Abstract
Background and Purpose
Epoxyeicosatrienoic acids (EETs) are arachidonic acid metabolites that play a protective role against damaging processes that may occur after re-oxygenation of the graft. We aimed to investigate whether the presence of functional polymorphisms in the gene encoding soluble epoxy hydrolase (EPHX2), which metabolizes EETs to less active compounds, may play a role in the outcome of renal transplantation.
Methods
In a group of 259 Caucasian renal transplant recipients and 183 deceased donors, we determined the presence of three common EPHX2 SNPs, namely rs41507953 (K55R), rs751141 (R287Q) and rs1042032 A/G. Associations with parameters of graft function and the incidence of acute rejection were retrospectively investigated throughout the first year after grafting by logistic regression adjusting for clinical and demographic variables.
Results
Carriers of the rs1042032 GG genotype displayed significantly lower estimated glomerular filtration rate (eGFR) (38.15 ± 15.57 vs. 45.99 ± 16.05; p = 0.04) and higher serum creatinine values (1.57 ± 0.58 vs. 1.30 ± 0.47 g/dL; p=0.02) one year after grafting, compared to patients carrying the wildtype A-allele. The same GG genotype was also associated to increased risk of acute rejection. Interestingly, this association was observed for the genotype of both recipients [OR =6.34 (1.35-29.90); p = 0.015] and donors [OR = 5.53 (1.10-27.80); p=0.042]. A statistical model including both genotypes along with other meaningful demographic and clinical variables resulted in an increased significance for the association with the recipients’ genotype [OR=8.28 (1.21-74.27); p=0.031].
Conclusions
Our results suggest that genetic variability in the EETs-metabolizing gene, EPHX2, may have a significant impact on the outcome of deceased-donor renal transplantation.
Introduction
The arachidonic acid (AA) is metabolized by cytochrome P450 (CYP) enzymes to a number of compounds with important biological functions. The epoxygenase branch of this pathway leads to the synthesis of epoxyeicosatrienoic acids (EETs), which are considered to be endothelium-derived hyperpolarizing factors that regulate the intracellular transport of electrolytes and maintain vascular smooth muscle tone with vasodilator and anti-inflammatory properties [1–3]. In turn, EETs are metabolized to less active dihydroxyeicosatrienoic acids (DHETs) by soluble epoxide hydrolase (sEH) [4].
There presently is an increasing body of evidence suggesting that these EETs may have a significant function in organ transplantation. Thus, these compounds have been shown to play a protective role in the kidney [5,6], particularly against damaging processes that may occur after re-oxygenation of the graft [7–9]. Indeed, increasing the levels of EETs, via sEH inhibition, has been proposed as a therapeutic strategy in renal diseases [2].
The EET-metabolizing enzyme sEH is encoded by the EPHX2 gene, which is expressed in the kidney and presents single nucleotide polymorphisms (SNPs) that have been associated with altered enzyme activity [10,11]. Previous disease-association studies have shown that certain genetic variations in EPHX2 are related to the risk of coronary heart disease and ischemic stroke [12,13]. In addition, one recent study in rodents has reported that sEH activity determines the severity of ischemia-reperfusion injury in the kidney [14]. To our knowledge, only two studies by the same research group have analyzed the impact of EPHX2 genetic variability on kidney disease and transplantation. The authors showed that some of these variants can affect the progression of human IgA nephropathy and may be predictive of allograft dysfunction, but no assessment was made on their impact on acute rejection [15,16].
With this background, we hypothesize that the presence of polymorphisms with an established functional and/or clinical relevance in the EPHX2 gene, namely rs41507953 (K55R), rs751141 (R287Q) and rs1042032 in the 3’ untranslated region (UTR), may play a role in the outcome of renal transplantation. To test this hypothesis, we retrospectively analyzed these variants in a population of renal transplant recipients and donors and searched for associations with graft dysfunction and acute rejection episodes (ARE).
Subjects and Methods
From an initial number of 354 clinical records reviewed, a total of 259 adult renal transplant recipients were included in the final study sample (patients with incomplete records of clinical parameters, demographic characteristics or those with failed genotyping were ruled out from the study). Part of these patients had already been studied in previous works by our group [17–19]. The patients were all of Caucasian origin and received a single kidney at two Spanish centers, the Infanta Cristina Hospital in Badajoz and the Hospital Universitario Central de Asturias in Oviedo. All transplants were carried out with deceased donors from whom genetic material was available in 183 cases.
After the transplant, a triple immunosuppressive therapy was implemented with mycophenolate mofetil (2 g/day), a tapering schedule of corticoids (500 mg IV methylprednisolone at the time of surgery, 125 mg intravenously (IV) the following day and then 20 mg of oral prednisone daily, progressively tapered to 5 mg daily at 2 months after transplantation) and either cyclosporine or tacrolimus. Tacrolimus starting dose was set to 0.1 mg/kg administered twice a day. Initial dosage of Cyclosporine was 4–10 mg/kg/day divided into two administrations. The first dose was administered orally shortly before transplantation or IV in the perioperative period when the patient's condition did not support the enteral route. The amount of drug administered IV was one third of the oral dose. Further doses of immunosupressants were subsequently adjusted according to blood concentrations. Tacrolimus and cyclosporine blood concentrations were routinely measured using an immunoassay performed on a Cobas Mira Plus analyzer (Roche Diagnostics).
Acute allograft rejection was established by histological findings in renal biopsies according to the Banff classification and/or by clinical evaluation as previously described [18,20]. Delayed graft function (DGF) was defined as the need for dialysis within the first week after transplantation. DGF and ARE data were retrospectively retrieved from clinical records up until the first year after grafting. To estimate death-censored allograft survival, patients were followed up until the earliest of graft loss (defined as the absence of kidney function, occurring any time after transplantation due to irreversible graft injury requiring chronic dialysis and/or re-transplantation), death with a functioning graft or December 31, 2013.
Renal function was assessed by estimating the glomerular filtration rate (eGFR) from serum creatinine using the Modification of Diet in Renal Disease (MDRD) formula [21]: eGFR (ml min-1 1.73 m-2) = 186 x (Serum Creatinin-1.154 x Age-0.203) x (1.212 if Black) x (0.742 if Female). Several studies have reported that among renal transplanted patients the MDRD values show a better correlation with the true filtration rate in comparison with other renal function estimates, e.g. raw serum creatinine or the Cockcroft-Gault formula [22].
Ethics Statement
All participants gave oral and written consent for their participation. The study was approved by the Ethics Committee of the Infanta Cristina Hospital (Reference No. 18002657), and was conducted in accordance with the Declaration of Helsinki and its subsequent revisions.
Genotype analysis
Genomic DNA was isolated by using a QIAamp DNA Blood Kit (Qiagen, Hilden, Germany) from either whole blood samples, in the case of the recipients, or from previously frozen lymphocytes obtained from donors. All three EPHX2 SNPs (Table 1) were identified by real-time PCR using TaqMan SNP Genotype Assays from Life Technologies (Rockville, MD, USA).
Table 1. Location, consequence and context sequence of the three EPHX2 SNPs analyzed in this study, as provided by the manufacturer of the TaqMan SNP genotyping assays (Life Technologies, Maryland, USA).
| SNP | Transition | Aminoacid change | Location | Context sequence [VIC/FAM] |
|---|---|---|---|---|
| rs41507953 | A/G | K55R | Chr.8: 27358505 on NCBI Build 37 | GAGGGTGCCACTACCCGGCTTATGA[A/G]AGGAGAGATCACACTTTCCCAGGTG |
| rs751141 | A/G | R287Q | Chr.8: 27373865 on NCBI Build 37 | CCTGCTCTGGCCCAGGCAGGTTACC[A/G]GGTCCTAGCTATGGACATGAAAGGC |
| rs1042032 | A/G | None (3’-UTR) | Chr.8: 27402074 on NCBI Build 37 | TGTGCCCACGCTCAGCAGGTGTGCC[A/G]TCCTTCCACCTGCTGGGGCACCATT |
UTR, untranslated region.
For the haplotype study, the SNPstats platform [23] was utilized to provide linkage disequilibrium data and to estimate the effect of haplotypes on the risk for acute rejection by linear regression modelling; regression parameters pertained to the log odds ratios adjusted by the same clinical and demographic variables used in the single-SNP study (see below). Frequency threshold for rare haplotypes was set at 0.01.
Statistical analyses
Fisher’s exact or Pearson’s X2 test were used for the univariate analysis of the associations between categorical data (i.e. genotypes vs. clinical events). In order to compare quantitative variables (e.g. eGFR) between the different genotype groups, T-student or ANOVA tests were used depending on the number of groups considered. Multivariate regression analysis was performed to collectively assess the impact of both genetic and non-genetic parameters. Analyses were adjusted for demographic and clinical covariates according to statistical significance in univariate studies and/or clinical criteria described elsewhere [18,24]. Variables included were age of donors and recipients, type of immunosuppression, presence of DGF, cold ischemia time > 24 hours, number of HLA mismatches ≥ 3, and peak cytotoxic PRA ≥50%. The age of both donors and recipients was transformed into categorical variables using the median values as cut-off points (49 and 51 years for recipients and donors, respectively). Association of the three EPHX2 SNPs with death-censored allograft survival was analyzed by Cox regression analyses.
SNPstats was used to determine the adequate model of inheritance (additive, dominant or recessive). Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 15.0 for Windows (SPSS Inc., Chicago, Ill. USA). In all instances differences were considered to be significant when p values were lower than 0.05.
Results
Demographic and clinical characteristics of the 259 transplant recipients analyzed in the study are described in Table 2. The most common primary kidney disease was glomerulonephritis (42.1%), followed by chronic interstitial nephritis (11.2%) and polycystic kidney disease (8.7%). Several other conditions accounted for 14.2% of cases. In 23.8% of the patients the specific condition could not be determined. Induction therapy was implemented in 53 subjects (20.5%) with antibodies against interleukin-2 receptor (basiliximab) and in 9 patients (3.5%) with thymoglobulin.
Table 2. Clinical and demographic parameters of the study population.
Four time-points within a one-year follow-up were considered.
| Time-point | One week | One month | 5 months | One year | |
|---|---|---|---|---|---|
| Creatinine serum concentration (mg/dL) | 2.48 ± 2.07 | 1.69 ± 0.93 | 1.49 ± 0.72 | 1.38 ± 0.62 | |
| eGFR (ml min-1 1.73 m-2) | 33.29 ± 14.10 | 36.90 ± 13.73 | 41.60 ± 13.98 | 44.02 ± 14.83 | |
| Recipients on tacrolimus | 208 (80.3) | ||||
| Dose (mg/kg) | 0.14 ± 0.07 | 0.11 ± 0.06 | 0.08 ± 0.04 | 0.06 ± 0.06 | |
| Dose-normalized Tac blood concentration (ng/ml per mg/day per kg) | 111.0 ± 80.1 | 121.1 ± 66.7 | 162.5± 77.0 | 176.3 ± 110.1 | |
| Recipients on cyclosporine | 51 (19.7) | ||||
| Dose (mg/kg) | 8.0 ± 1.9 | 6.3 ± 1.6 | 4.5 ±1.7 | 3.5 ± 1.1 | |
| Dose-normalized CsA blood concentration (ng/ml per mg/day per kg) | 42.32 ± 20.13 | 53.91 ± 22.48 | 51.04 ± 17.96 | 50.16 ± 18.98 | |
| Recipients sex (male/female) | 158 (61.0) / 101 (39.0) | ||||
| Recipients age (years) | 48.18 ± 14.31 | ||||
| Type of dialysis (hemodialysis/peritoneal) | 174 (67.2)/85 (32.8) | ||||
| Duration of dialysis before transplantation (months) | 38.16 ± 30.09 | ||||
| Donor age (years) | 47.63 ± 17.51 | ||||
| Number of transplants (first/second/third) | 246 (95.0)/11 (4.2)/1 (0.8) | ||||
| Cold ischemia time (hours) | 16.22 ± 5.01 | ||||
| Peak cytotoxic PRA ≥ 50% | 20 (7.72) | ||||
| HLA mismatch | |||||
| 0–2 | 69 (26.64) | ||||
| 3–4 | 162 (62.54) | ||||
| 5–6 | 28 (10.81) |
Data are shown as number (percentage) or mean ± standard deviation.
Genotype and minor allele frequencies, both in donors and recipients, are shown in Table 3. Allele frequencies in the population of study were all in Hardy-Weinberg equilibrium (p-values > 0.05).
Table 3. Genotypic and allelic frequencies in donors and renal transplant recipients.
| Recipients | Donors | |||||
|---|---|---|---|---|---|---|
| Polymorphism | N | % | N | % | MAF | |
| EPHX2 K55R, rs41507953 | KK | 215 | 83.0 | 133 | 72.7 | 0.114 |
| KR | 40 | 15.4 | 47 | 25.7 | ||
| RR | 4 | 1.5 | 3 | 1.6 | ||
| EPHX2 R287Q, rs751141 | RR | 223 | 86.1 | 153 | 83.6 | 0.076 |
| RQ | 36 | 13.9 | 29 | 15.8 | ||
| 0 | 0.0 | 1 | 0.5 | |||
| EPHX2 3’UTR A>G, rs1042032 | AA | 153 | 59.1 | 81 | 44.3 | 0.273 |
| AG | 85 | 32.8 | 90 | 49.2 | ||
| GG | 21 | 8.1 | 12 | 6.6 | ||
N, number of subjects; MAF, minor allele frequency.
Association of genetic polymorphisms in donors and recipients with graft function and survival
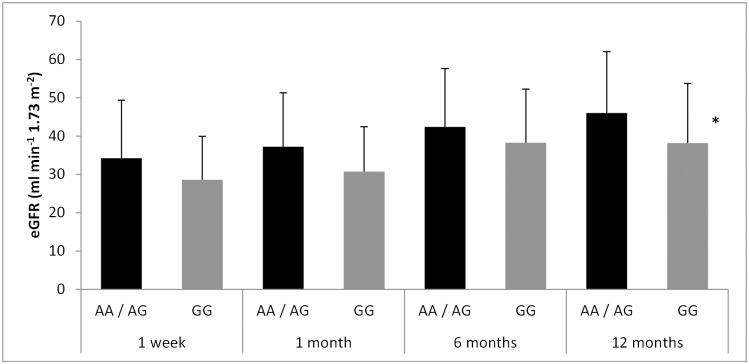
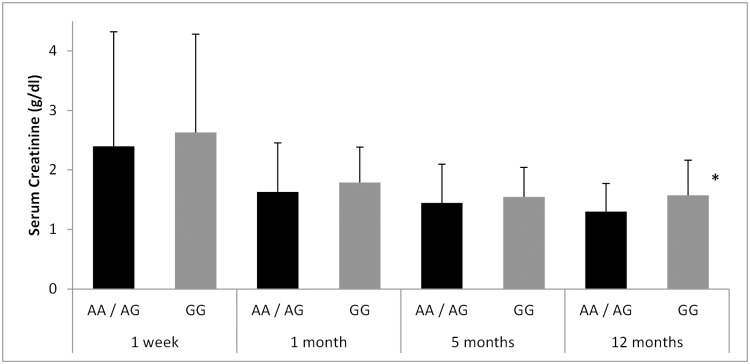
The eGFR in the recipients increased from 33.29 ± 14.07 to 44.02 ± 14.83 in the one-year follow-up period (p = 9.3 e-22). Amongst the SNPs analyzed, we found that subjects who carried the rs1042032 GG genotype displayed worse estimated renal function at all four time-points considered, compared to carriers of the wild type A-allele (Fig 1). Differences reached statistical significance at the end of the one-year follow-up (38.15 ± 15.57 vs. 45.99 ± 16.05 for GG and AA/AG respectively; p = 0.04). In a similar manner, the same genotype was also associated to higher serum creatinine values throughout the study (Fig 2) and again the difference was significant twelve months after grafting (1.57 ± 0.58 vs. 1.30 ± 0.47 g/dL for GG and AA/AG respectively; p = 0.02). In contrast, neither the rs1042032 GG genotype in the donor nor the other two SNPs analyzed showed a relevant effect on the measured renal function (S1 and S2 Tables show mean eGFR and serum creatinine values for the rs41507953 and rs751141 polymorphisms).
Fig 1. Effect of the EPHX2 3’ UTR A/G polymorphism (rs1042032) of the recipient on the estimated glomerular filtration rate throughout the one-year follow-up.
*p<0.05 vs. AA/AG group.
Fig 2. Effect of the EPHX2 3’ UTR A/G polymorphism (rs1042032) of the recipient on serum creatinine concentrations throughout the one-year follow-up.
*p<0.05 vs. AA/AG group.
The mean follow-up time for the graft survival study was 114.65 ± 60.07 months. There were no differences with regard to death-censored allograft survival when patients were stratified according to their genotype or that of their donors. Hazard ratios with 95% confidence intervals obtained by Cox regression analyses are shown in S3 Table.
Association of genetic polymorphisms in donors and recipients with acute rejection
Thirty-one patients (11.96%) experienced ARE according to the diagnostic criteria described in Methods. The incidence of acute rejection was significantly more frequent in carriers of the rs1042032 GG genotype, compared to subjects with the wildtype AA genotype (Table 4). In addition, a borderline association was observed for the GG genotype of the donor [OR = 4.80 (0.96–26.54)]. When the participants were analyzed using a recessive model (rs1042032 GG vs. AA/AG) the significance level of the association further increased [OR = 6.34 (1.35–29.90), p = 0.015 and OR = 5.53 (1.10–27.80), p = 0.042 for recipient and donor genotypes, respectively]. In the case of the recipients’ genotype, the association remained significant after correction for multiple testing.
Table 4. Odds ratios (OR) with 95% confidence intervals (CI) for the association of EPHX2 SNPs with acute rejection in renal transplant recipients.
| Recipients | Donors | ||||
|---|---|---|---|---|---|
| Polymorphism | OR (CI) | p | OR (CI) | p | |
| EPHX2 K55R | KK | Ref. | 0.83 | Ref. | 0.43 |
| KR | 1.23 (0.35–4.28) | 1.53 (0.49–4.79) | |||
| RR | NC | 6.61 (0.28–156.51) | |||
| EPHX2 R287Q | RR | Ref. | 0.38 | Ref. | 0.48 |
| RQ | 1.93 (0.46–8.01) | 1.77 (0.35–7.24) | |||
| NC | NC | ||||
| EPHX2 3’UTR A>G | AA | Ref. | 0.04 | Ref. | 0.11 |
| AG | 0.64 (0.15–2.67) | 0.76 (0.25–2.33) | |||
| GG | 5.45 (1.09–27.21) | 4.80 (0.96–26.54) | |||
NC, non-calculable.
In order to determine the relative weight of the donor vs. recipient rs1042032 GG genotype in the risk for ARE, we created a statistical model that included both genotypes along with significant demographic and clinical covariates described in methods. The results shows that the significance of the donor rs1042032 GG genotype decreased slightly [OR = 5.84 (0.82–37.88), p = 0.078]; in contrast, the OR value for the recipient’s GG genotype increased up to an OR of 8.28 (1.21–74.27) with a p-value of 0.031. This genotype presented the highest regression coefficient of all variables tested (B = 2.11). Amongst non-genetic factors, the impact of DGF, which was present in 29.1% of the patients and had shown a strong association with ARE in univariate analysis [OR = 9.66 (3.68–25.35); p < 0.001] decreased to a borderline association [OR = 2.72 (0.94–11.66)]. DGF occurrence was not significantly associated with any of the studied SNPs. The model explained 36.3% of the variability in the sample.
Finally, we tested whether there was any modification of ARE risk when the three EPHX2 loci were analyzed combined. Linkage disequilibrium data (D’, r2) were 0.99, 0.008 for rs41507953/rs751141; 0.78, 0.186 for rs41507953/rs1042032 and 0.92, 0.196 for rs751141/ rs1042032 in recipients and 0.99, 0.016; 0.75, 0.109 and 0.95, 0.307 for the same SNP pairs in donors. Table 5 shows the different haplotypes identified and their association with the risk for ARE. Haplotype distribution was not different between donors and recipients (Chi-square p > 0.05). Only haplotype *2 in the recipient (rs41507953 A / rs751141 A/ rs1042032 G) displayed a statistical trend towards higher risk of ARE [OR = 2.28 (0.92–5.67), p = 0.08]. The analyses were adjusted by the same variables as in the single-SNP study.
Table 5. Effect of EPHX2 haplotypes both in donors and recipients on the risk for acute rejection.
| Haplotype | Frequency | rs41507953 | rs751141 | rs1042032 | OR (CI) | p |
|---|---|---|---|---|---|---|
| Recipients | ||||||
| *1 | 0.732 | wt | wt | wt | Reference | |
| *2 | 0.114 | wt | wt | M | 2.28 (0.92–5.67) | 0.08 |
| *3 | 0.082 | M | wt | M | 0.24 (0.01–4.14) | 0.33 |
| *4 | 0.065 | wt | M | M | 3.09 (0.76–12.52) | 0.12 |
| *5 | <0.01 | M | wt | wt | - | |
| Donors | ||||||
| *1 | 0.662 | wt | wt | wt | Reference | |
| *2 | 0.116 | wt | wt | M | 0.94 (0.26–3.37) | 0.93 |
| *3 | 0.077 | M | wt | M | 1.96 (0.53–7.29) | 0.32 |
| *4 | 0.137 | wt | M | M | 2.21 (0.81–6.00) | 0.12 |
| *5 | <0.01 | M | wt | wt | - |
wt, wild type allele. M, variant allele.
Discussion
Some ten years ago, two studies revealed that a CYP3A5 allele was able to predict tacrolimus concentration-to-dose ratios in renal transplant recipients [25,26]. This finding prompted the publication of a great number of reports aimed to determine an association between genetic variants and meaningful clinical parameters in renal transplantation. The vast majority of these studies have focused on the variability in the CYP3A and ABCB1 genes, which are responsible for the metabolism and transport of anticalcineurin inhibitors [27,28]. However, it is somewhat surprising the lack of attention that has attracted the role of EPHX2 variants in the field of renal transplantation. It is even more so when EETs, the substrates for the EPHX2-encoded sEH, are vital components of the renal and vascular response to injury such as that produced in ischemia-reperfusion [2,29]. Indeed, AR9281 and GSK2256294 are new sEH inhibitors that are being tested in clinical trials to assess their efficacy and safety for the treatment of hypertension and other cardiovascular diseases [30,31].
In the present paper we have evaluated the impact of common genetic variability in the gene coding for sEH, the enzyme responsible for EETs degradation in the kidney. We observed that the GG genotype of the rs1042032 A/G SNP was associated with lower eGFR and higher serum creatinine values, particularly late in the study period. Only one previous study by Lee et al. [16] has analyzed the clinical role of this SNP in renal transplantation. The authors did not find a relevant impact of the GG genotype on their patients’ renal function, quite the opposite; they reported an association of the wild type AA genotype with allograft dysfunction. In contrast with recent evidence (see below) this study assumed that the SNP resulted in reduced enzyme activity, although the authors failed to confirm this in vitro. Some facts could also explain the discrepancy with our results. First, the patients in the study by Lee et al. were Asian who displayed a much higher MAF for the SNP (0.445 vs. 0.273 in our patients). Second, their study setting was living-donor transplantation, which generally means lower rates of graft dysfunction and acute rejection [32]; and last but not least, the study by Lee et al. only detected an association of the AA genotype with worse renal function when participants were stratified into two groups (High/Low eGFR), because when raw numbers were considered eGFR values were not significantly different. In fact, it is interesting that, in line with our results, serum creatinine values were higher in carriers of the variant G-allele [16]. In any case, our findings should be interpreted cautiously, given the existence of contradictory results. On the other hand, we did not observe a significant effect of the rs1042032 SNP on long-term graft survival. It is tempting to speculate that, in the long term, the protective effect of EETs [2,14] may be eclipsed by other factors such as chronic exposure to medication or the occurrence of infections.
The most relevant finding of this study was the fact that the rs1042032 GG genotype, both in donors and recipients, was associated with a higher risk of ARE after adjusting for demographic and clinical covariates. Interestingly, the only two recipients that carried the GG genotype and also received a kidney from a GG donor experienced ARE. The most likely explanation for this observation must be an increase of enzymatic activity produced by the SNP, because that would imply a faster rate of EETs degradation, subsequent endothelial dysfunction, increased blood pressure and, eventually, glomerular injury, as it has previously suggested [2]. In line with this hypothesis, two recent studies have demonstrated that enhanced activity of sEH increases the severity of the ischemia-reperfusion injury in the kidney [14] and is also associated with more advanced endothelial dysfunction [33], which are common complications in kidney transplantation [34]. Moreover, recent data available in the Genevar (GENe Expression VARiation) database [35] at the Sanger Institute website (http://www.sanger.ac.uk/resources/software/genevar) seem to confirm that this SNP is associated with increased enzyme activity. Genevar charts show that subjects from different ethnicities carrying the rs1042032 GG genotype, which we found to be related to increased risk of acute rejection and worse renal function, display significantly higher expression of sEH (S1 Fig). This would presumably lead to lower levels of EETs and, consequently, to a lower endogenous capacity of counteracting damaging processes in the graft. Furthermore, an increased sEH activity could also be the explanation for the borderline association with ARE observed with haplotype *2 in the recipient, since this allele combination contained the aforementioned 1042032 G variant and two wild type alleles in the other loci; an allele constellation that would presumably lead to a faster rate of EETs degradation.
The statistical model proposed in the present work indicates that the EPHX2 genotype of the recipient was more important to predict ARE than that of the donor, which underlines the importance of vascular and/or inflammatory mechanisms at a systemic level. In line with this finding, it was also the 1042032 GG genotype of the recipient, but not that of the donor, that was associated with worse graft function. Indeed, along with kidney EETs concentrations, decreased levels of vascular EETs have also been associated with renal diseases [2]. Future studies including circulating levels of EETs and, particularly, the actual expression of these compounds in the graft may help elucidate this question.
In any case, the statistical model presented herein should be validated in larger cohorts and, as such, has more of an academic than a clinical value. In this regard, a limitation of this work was its relatively small sample size, which may have been particularly relevant for some wide, although significant, confidence intervals. In addition, availability of genetic material from deceased donors was incomplete, which was not unexpected as DNA was obtained from lymphocytes that in some cases had been frozen for years. Another limitation was the lack of data on circulating levels of EETs (a parameter not available in a retrospective study) or on enzyme expression in the graft, which could have been helpful to confirm the proposed hypotheses. In this regard, protocol biopsies in subjects with stable graft function or in those with unambiguous clinical data are not performed in our centers, and therefore there was not enough tissue available as to carry out protein expression studies in this series of patients. This lack of histological confirmation for all acute rejection episodes may have also affected the reported incidence of this complication. On the other hand, the fact that the donor genotype was available greatly increased the significance of some findings, such as the aforementioned association with acute rejection observed for the rs1042032 SNP of both donors and recipients.
In conclusion, in a study designed with mechanism-linked, established polymorphisms, we have shown for the first time to our knowledge, that genetic variability in the EETs-metabolizing gene, EPHX2, may have a significant impact on the outcome of deceased-donor renal transplantation. The explanation for this finding most likely implies the modulation of the levels of EETs, with vasoactive and anti-inflammatories properties. The findings described in the present work may be the first step in a new field of research on the role of genetic variants affecting EETs availability in renal transplant. Further work with larger, independent series of patients and/or additional genes involved in EETs synthesis is warranted to confirm the findings described herein.
Supporting Information
Spearman's rho, nominal p-value and permutation p-value are shown above each plot (https://www.sanger.ac.uk/resources/software/genevar).
(TIF)
Mean and standard deviation (SD) values are shown. *Only one donor carried the 287QQ genotype.
(DOCX)
Mean and standard deviation (SD) values are shown. *Only one donor carried the 287QQ genotype.
(DOCX)
Hazard ratio values were adjusted by donor age, acute rejection, delayed graft function, type of immunosuppression and PRA peak. HR, hazard ratio; CI, 95% confidence intervals. aIndividuals were grouped in AA vs. AG+GG to make it up for the low number of GG carriers. bThere were no recipients with the GG genotype. cIn order to keep consistency with the other analyses performed in the study, the recessive model of inheritance is shown for the rs1042032 SNP. dOnly one donor carried the GG genotype and was ruled out from the analysis.
(DOCX)
Acknowledgments
The authors want to thank the technical and human support provided by Facility of Bioscience Applied Techniques of SAIUEx (financed by UEX, Junta de Extremadura, MICINN, FEDER and FSE).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work has been supported in part by the Association for the Study and Prevention of Renal Diseases (ASEPER), Badajoz, Spain, and by grant GR10022 from Junta de Extremadura, Consejeria de Economia, Comercio e Innovacion, Merida, Spain. Authors EC, CD-C and CL-L are supported by RED de Investigación Renal - REDINREN (Instituto de Salud Carlos III - European FEDER funds).
References
- 1. Yang L, Maki-Petaja K, Cheriyan J, McEniery C, Wilkinson IB. The role of epoxyeicosatrienoic acids in the cardiovascular system. Br J Clin Pharmacol. 2015. 10.1111/bcp.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Imig JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol. 2005; 289: F496–503. [DOI] [PubMed] [Google Scholar]
- 3. Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999; 285: 1276–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002; 82: 131–185. [DOI] [PubMed] [Google Scholar]
- 5. Lee CR, Imig JD, Edin ML, Foley J, DeGraff LM, Bradbury JA, et al. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 2010; 24: 3770–3781. 10.1096/fj.10-160119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma M, McCarthy ET, Reddy DS, Patel PK, Savin VJ, Medhora M, et al. 8,9-Epoxyeicosatrienoic acid protects the glomerular filtration barrier. Prostaglandins Other Lipid Mediat. 2009; 89: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alkayed NJ, Goyagi T, Joh HD, Klaus J, Harder DR, Traystman RJ, et al. Neuroprotection and P450 2C11 upregulation after experimental transient ischemic attack. Stroke. 2002; 33: 1677–1684. [DOI] [PubMed] [Google Scholar]
- 8. Fisslthaler B, Popp R, Michaelis UR, Kiss L, Fleming I, Busse R. Cyclic stretch enhances the expression and activity of coronary endothelium-derived hyperpolarizing factor synthase. Hypertension. 2001; 38: 1427–1432. [DOI] [PubMed] [Google Scholar]
- 9. Paller MS, Jacob HS. Cytochrome P-450 mediates tissue-damaging hydroxyl radical formation during reoxygenation of the kidney. Proc Natl Acad Sci U S A. 1994; 91: 7002–7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Przybyla-Zawislak BD, Srivastava PK, Vazquez-Matias J, Mohrenweiser HW, Maxwell JE, Hammock BD, et al. Polymorphisms in human soluble epoxide hydrolase. Mol Pharmacol. 2003; 64: 482–490. [DOI] [PubMed] [Google Scholar]
- 11. Srivastava PK, Sharma VK, Kalonia DS, Grant DF. Polymorphisms in human soluble epoxide hydrolase: effects on enzyme activity, enzyme stability, and quaternary structure. Arch Biochem Biophys. 2004; 427: 164–169. [DOI] [PubMed] [Google Scholar]
- 12. Lee CR, North KE, Bray MS, Fornage M, Seubert JM, Newman JW, et al. Genetic variation in soluble epoxide hydrolase (EPHX2) and risk of coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. Hum Mol Genet. 2006; 15: 1640–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fornage M, Lee CR, Doris PA, Bray MS, Heiss G, Zeldin DC, et al. The soluble epoxide hydrolase gene harbors sequence variation associated with susceptibility to and protection from incident ischemic stroke. Hum Mol Genet. 2005; 14: 2829–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee JP, Yang SH, Lee HY, Kim B, Cho JY, Paik JH, et al. Soluble epoxide hydrolase activity determines the severity of ischemia-reperfusion injury in kidney. PLoS One. 2012; 7: e37075 10.1371/journal.pone.0037075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee JP, Yang SH, Kim DK, Lee H, Kim B, Cho JY, et al. In vivo activity of epoxide hydrolase according to sequence variation affects the progression of human IgA nephropathy. Am J Physiol Renal Physiol. 2011; 300: F1283–1290. 10.1152/ajprenal.00733.2010 [DOI] [PubMed] [Google Scholar]
- 16. Lee SH, Lee J, Cha R, Park MH, Ha JW, Kim S, et al. Genetic variations in soluble epoxide hydrolase and graft function in kidney transplantation. Transplant Proc. 2008; 40: 1353–1356. 10.1016/j.transproceed.2008.03.137 [DOI] [PubMed] [Google Scholar]
- 17. Garcia M, Macias RM, Cubero JJ, Benitez J, Caravaca F, Gervasini G. ABCB1 polymorphisms are associated with cyclosporine-induced nephrotoxicity and gingival hyperplasia in renal transplant recipients. Eur J Clin Pharmacol. 2013; 69: 385–393. 10.1007/s00228-012-1355-x [DOI] [PubMed] [Google Scholar]
- 18. Gervasini G, Garcia M, Macias RM, Benitez J, Caravaca F, Cubero JJ. CYP2C8*3 Polymorphism and Donor Age are Associated With Allograft Dysfunction in Kidney Transplant Recipients Treated With Calcineurin Inhibitors. J Clin Pharmacol. 2013; 53: 427–434. 10.1002/jcph.15 [DOI] [PubMed] [Google Scholar]
- 19. Gervasini G, Garcia M, Macias RM, Cubero JJ, Caravaca F, Benitez J. Impact of genetic polymorphisms on tacrolimus pharmacokinetics and the clinical outcome of renal transplantation. Transpl Int. 2012; 25: 471–480. 10.1111/j.1432-2277.2012.01446.x [DOI] [PubMed] [Google Scholar]
- 20. Joosten SA, Sijpkens YW, van Kooten C, Paul LC. Chronic renal allograft rejection: pathophysiologic considerations. Kidney International. 2005; 68: 1–13. [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Greene T, Kuske J, Beck GJ, Group MS. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000; 11: 155A. [Google Scholar]
- 22. Poge U, Gerhardt T, Palmedo H, Klehr HU, Sauerbruch T, Woitas RP. MDRD equations for estimation of GFR in renal transplant recipients. Am J Transplant. 2005; 5: 1306–1311. [DOI] [PubMed] [Google Scholar]
- 23. Sole X, Guino E, Valls J, Iniesta R, Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics. 2006; 22: 1928–1929. [DOI] [PubMed] [Google Scholar]
- 24. Lebranchu Y, Baan C, Biancone L, Legendre C, Morales JM, Naesens M, et al. Pretransplant identification of acute rejection risk following kidney transplantation. Transplant International. 2014; 27: 129–138. 10.1111/tri.12205 [DOI] [PubMed] [Google Scholar]
- 25. Macphee IA, Fredericks S, Tai T, Syrris P, Carter ND, Johnston A, et al. Tacrolimus pharmacogenetics: polymorphisms associated with expression of cytochrome p4503A5 and P-glycoprotein correlate with dose requirement. Transplantation. 2002; 74: 1486–1489. [DOI] [PubMed] [Google Scholar]
- 26. Thervet E, Anglicheau D, King B, Schlageter MH, Cassinat B, Beaune P, et al. Impact of cytochrome p450 3A5 genetic polymorphism on tacrolimus doses and concentration-to-dose ratio in renal transplant recipients. Transplantation. 2003; 76: 1233–1235. [DOI] [PubMed] [Google Scholar]
- 27. Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I. Clin Pharmacokinet. 2010; 49: 141–175. 10.2165/11317350-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 28. Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part II. Clin Pharmacokinet. 2010; 49: 207–221. 10.2165/11317550-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 29. Gross GJ, Falck JR, Gross ER, Isbell M, Moore J, Nithipatikom K. Cytochrome P450 and arachidonic acid metabolites: role in myocardial ischemia/reperfusion injury revisited. Cardiovasc Res. 2005; 68: 18–25. [DOI] [PubMed] [Google Scholar]
- 30. Chen D, Whitcomb R, MacIntyre E, Tran V, Do ZN, Sabry J, et al. Pharmacokinetics and pharmacodynamics of AR9281, an inhibitor of soluble epoxide hydrolase, in single- and multiple-dose studies in healthy human subjects. J Clin Pharmacol. 2012; 52: 319–328. 10.1177/0091270010397049 [DOI] [PubMed] [Google Scholar]
- 31. Podolin PL, Bolognese BJ, Foley JF, Long E 3rd, Peck B, Umbrecht S, et al. In vitro and in vivo characterization of a novel soluble epoxide hydrolase inhibitor. Prostaglandins Other Lipid Mediat. 2013; 104–105: 25–31. 10.1016/j.prostaglandins.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 32. Naderi GH, Mehraban D, Kazemeyni SM, Darvishi M, Latif AH. Living or deceased donor kidney transplantation: a comparison of results and survival rates among Iranian patients. Transplant Proc. 2009; 41: 2772–2774. 10.1016/j.transproceed.2009.07.041 [DOI] [PubMed] [Google Scholar]
- 33. Schuck RN, Theken KN, Edin ML, Caughey M, Bass A, Ellis K, et al. Cytochrome P450-derived eicosanoids and vascular dysfunction in coronary artery disease patients. Atherosclerosis. 2013; 227: 442–448. 10.1016/j.atherosclerosis.2013.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morris ST, McMurray JJ, Rodger RS, Farmer R, Jardine AG. Endothelial dysfunction in renal transplant recipients maintained on cyclosporine. Kidney Int. 2000; 57: 1100–1106. [DOI] [PubMed] [Google Scholar]
- 35. Yang TP, Beazley C, Montgomery SB, Dimas AS, Gutierrez-Arcelus M, Stranger BE, et al. Genevar: a database and Java application for the analysis and visualization of SNP-gene associations in eQTL studies. Bioinformatics. 2010; 26: 2474–2476. 10.1093/bioinformatics/btq452 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spearman's rho, nominal p-value and permutation p-value are shown above each plot (https://www.sanger.ac.uk/resources/software/genevar).
(TIF)
Mean and standard deviation (SD) values are shown. *Only one donor carried the 287QQ genotype.
(DOCX)
Mean and standard deviation (SD) values are shown. *Only one donor carried the 287QQ genotype.
(DOCX)
Hazard ratio values were adjusted by donor age, acute rejection, delayed graft function, type of immunosuppression and PRA peak. HR, hazard ratio; CI, 95% confidence intervals. aIndividuals were grouped in AA vs. AG+GG to make it up for the low number of GG carriers. bThere were no recipients with the GG genotype. cIn order to keep consistency with the other analyses performed in the study, the recessive model of inheritance is shown for the rs1042032 SNP. dOnly one donor carried the GG genotype and was ruled out from the analysis.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.