, to induce endoderm in animal cap explants is repressed by the ventralizing factor BMP-4. When FGF signaling is blocked by a dominant-negative FGF receptor in chd-injected animal caps, neural induction is inhibited and most of the explant is induced to become endoderm. The results suggest that proteins secreted by the organizer, acting together with known peptide growth factors, regulate differentiation of the endodermal germ layer.
Full text
PDF
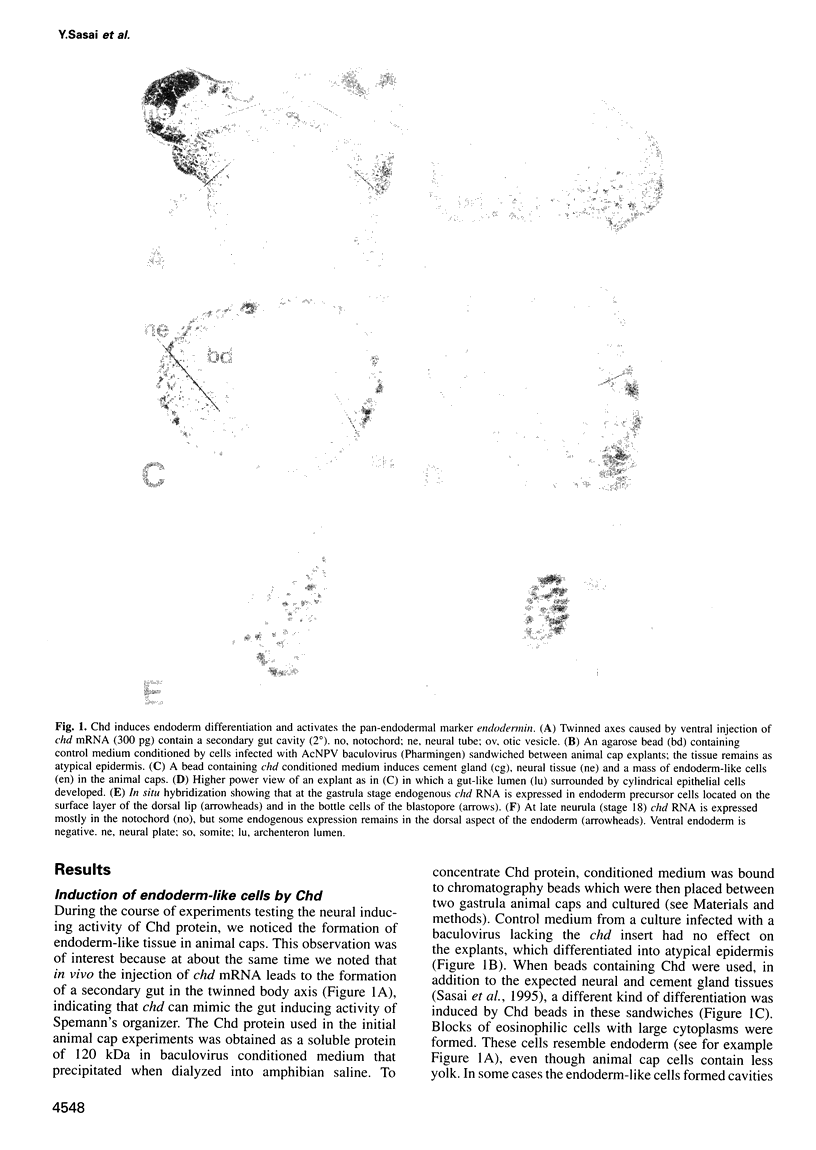




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaya E., Musci T. J., Kirschner M. W. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991 Jul 26;66(2):257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Asashima M., Uchiyama H., Nakano H., Eto Y., Ejima D., Sugino H., Davids M., Plessow S., Born J., Hoppe P. The vegetalizing factor from chicken embryos: its EDF (activin A)-like activity. Mech Dev. 1991 Jun;34(2-3):135–141. doi: 10.1016/0925-4773(91)90050-g. [DOI] [PubMed] [Google Scholar]
- Cho K. W., Blumberg B., Steinbeisser H., De Robertis E. M. Molecular nature of Spemann's organizer: the role of the Xenopus homeobox gene goosecoid. Cell. 1991 Dec 20;67(6):1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J., Smith J. C., Smith E. J., Yaqoob M. The organization of mesodermal pattern in Xenopus laevis: experiments using a Xenopus mesoderm-inducing factor. Development. 1987 Dec;101(4):893–908. doi: 10.1242/dev.101.4.893. [DOI] [PubMed] [Google Scholar]
- Cornell R. A., Kimelman D. Activin-mediated mesoderm induction requires FGF. Development. 1994 Feb;120(2):453–462. doi: 10.1242/dev.120.2.453. [DOI] [PubMed] [Google Scholar]
- Cox W. G., Hemmati-Brivanlou A. Caudalization of neural fate by tissue recombination and bFGF. Development. 1995 Dec;121(12):4349–4358. doi: 10.1242/dev.121.12.4349. [DOI] [PubMed] [Google Scholar]
- De Robertis E. M., Sasai Y. A common plan for dorsoventral patterning in Bilateria. Nature. 1996 Mar 7;380(6569):37–40. doi: 10.1038/380037a0. [DOI] [PubMed] [Google Scholar]
- Dirksen M. L., Jamrich M. A novel, activin-inducible, blastopore lip-specific gene of Xenopus laevis contains a fork head DNA-binding domain. Genes Dev. 1992 Apr;6(4):599–608. doi: 10.1101/gad.6.4.599. [DOI] [PubMed] [Google Scholar]
- Fainsod A., Steinbeisser H., De Robertis E. M. On the function of BMP-4 in patterning the marginal zone of the Xenopus embryo. EMBO J. 1994 Nov 1;13(21):5015–5025. doi: 10.1002/j.1460-2075.1994.tb06830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson E. L., Anderson K. V. Decapentaplegic acts as a morphogen to organize dorsal-ventral pattern in the Drosophila embryo. Cell. 1992 Oct 30;71(3):451–461. doi: 10.1016/0092-8674(92)90514-d. [DOI] [PubMed] [Google Scholar]
- Ferguson E. L., Anderson K. V. Localized enhancement and repression of the activity of the TGF-beta family member, decapentaplegic, is necessary for dorsal-ventral pattern formation in the Drosophila embryo. Development. 1992 Mar;114(3):583–597. doi: 10.1242/dev.114.3.583. [DOI] [PubMed] [Google Scholar]
- Francois V., Solloway M., O'Neill J. W., Emery J., Bier E. Dorsal-ventral patterning of the Drosophila embryo depends on a putative negative growth factor encoded by the short gastrulation gene. Genes Dev. 1994 Nov 1;8(21):2602–2616. doi: 10.1101/gad.8.21.2602. [DOI] [PubMed] [Google Scholar]
- Gamer L. W., Wright C. V. Autonomous endodermal determination in Xenopus: regulation of expression of the pancreatic gene XlHbox 8. Dev Biol. 1995 Sep;171(1):240–251. doi: 10.1006/dbio.1995.1275. [DOI] [PubMed] [Google Scholar]
- Gont L. K., Steinbeisser H., Blumberg B., de Robertis E. M. Tail formation as a continuation of gastrulation: the multiple cell populations of the Xenopus tailbud derive from the late blastopore lip. Development. 1993 Dec;119(4):991–1004. doi: 10.1242/dev.119.4.991. [DOI] [PubMed] [Google Scholar]
- Graff J. M., Thies R. S., Song J. J., Celeste A. J., Melton D. A. Studies with a Xenopus BMP receptor suggest that ventral mesoderm-inducing signals override dorsal signals in vivo. Cell. 1994 Oct 7;79(1):169–179. doi: 10.1016/0092-8674(94)90409-x. [DOI] [PubMed] [Google Scholar]
- Green J. B., Howes G., Symes K., Cooke J., Smith J. C. The biological effects of XTC-MIF: quantitative comparison with Xenopus bFGF. Development. 1990 Jan;108(1):173–183. doi: 10.1242/dev.108.1.173. [DOI] [PubMed] [Google Scholar]
- Harland R. M. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A., Kelly O. G., Melton D. A. Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell. 1994 Apr 22;77(2):283–295. doi: 10.1016/0092-8674(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Henry G. L., Brivanlou I. H., Kessler D. S., Hemmati-Brivanlou A., Melton D. A. TGF-beta signals and a pattern in Xenopus laevis endodermal development. Development. 1996 Mar;122(3):1007–1015. doi: 10.1242/dev.122.3.1007. [DOI] [PubMed] [Google Scholar]
- Holley S. A., Jackson P. D., Sasai Y., Lu B., De Robertis E. M., Hoffmann F. M., Ferguson E. L. A conserved system for dorsal-ventral patterning in insects and vertebrates involving sog and chordin. Nature. 1995 Jul 20;376(6537):249–253. doi: 10.1038/376249a0. [DOI] [PubMed] [Google Scholar]
- Keller R. E. Vital dye mapping of the gastrula and neurula of Xenopus laevis. I. Prospective areas and morphogenetic movements of the superficial layer. Dev Biol. 1975 Feb;42(2):222–241. doi: 10.1016/0012-1606(75)90331-0. [DOI] [PubMed] [Google Scholar]
- Kengaku M., Okamoto H. Basic fibroblast growth factor induces differentiation of neural tube and neural crest lineages of cultured ectoderm cells from Xenopus gastrula. Development. 1993 Dec;119(4):1067–1078. doi: 10.1242/dev.119.4.1067. [DOI] [PubMed] [Google Scholar]
- Kengaku M., Okamoto H. bFGF as a possible morphogen for the anteroposterior axis of the central nervous system in Xenopus. Development. 1995 Sep;121(9):3121–3130. doi: 10.1242/dev.121.9.3121. [DOI] [PubMed] [Google Scholar]
- LaBonne C., Whitman M. Mesoderm induction by activin requires FGF-mediated intracellular signals. Development. 1994 Feb;120(2):463–472. doi: 10.1242/dev.120.2.463. [DOI] [PubMed] [Google Scholar]
- Lamb T. M., Harland R. M. Fibroblast growth factor is a direct neural inducer, which combined with noggin generates anterior-posterior neural pattern. Development. 1995 Nov;121(11):3627–3636. doi: 10.1242/dev.121.11.3627. [DOI] [PubMed] [Google Scholar]
- Lamb T. M., Knecht A. K., Smith W. C., Stachel S. E., Economides A. N., Stahl N., Yancopolous G. D., Harland R. M. Neural induction by the secreted polypeptide noggin. Science. 1993 Oct 29;262(5134):713–718. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- Launay C., Fromentoux V., Shi D. L., Boucaut J. C. A truncated FGF receptor blocks neural induction by endogenous Xenopus inducers. Development. 1996 Mar;122(3):869–880. doi: 10.1242/dev.122.3.869. [DOI] [PubMed] [Google Scholar]
- Levin M., Johnson R. L., Stern C. D., Kuehn M., Tabin C. A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell. 1995 Sep 8;82(5):803–814. doi: 10.1016/0092-8674(95)90477-8. [DOI] [PubMed] [Google Scholar]
- Niehrs C., Steinbeisser H., De Robertis E. M. Mesodermal patterning by a gradient of the vertebrate homeobox gene goosecoid. Science. 1994 Feb 11;263(5148):817–820. doi: 10.1126/science.7905664. [DOI] [PubMed] [Google Scholar]
- Padgett R. W., Wozney J. M., Gelbart W. M. Human BMP sequences can confer normal dorsal-ventral patterning in the Drosophila embryo. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2905–2909. doi: 10.1073/pnas.90.7.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Cox C., Jessell T. M., Klar A. Ectopic neural expression of a floor plate marker in frog embryos injected with the midline transcription factor Pintallavis. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8268–8272. doi: 10.1073/pnas.90.17.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H., De Robertis E. M. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature. 1995 Jul 27;376(6538):333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H., Geissert D., Gont L. K., De Robertis E. M. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994 Dec 2;79(5):779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. E., Suzuki A., Ueno N., Kimelman D. Localized BMP-4 mediates dorsal/ventral patterning in the early Xenopus embryo. Dev Biol. 1995 May;169(1):37–50. doi: 10.1006/dbio.1995.1124. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S., Smith J. C. Mesoderm formation in response to Brachyury requires FGF signalling. Curr Biol. 1995 Jan 1;5(1):62–67. doi: 10.1016/s0960-9822(95)00017-0. [DOI] [PubMed] [Google Scholar]
- Shih J., Keller R. The epithelium of the dorsal marginal zone of Xenopus has organizer properties. Development. 1992 Dec;116(4):887–899. doi: 10.1242/dev.116.4.887. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Harland R. M. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992 Sep 4;70(5):829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Steinbeisser H., Fainsod A., Niehrs C., Sasai Y., De Robertis E. M. The role of gsc and BMP-4 in dorsal-ventral patterning of the marginal zone in Xenopus: a loss-of-function study using antisense RNA. EMBO J. 1995 Nov 1;14(21):5230–5243. doi: 10.1002/j.1460-2075.1995.tb00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M. W., Suzuki H. R., Bieker J. J., Solursh M., Ramirez F. Expression of two nonallelic type II procollagen genes during Xenopus laevis embryogenesis is characterized by stage-specific production of alternatively spliced transcripts. J Cell Biol. 1991 Oct;115(2):565–575. doi: 10.1083/jcb.115.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Thies R. S., Yamaji N., Song J. J., Wozney J. M., Murakami K., Ueno N. A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10255–10259. doi: 10.1073/pnas.91.22.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira M., Jamrich M., Good P. J., Dawid I. B. The LIM domain-containing homeo box gene Xlim-1 is expressed specifically in the organizer region of Xenopus gastrula embryos. Genes Dev. 1992 Mar;6(3):356–366. doi: 10.1101/gad.6.3.356. [DOI] [PubMed] [Google Scholar]
- Thomsen G. H., Melton D. A. Processed Vg1 protein is an axial mesoderm inducer in Xenopus. Cell. 1993 Aug 13;74(3):433–441. doi: 10.1016/0092-8674(93)80045-g. [DOI] [PubMed] [Google Scholar]
- Tiedemann H., Lottspeich F., Davids M., Knöchel S., Hoppe P., Tiedemann H. The vegetalizing factor. A member of the evolutionarily highly conserved activin family. FEBS Lett. 1992 Mar 30;300(2):123–126. doi: 10.1016/0014-5793(92)80178-j. [DOI] [PubMed] [Google Scholar]
- Vaughan J. M., Vale W. W. Alpha 2-macroglobulin is a binding protein of inhibin and activin. Endocrinology. 1993 May;132(5):2038–2050. doi: 10.1210/endo.132.5.7682939. [DOI] [PubMed] [Google Scholar]
- Webb D. J., Atkins T. L., Crookston K. P., Burmester J. K., Qian S. W., Gonias S. L. Transforming growth factor beta isoform 2-specific high affinity binding to native alpha 2-macroglobulin. Chimeras identify a sequence that determines affinity for native but not activated alpha 2-macroglobulin. J Biol Chem. 1994 Dec 2;269(48):30402–30406. [PubMed] [Google Scholar]
- Wharton K. A., Ray R. P., Gelbart W. M. An activity gradient of decapentaplegic is necessary for the specification of dorsal pattern elements in the Drosophila embryo. Development. 1993 Feb;117(2):807–822. doi: 10.1242/dev.117.2.807. [DOI] [PubMed] [Google Scholar]
- Wilson P. A., Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 1995 Jul 27;376(6538):331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- Xu R. H., Kim J., Taira M., Zhan S., Sredni D., Kung H. F. A dominant negative bone morphogenetic protein 4 receptor causes neuralization in Xenopus ectoderm. Biochem Biophys Res Commun. 1995 Jul 6;212(1):212–219. doi: 10.1006/bbrc.1995.1958. [DOI] [PubMed] [Google Scholar]
- Zusman S. B., Sweeton D., Wieschaus E. F. short gastrulation, a mutation causing delays in stage-specific cell shape changes during gastrulation in Drosophila melanogaster. Dev Biol. 1988 Oct;129(2):417–427. doi: 10.1016/0012-1606(88)90389-2. [DOI] [PubMed] [Google Scholar]
- von Dassow G., Schmidt J. E., Kimelman D. Induction of the Xenopus organizer: expression and regulation of Xnot, a novel FGF and activin-regulated homeo box gene. Genes Dev. 1993 Mar;7(3):355–366. doi: 10.1101/gad.7.3.355. [DOI] [PubMed] [Google Scholar]