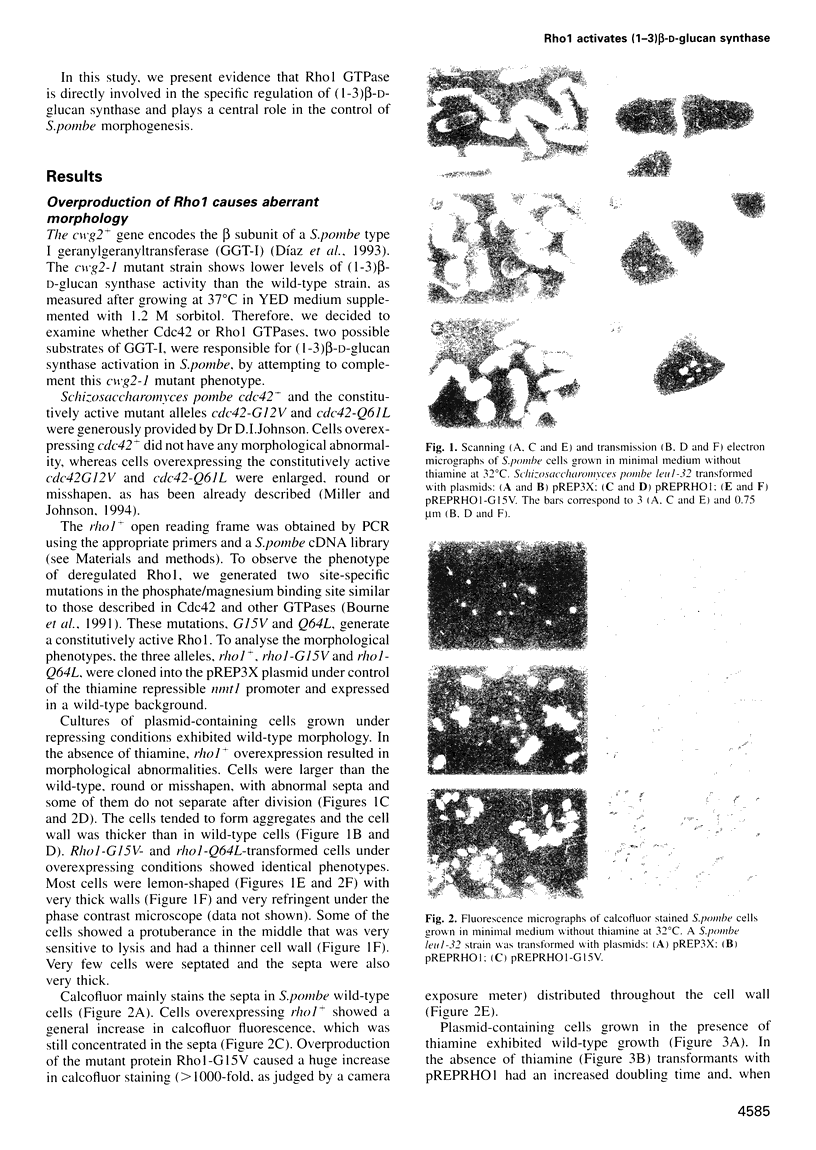
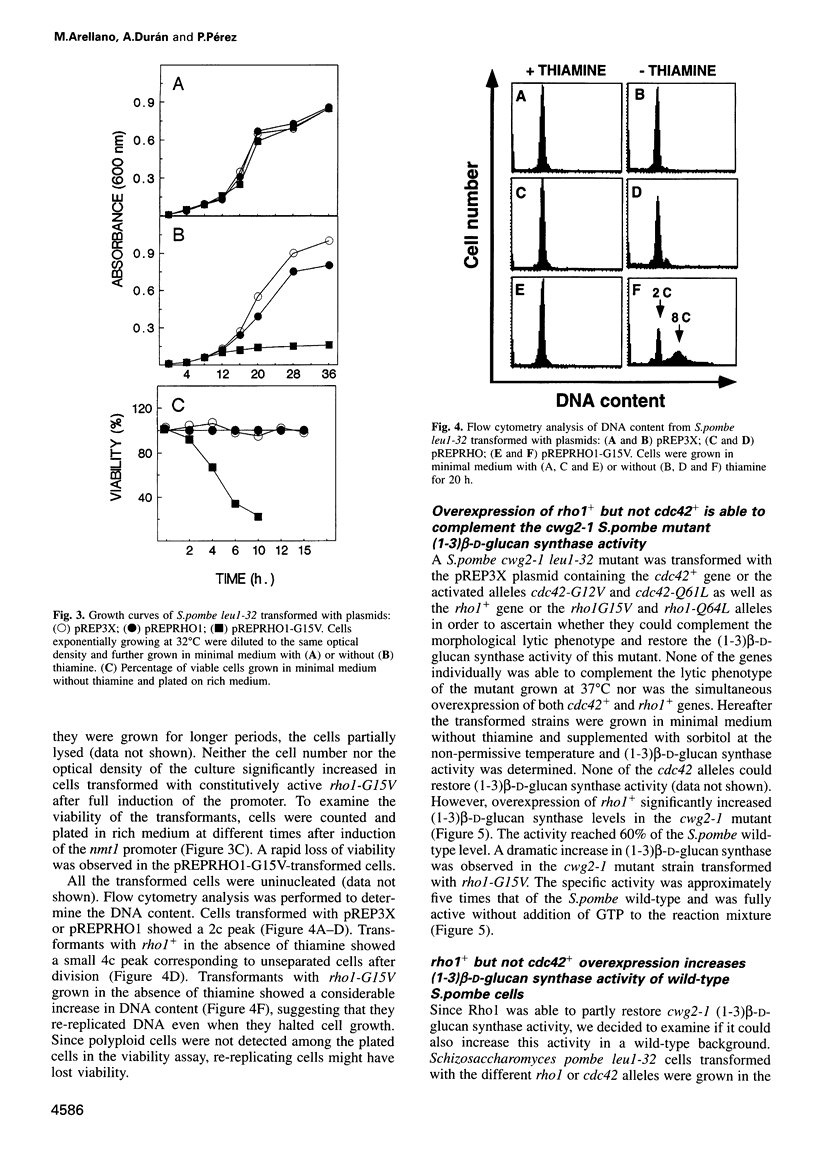
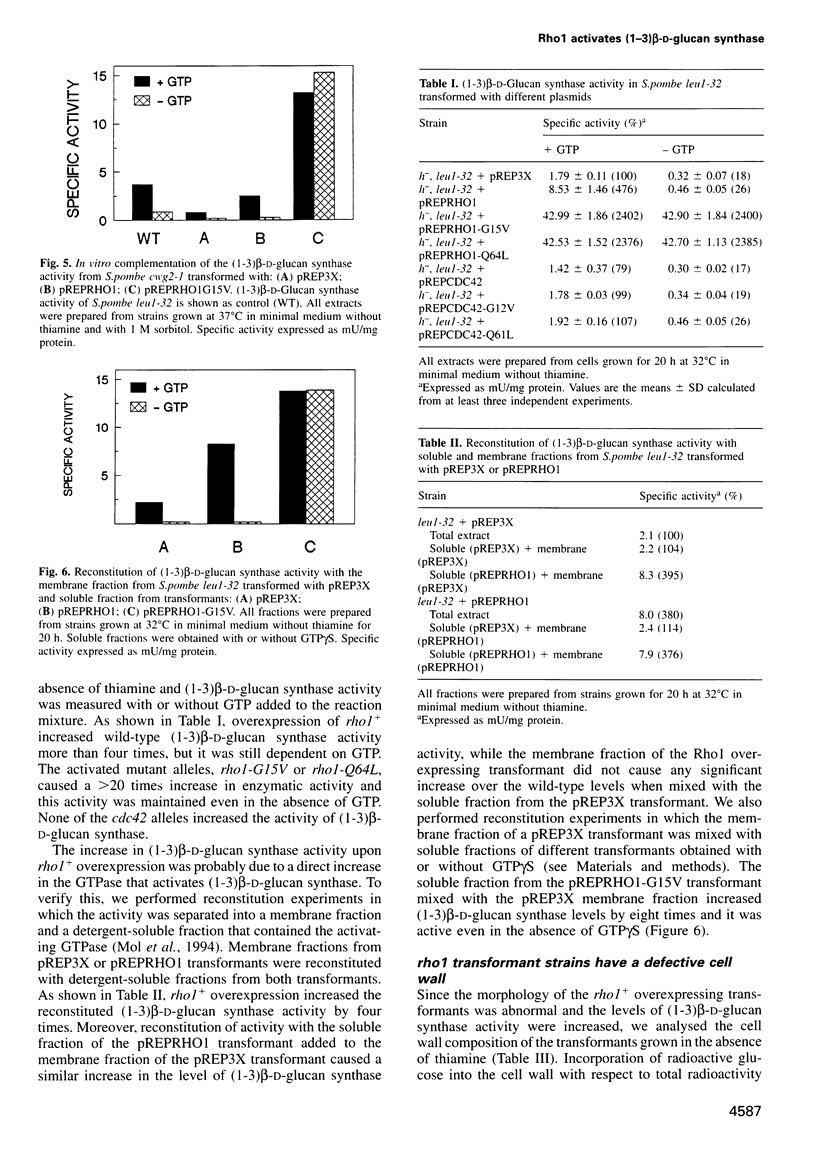
Abstract
The Schizosaccharomyces pombe Cdc42 and Rho1 GTPases were tested for their ability to complement the cwg2-1 mutant phenotype of a decrease in (1-3)beta-D-glucan synthase activity when grown at the non-permissive temperature. Only Rho1 is able to partly complement the defect in glucan synthase associated with the cwg2-1 mutation. Moreover, overexpression of the rho1 gene in wild-type S.pombe cells causes aberrant morphology with loss of polarity and cells with several septa. Under this condition (1-3)beta-D-glucan synthase activity is increased four times, but is still dependent on GTP. When S.pombe is transformed with constitutively active rho1 mutant alleles (rho1-G15V or rho1-Q64L), cells stop growing and show a very thick cell wall with hardly any septum. Under this condition the level of (1-3)beta-D-glucan synthase activity is at least 20 times higher than wild-type and is independent of GTP. Neither cdc42+ nor the cdc42-V12G or cdc42-Q61L constitutively active mutant alleles affect (1-3)beta-D-glucan synthase activity when overexpressed in S.pombe. Cells overproducing Rho1 are hypersensitive to inhibitors of cell wall biosynthesis or to cell wall degrading enzymes. We conclude that Rho1 GTPase directly activates (1-3)beta-D-glucan synthase and regulates S.pombe morphogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baguley B. C., Römmele G., Gruner J., Wehrli W. Papulacandin B: an inhibitor of glucan synthesis in yeast spheroplasts. Eur J Biochem. 1979 Jul;97(2):345–351. doi: 10.1111/j.1432-1033.1979.tb13120.x. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Bulawa C. E., Slater M., Cabib E., Au-Young J., Sburlati A., Adair W. L., Jr, Robbins P. W. The S. cerevisiae structural gene for chitin synthase is not required for chitin synthesis in vivo. Cell. 1986 Jul 18;46(2):213–225. doi: 10.1016/0092-8674(86)90738-5. [DOI] [PubMed] [Google Scholar]
- Chang E. C., Barr M., Wang Y., Jung V., Xu H. P., Wigler M. H. Cooperative interaction of S. pombe proteins required for mating and morphogenesis. Cell. 1994 Oct 7;79(1):131–141. doi: 10.1016/0092-8674(94)90406-5. [DOI] [PubMed] [Google Scholar]
- Chant J., Stowers L. GTPase cascades choreographing cellular behavior: movement, morphogenesis, and more. Cell. 1995 Apr 7;81(1):1–4. doi: 10.1016/0092-8674(95)90363-1. [DOI] [PubMed] [Google Scholar]
- Cvrcková F., De Virgilio C., Manser E., Pringle J. R., Nasmyth K. Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev. 1995 Aug 1;9(15):1817–1830. doi: 10.1101/gad.9.15.1817. [DOI] [PubMed] [Google Scholar]
- Drgonová J., Drgon T., Tanaka K., Kollár R., Chen G. C., Ford R. A., Chan C. S., Takai Y., Cabib E. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science. 1996 Apr 12;272(5259):277–279. doi: 10.1126/science.272.5259.277. [DOI] [PubMed] [Google Scholar]
- Díaz M., Sanchez Y., Bennett T., Sun C. R., Godoy C., Tamanoi F., Duran A., Perez P. The Schizosaccharomyces pombe cwg2+ gene codes for the beta subunit of a geranylgeranyltransferase type I required for beta-glucan synthesis. EMBO J. 1993 Dec 15;12(13):5245–5254. doi: 10.1002/j.1460-2075.1993.tb06220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell E., Bowden S., Armstrong J. A homologue of the ras-related CDC42 gene from Schizosaccharomyces pombe. Gene. 1992 May 1;114(1):153–154. doi: 10.1016/0378-1119(92)90724-4. [DOI] [PubMed] [Google Scholar]
- Forsburg S. L. Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 1993 Jun 25;21(12):2955–2956. doi: 10.1093/nar/21.12.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Imai J., Toh-e A., Matsui Y. Genetic analysis of the Saccharomyces cerevisiae RHO3 gene, encoding a rho-type small GTPase, provides evidence for a role in bud formation. Genetics. 1996 Feb;142(2):359–369. doi: 10.1093/genetics/142.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S. B., Takewaki N., Takasuka T., Mio T., Adachi M., Fujii Y., Miyamoto C., Arisawa M., Furuichi Y., Watanabe T. Characterization and gene cloning of 1,3-beta-D-glucan synthase from Saccharomyces cerevisiae. Eur J Biochem. 1995 Aug 1;231(3):845–854. doi: 10.1111/j.1432-1033.1995.tb20770.x. [DOI] [PubMed] [Google Scholar]
- Kang M. S., Cabib E. Regulation of fungal cell wall growth: a guanine nucleotide-binding, proteinaceous component required for activity of (1----3)-beta-D-glucan synthase. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5808–5812. doi: 10.1073/pnas.83.16.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. E., Bartlett-Heubusch E. Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. J Cell Biol. 1992 Mar;116(5):1221–1229. doi: 10.1083/jcb.116.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaule P., Axel R., Myers A. M. Characterization of two members of the rho gene family from the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1987 Feb;84(3):779–783. doi: 10.1073/pnas.84.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus S., Polverino A., Chang E., Robbins D., Cobb M. H., Wigler M. H. Shk1, a homolog of the Saccharomyces cerevisiae Ste20 and mammalian p65PAK protein kinases, is a component of a Ras/Cdc42 signaling module in the fission yeast Schizosaccharomyces pombe. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):6180–6184. doi: 10.1073/pnas.92.13.6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y., Toh-E A. Yeast RHO3 and RHO4 ras superfamily genes are necessary for bud growth, and their defect is suppressed by a high dose of bud formation genes CDC42 and BEM1. Mol Cell Biol. 1992 Dec;12(12):5690–5699. doi: 10.1128/mcb.12.12.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur P., Morin N., Baginsky W., el-Sherbeini M., Clemas J. A., Nielsen J. B., Foor F. Differential expression and function of two homologous subunits of yeast 1,3-beta-D-glucan synthase. Mol Cell Biol. 1995 Oct;15(10):5671–5681. doi: 10.1128/mcb.15.10.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni C., Zarov P., Rambourg A., Mann C. The SLT2 (MPK1) MAP kinase homolog is involved in polarized cell growth in Saccharomyces cerevisiae. J Cell Biol. 1993 Dec;123(6 Pt 2):1821–1833. doi: 10.1083/jcb.123.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. J., Johnson D. I. Cdc42p GTPase is involved in controlling polarized cell growth in Schizosaccharomyces pombe. Mol Cell Biol. 1994 Feb;14(2):1075–1083. doi: 10.1128/mcb.14.2.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol P. C., Park H. M., Mullins J. T., Cabib E. A GTP-binding protein regulates the activity of (1-->3)-beta-glucan synthase, an enzyme directly involved in yeast cell wall morphogenesis. J Biol Chem. 1994 Dec 9;269(49):31267–31274. [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Nakano K., Mabuchi I. Isolation and sequencing of two cDNA clones encoding Rho proteins from the fission yeast Schizosaccharomyces pombe. Gene. 1995 Mar 21;155(1):119–122. doi: 10.1016/0378-1119(94)00917-h. [DOI] [PubMed] [Google Scholar]
- Nobes C. D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995 Apr 7;81(1):53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nonaka H., Tanaka K., Hirano H., Fujiwara T., Kohno H., Umikawa M., Mino A., Takai Y. A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J. 1995 Dec 1;14(23):5931–5938. doi: 10.1002/j.1460-2075.1995.tb00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya Y., Qadota H., Anraku Y., Pringle J. R., Botstein D. Suppression of yeast geranylgeranyl transferase I defect by alternative prenylation of two target GTPases, Rho1p and Cdc42p. Mol Biol Cell. 1993 Oct;4(10):1017–1025. doi: 10.1091/mbc.4.10.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M. F., Ashworth A., Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995 Sep 1;269(5228):1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- Omer C. A., Gibbs J. B. Protein prenylation in eukaryotic microorganisms: genetics, biology and biochemistry. Mol Microbiol. 1994 Jan;11(2):219–225. doi: 10.1111/j.1365-2958.1994.tb00302.x. [DOI] [PubMed] [Google Scholar]
- Ottilie S., Miller P. J., Johnson D. I., Creasy C. L., Sells M. A., Bagrodia S., Forsburg S. L., Chernoff J. Fission yeast pak1+ encodes a protein kinase that interacts with Cdc42p and is involved in the control of cell polarity and mating. EMBO J. 1995 Dec 1;14(23):5908–5919. doi: 10.1002/j.1460-2075.1995.tb00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paravicini G., Cooper M., Friedli L., Smith D. J., Carpentier J. L., Klig L. S., Payton M. A. The osmotic integrity of the yeast cell requires a functional PKC1 gene product. Mol Cell Biol. 1992 Nov;12(11):4896–4905. doi: 10.1128/mcb.12.11.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadota H., Python C. P., Inoue S. B., Arisawa M., Anraku Y., Zheng Y., Watanabe T., Levin D. E., Ohya Y. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-beta-glucan synthase. Science. 1996 Apr 12;272(5259):279–281. doi: 10.1126/science.272.5259.279. [DOI] [PubMed] [Google Scholar]
- Ribas J. C., Diaz M., Duran A., Perez P. Isolation and characterization of Schizosaccharomyces pombe mutants defective in cell wall (1-3)beta-D-glucan. J Bacteriol. 1991 Jun;173(11):3456–3462. doi: 10.1128/jb.173.11.3456-3462.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. J. Rho-related proteins: actin cytoskeleton and cell cycle. Curr Opin Genet Dev. 1995 Feb;5(1):24–30. doi: 10.1016/s0959-437x(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Roemer T., Paravicini G., Payton M. A., Bussey H. Characterization of the yeast (1-->6)-beta-glucan biosynthetic components, Kre6p and Skn1p, and genetic interactions between the PKC1 pathway and extracellular matrix assembly. J Cell Biol. 1994 Oct;127(2):567–579. doi: 10.1083/jcb.127.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazer S., Sherwood S. W. Mitochondrial growth and DNA synthesis occur in the absence of nuclear DNA replication in fission yeast. J Cell Sci. 1990 Nov;97(Pt 3):509–516. doi: 10.1242/jcs.97.3.509. [DOI] [PubMed] [Google Scholar]
- Simon M. N., De Virgilio C., Souza B., Pringle J. R., Abo A., Reed S. I. Role for the Rho-family GTPase Cdc42 in yeast mating-pheromone signal pathway. Nature. 1995 Aug 24;376(6542):702–705. doi: 10.1038/376702a0. [DOI] [PubMed] [Google Scholar]
- Trueblood C. E., Ohya Y., Rine J. Genetic evidence for in vivo cross-specificity of the CaaX-box protein prenyltransferases farnesyltransferase and geranylgeranyltransferase-I in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Jul;13(7):4260–4275. doi: 10.1128/mcb.13.7.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe T., Yamada-Okabe T., Kasahara S., Furuichi Y., Nakajima T., Ichishima E., Arisawa M., Yamada-Okabe H. HKR1 encodes a cell surface protein that regulates both cell wall beta-glucan synthesis and budding pattern in the yeast Saccharomyces cerevisiae. J Bacteriol. 1996 Jan;178(2):477–483. doi: 10.1128/jb.178.2.477-483.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamochi W., Tanaka K., Nonaka H., Maeda A., Musha T., Takai Y. Growth site localization of Rho1 small GTP-binding protein and its involvement in bud formation in Saccharomyces cerevisiae. J Cell Biol. 1994 Jun;125(5):1077–1093. doi: 10.1083/jcb.125.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Bender A., Cerione R. A. Interactions among proteins involved in bud-site selection and bud-site assembly in Saccharomyces cerevisiae. J Biol Chem. 1995 Jan 13;270(2):626–630. doi: 10.1074/jbc.270.2.626. [DOI] [PubMed] [Google Scholar]
- Ziman M., O'Brien J. M., Ouellette L. A., Church W. R., Johnson D. I. Mutational analysis of CDC42Sc, a Saccharomyces cerevisiae gene that encodes a putative GTP-binding protein involved in the control of cell polarity. Mol Cell Biol. 1991 Jul;11(7):3537–3544. doi: 10.1128/mcb.11.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman M., Preuss D., Mulholland J., O'Brien J. M., Botstein D., Johnson D. I. Subcellular localization of Cdc42p, a Saccharomyces cerevisiae GTP-binding protein involved in the control of cell polarity. Mol Biol Cell. 1993 Dec;4(12):1307–1316. doi: 10.1091/mbc.4.12.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Sherbeini M., Clemas J. A. Cloning and characterization of GNS1: a Saccharomyces cerevisiae gene involved in synthesis of 1,3-beta-glucan in vitro. J Bacteriol. 1995 Jun;177(11):3227–3234. doi: 10.1128/jb.177.11.3227-3234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]