Abstract
Purpose of review
To review recent advances in the management strategies of polyarticular course juvenile idiopathic arthritis (JIA) and identify unanswered questions and avenues for further research.
Recent findings
There is evidence for an early, aggressive, treat-to-target approach for polyarticular JIA. Clinical disease activity criteria have been recently defined and validated, including criteria for inactive disease and the juvenile arthritis disease activity score (JADAS). There is a need for evidence-based, defined disease targets and biomarkers for prediction of response, including targets for remission induction, and guidelines on drug withdrawal. Recent treatment consensus plans and guidelines are discussed and compared, including the 2015 NHS England clinical policy statement, the 2014 Childhood Arthritis and Rheumatology Research Alliance (CARRA) treatment plans and the 2011 American College of Rheumatology (ACR) guidelines. Evidence for new agents such as tocilizumab, rituximab, golimumab, ustekinumab, certolizumab and tofacitinib is promising: the recent clinical trials are summarized here. Stratification of individual patient treatment remains a goal, and predictive biomarkers have been shown to predict success in the withdrawal of methotrexate therapy.
Summary
There are promising advances in the treatment approaches, disease activity criteria, clinical guidelines, pharmaceutical choices and individually stratified therapy choices for polyarticular JIA.
Keywords: juvenile idiopathic arthritis, treat to target, treatment
INTRODUCTION
Juvenile idiopathic arthritis is defined as arthritis of unknown etiology, presenting in children less than 16 years old and persisting for at least 6 weeks. It is classified by ILAR into six subtypes [1]. Polyarticular juvenile idiopathic arthritis (pJIA) is defined as disease involving more than five joints in the first 6 months of disease. A recent Canadian study [2] of 1104 JIA patients showed that patients with pJIA, particularly rheumatoid factor (RF)-positive pJIA, were less likely to go into remission, more likely to have worse outcomes and be treated with steroids and biologic agents than the other subtypes. Some studies use the term polyarticular course JIA to denote any disease with more than five joints involved, which then may include extended oligoarticular JIA, enthesitis-related arthritis (ERA), psoriatic JIA and systemic-onset JIA. For the purposes of this review, data and evidence may be relevant to all of these subtypes, except for systemic-onset JIA, which has been extensively reviewed elsewhere [3▪]. In this review, we will discuss recent advances in the management strategies of pJIA as well as identify unanswered questions and avenues for further research.
Box 1.
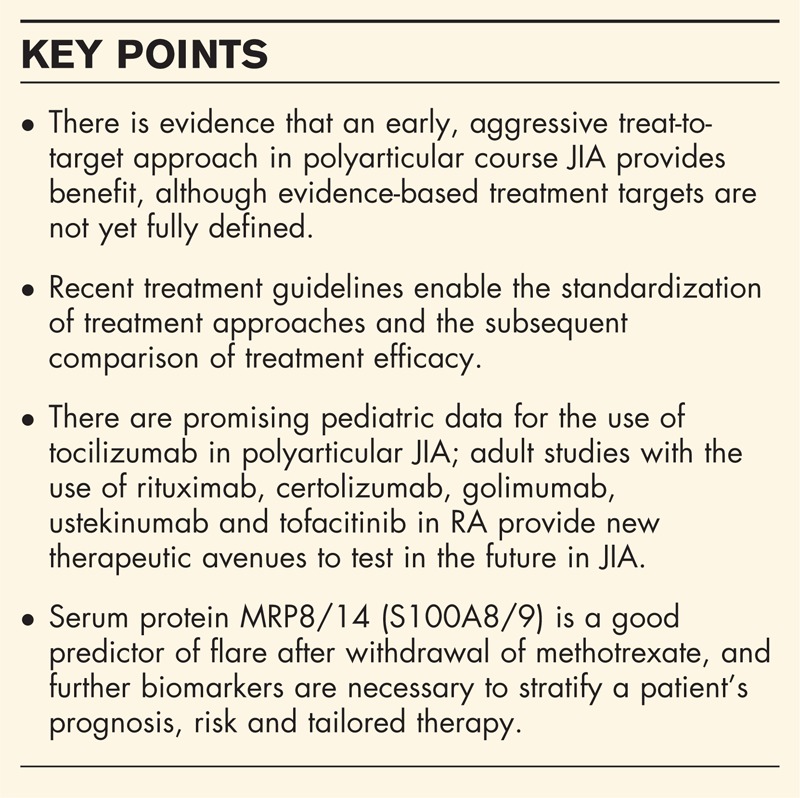
no caption available
TREAT TO TARGET
Strategies for early, aggressive treatment of adult inflammatory arthritis now use defined disease targets. Tight disease control is beneficial in treatment of adult-onset rheumatoid arthritis (RA) [4–6] and is included in the adult recommendations [7,8]. Similarly, the pediatric rheumatology community has recently considered whether this applies to JIA. The Aggressive Combination Drug Therapy in Very Early Polyarticular Juvenile Idiopathic Arthritis (ACUTE-JIA) trial published in 2011 compared biologic combination therapy [methotrexate plus tumor necrosis factor (TNF)α inhibitor], conventional synthetic DMARD combination (methotrexate, sulphasalazine and chloroquine) or methotrexate alone. Patients had at least five active joints and included patients with ERA and psoriatic JIA [9]. Patients on the biologic and methotrexate combination arm achieved the primary outcome response (ACR Pedi 75), and spent significantly more time in clinically inactive disease (CID) [10] during the study. Of note is that only 1 out of 59 patients recruited was RF+. The Trial of Early Aggressive Therapy in pJIA trial (TREAT) [11] enrolled polyarticular RF+ or RF− patients according to the ILAR classification, including patients that had a positive family history of psoriasis, but no evidence of psoriasis. Thirty-three to thirty-nine percent were RF+. Patients were stratified to receive: an aggressive regimen of high dose oral prednisone, subcutaneous methotrexate and etanercept, or subcutaneous methotrexate alone with placebo oral steroids and placebo etanercept. The primary end point was achievement of CID [12] at 6 months. Forty percent of those on the aggressive arm achieved this, vs 23% on methotrexate alone: this difference did not reach statistical significance (P = 0.088). The response to methotrexate was higher than in some studies, which may reflect the use of the subcutaneous route as first line. There was however a significant difference in the percentage of patients achieving an ACR 70 response at 4 months (P = 0.01). Interestingly, this study found that the only predictor of achievement of CID was disease duration at onset of treatment, not ESR, joint count or RF positivity. Subsequent analysis reiterated that shorter disease duration prior to treatment, a robust response at 4 months (with achievement of ACR 70) and more aggressive therapy result in a higher likelihood and longer duration of CID in patients with pJIA [13▪]. This supports earlier evidence that predictors of good response to methotrexate in JIA include short time to treatment initiation [14]. Therefore, together several strands of evidence suggest benefit from early, aggressive therapy for pJIA.
DEFINING TARGETS
A major recent advance is the definition and clinical validation of disease activity in children with JIA [12,15,16]. These include the Wallace criteria for CID: no active joints, no uveitis, a normal CRP and ESR, a physician global assessment of 0 and morning stiffness for less than 15 min [12]. These criteria include CID on medication (CID for >6 months on medication) and CID off medication (CID for >12 months off medication). The Juvenile Arthritis Disease Activity Score (JADAS), constructed from four variables [active joint count (in 10, 27 or 71 joints), patient global assessment, physician global assessment and ESR], has been validated clinically in JIA [15]. It has also been shown that a three-item JADAS that excludes use of the ESR (JADAS3) correlates well with conventional JADAS and that there is good correlation between the JADAS 10, 27 and 71 [16], suggesting that joint count, combined with parent/patient and physician's score, without ESR measurement may be sufficient for robust assessment of disease activity.
To have a successful treat-to-target guideline, there need to be defined targets for disease control within current practice guidelines [17]. The 2015 NHS England consensus statement on JIA treatment includes a statement on the goals of therapy [18▪] (see below), but evidence-based consensus targets, including targets for remission induction, maintenance therapy and guidelines on drug withdrawal, are not yet uniformly defined in JIA [17].
TREATMENT PLANS, GUIDELINES AND RECOMMENDATIONS
There is a lack of consensus-driven, evidence-based treatment guidelines for pJIA. Owing to this and variable availability of drugs, there are variations in practice within countries and worldwide. Inception cohorts, therefore, vary in patient recruitment and treatment regimens, making it more difficult to directly compare drug efficacy between them. In 2015, NHS England released a policy statement, ‘Biologic Therapies for the treatment of Juvenile Idiopathic Arthritis’ [18▪]. This is a clinical guideline for clinicians and funders, which defined goals of therapy: ‘to induce and maintain a complete remission of all symptoms, and thus to allow a child to achieve normal growth, development, and allow full participation in school, career, sport and all other aspects of normal life.’ Intravenous or intra-articular steroids are recommended for induction, and methotrexate is advised uniformly for all types of JIA involving more than four joints as an initial treatment. Importantly, these guidelines include a definition of clinical response, clinically inactive disease, clinical remission on and off medication and treatment failure. The use of consensus definitions for these outcomes will provide for the comparison of countrywide patient cohorts. These guidelines advise starting a biologic agent in polyarticular disease where there has been the use of methotrexate at 15 mg/m2 subcutaneously for at least 3 months with poor response, except where axial disease is present, where an anti-TNFα agent can be started immediately. If CID is not achieved after 3 months, all patients then start on a TNFα inhibitor. If there is inadequate response, patients may switch to a second TNFα inhibitor. With further lack of effect, the recommendations for polyarticular RF+ JIA are to start rituximab, whereas tocilizumab or abatacept is recommended for all others who have failed to respond to two TNFα inhibitors and are RF−.
In 2014, the Childhood Arthritis and Rheumatology Research Alliance (CARRA) developed standardized consensus-driven treatment plans for treatment of pJIA. The purpose was to develop guidelines to enable observational studies and comparative effectiveness studies and decrease variability of treatment practice. These plans enable physicians to choose the treatment plan they prefer, that may then form part of a standardized database for evaluation. Treatment plans include any JIA that involves more than four joints, and therefore includes ERA, psoriatic JIA, undifferentiated JIA and extended oligoarticular JIA, but specifically exclude systemic JIA. The three regimens include the step-up plan (conventional DMARD followed by biologic after nonresponse), early combination plan (conventional DMARD and biologic at onset) and biologic only plan. There are also recommendations on visit frequency, drug doses and steroid weaning [19▪].
These treatment plans differ from the 2011 ACR recommendations [20], which were developed by a Rand/UCLA appropriateness method, used to assess benefits vs risks of various therapies when definitive evidence does not exist to guide treatment. These are intended more as treatment recommendations, rather than treatment standardizations as in the CARRA initiative. The treatment algorithm is suggested as a recommendation and not a guideline. For JIA that involves more than four joints (which includes ERA without sacroiliitis, psoriatic, RF+ and RF−, extended oligoarticular and undifferentiated JIA), the recommendation is similar to the step-up plan from CARRA above. Notably, these recommendations also include rituximab for pJIA (especially RF+) that is resistant to treatment with TNFα inhibition and abatacept treatment. A large ongoing European collaborative network (SHARE) is being developed to standardize care, and create a basic minimum standard of care across Europe for young people with JIA: the SHARE recommendations have not yet been published [21].
DRUG ADVANCES AND NEW DRUGS FOR THE TREATMENT OF POLYARTICULAR JUVENILE IDIOPATHIC ARTHRITIS
Tocilizumab
The CHERISH trial, published in 2014, was a three-part, randomized, placebo-controlled, double-blind withdrawal study [22▪] of tocilizumab. This trial enrolled patients with pJIA (RF+ and RF−) or extended oligoarticular JIA with at least five active joints. Patients had failed or been intolerant to methotrexate, and may have had a previously ineffective biologic agent. In the open-label phase, 89% of patients achieved an ACR 30 response, and all core outcome variables measured showed improvement. Optimal dose of tocilizumab was 8 mg/kg if more than 30 kg and 10 mg/kg if less than 10-kg body weight. There was a significantly increased rate of flare in patients on the placebo arm during the withdrawal phase, compared with those who continued on tocilizumab. Concurrent methotrexate was shown to decrease risk of flare in both the placebo and tocilizumab groups. Patients who had previously failed another biologic agent were more likely to flare than biologic-naïve patients in the withdrawal arm, but 48% of patients with previous biologic failure still achieved an ACR 70 response on tocilizumab. These are encouraging results for the use of tocilizumab in patients with pJIA who have not achieved a good response with methotrexate and a TNFα inhibitor. To this end, tocilizumab is now included in the NHS England guidelines for use in RF− pJIA in patients who have failed two TNFα inhibitors [18▪].
Rituximab
Rituximab is a chimeric monoclonal antibody to the B cell antigen CD20, which has been shown to be effective in adult-onset RA refractory to anti-TNF therapy [23–25]. There is limited published evidence of use of rituximab for refractory pJIA. Most reports are case studies or small series and several are studies in systemic JIA patients [26,27]. An open-label prospective study [28] on the effectiveness of rituximab performed in Russia included 55 patients; 84% of these patients had systemic-onset JIA. Despite this lack of evidence, there is a recommendation from the ACR for the use of rituximab for severe, unresponsive pJIA [20]. NHS England guidelines recommend rituximab use in polyarticular RF+ patients who have failed two TNFα inhibitors [18▪].
Golimumab
Golimumab is a fully humanized monoclonal antibody to soluble and transmembranous TNFα. It can be used as a monthly subcutaneous dose (50 mg for adults), or a weight-based (2 mg/kg) 8 weekly intravenous infusion. This may be useful in JIA, as weight-based dosing and a relatively long interval between infusions have advantages in children. It is biologically similar to infliximab in molecular weight and constant region sequence, except that it is fully humanized and not chimeric [29]. GO-KIDS, a recent three part, placebo-controlled, withdrawal trial showed an 87% ACR 30 response rate and 36% CID achievement during the open-label first 16 weeks on golimumab. The study however failed to meet its primary endpoint, which was a comparison of rate of flare in responders during the withdrawal phase between placebo and golimumab (both with concurrent methotrexate). There was no significant difference in disease flare between groups, with a sustained ACR 30 response in both groups (89–95%). Safety profile was acceptable and injections were tolerable (reported in abstract form [30]). Further investigation in JIA is needed. In adults with RA, golimumab has been shown to be effective in patients with insufficient response to methotrexate alone as compared with placebo [31] and in patients on methotrexate who have had an insufficient response to another TNFα inhibitor [32]. It has been shown to be effective in adult psoriatic arthritis [33] and ankylosing spondylitis [34], with a safety profile comparable with other TNFα inhibitors, at least in the short term.
Certolizumab
Certolizumab pegol is a pegylated anti-TNFα inhibitor that in adults has shown to be effective and have a good comparative safety profile to other biologic agents [35–37]. Pegylation enhances the half-life of the drug and allows a 2–4-week dosing schedule. There is a clinical trial underway for the use of certolizumab in children with JIA [38].
Ustekinumab
Psoriatic arthritis and ankylosing spondylitis are unique subtypes of arthritis that have been associated with IL-23 receptor and other genetic polymorphisms [39,40]. The equivalent subcategories of JIA, namely psoriatic JIA and ERA, can be challenging to manage, and are less likely than other subtypes to fully respond to TNFα inhibition. Ustekinumab is a human monoclonal antibody directed against the combined interleukin-12 and interleukin-23 p40 subunit. In adults, ustekinumab has been shown to be effective and superior to etanercept for severe plaque psoriasis [41–43] and effective in adult patients with psoriatic arthritis who have had a previous failed TNFα inhibitor or are biologic-naive [44,45]. Results are awaited for a recent clinical trial on the use of ustekinumab in adolescents with recalcitrant plaque psoriasis [46]. An open-label proof of concept trial has shown promising results for the use of ustekinumab in ankylosing spondylitis [47]. There are currently no data on the use of ustekinumab for JIA, but the emerging adult data are promising.
Tofacitinib
Tofacitinib is an oral small molecule inhibitor of the Janus kinase (JAK) and signal transducers and activators of transcription (STAT) pathways, key signal transducers that transmit signals from various cytokine receptors (GMCSF, interleukin-6, IFN α,ϒ and β, interleukin-10 and others), which further translocate to the nucleus and regulate gene expression [48]. Tofacitinib has been shown to be effective in adult RA refractory to DMARD and biologic, noninferior to abatacept, and has been explored as first-line monotherapy vs methotrexate [49–52]. It has been approved by the FDA for treatment of RA refractory to methotrexate but not by the European Medicines Agency. Safety concerns exist regarding lymphopenia, risk of malignancy, elevations in low and high-density lipoproteins and creatinine [53,54]. There is currently no evidence for the use of tofacitinib in JIA, but pharmacokinetic studies in children are underway [55].
Tailored treatment for polyarticular JIA
Given the rapid increase in available choices of medication for JIA patients refractory to methotrexate, there is an urgent need for evidence-based, prognostic biomarkers, which provide estimates of risk of nonresponse to specific drugs. Thus, robust evidence for high risk of methotrexate failure would allow clinicians to argue for an early use of biologic, in combination or alone. Such biomarkers need to be readily measureable in the pediatric population for example in small blood volume, or urine. JIA was the first condition in which a robust biomarker for use to predict flare upon stopping methotrexate treatment was discovered. Children who have reached CID on methotrexate, in whom serum protein MRP8/14 (S100A8/9) is higher than normal, have a greater chance of flare after stopping methotrexate than those with normal MRP8/14 serum levels [56]. Progress in the field of predictive biomarkers for drug response in JIA has been encouraging with genetic, serum and transcriptome studies [57–61] suggesting that such biomarkers may become available. Validation of these studies requires large collaborative efforts. A UK wide consortium CHART (Childhood arthritis response to treatment) has been established to work in parallel with large international efforts to address this unmet need.
CONCLUSION
There is evidence that early aggressive treatment for pJIA is beneficial. There are defined and validated clinical disease activity criteria, but defined disease targets necessary for a treat-to-target approach are lacking in pJIA. Various treatment consensus plans and guidelines exist, which should allow standardization and direct comparison of drugs and treatment regimens. Tocilizumab and rituximab have been added to the pJIA armamentarium, and there are trials underway for novel agents such as golimumab, ustekinumab, certolizumab and tofacitinib in which the adult data are promising. Stratification of individual patient treatment remains a goal, and predictive biomarkers are promising for this purpose.
Acknowledgements
Both authors are supported by funding from Arthritis Research UK, as part of the Centre for Adolescent Rheumatology at UCL UCLH and GOSH strategic award (ref: 20164). LW's research group is also supported by Great Ormond Street Children's Charity and the GOSH/ICH NIHR Biomedical Research Centre. KW is an Action Medical research clinical training fellow (ref:GN2357).
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1. Petty R, Southwood T, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004; 31:390–392. [PubMed] [Google Scholar]
- 2. Guzman J, Oen K, Tucker LB, et al. The outcomes of juvenile idiopathic arthritis in children managed with contemporary treatments: results from the ReACCh-Out cohort. Ann Rheum Dis 2014; 0:1–7. [DOI] [PubMed] [Google Scholar]
- 3▪. Nigrovic PA. Review: is there a window of opportunity for treatment of systemic juvenile idiopathic arthritis? Arthritis Rheumatol 2014; 66:1405–1413. [DOI] [PubMed] [Google Scholar]; Review on the treatment of systemic-onset JIA, summarizing recent data, and discussion of the pathophysiological benefits of early treatment with interleukin-1 inhibition to possibly decrease the development of chronic arthritis.
- 4. Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004; 364:263–269. [DOI] [PubMed] [Google Scholar]
- 5. Goekoop-Ruiterman YPM, de Vries-Bouwstra JK, Allaart CF, et al. Comparison of Treatment Strategies in Early Rheumatoid ArthritisA Randomized Trial. Annals of Internal Medicine 2007; 146:406–415. [DOI] [PubMed] [Google Scholar]
- 6. Quinn M, Emery P. Window of opportunity in early rheumatoid arthritis: possibility of altering the disease process with early intervention. Clin Exp Rheumatol 2003; 21 (5; SUPP 31):S154–S157. [PubMed] [Google Scholar]
- 7. Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 2010; 69:964–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Annals of the Rheumatic Diseases 2014; 73:492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tynjälä P, Vähäsalo P, Tarkiainen M, et al. Aggressive Combination Drug Therapy in Very Early Polyarticular Juvenile Idiopathic Arthritis (ACUTE–JIA): a multicentre randomised open-label clinical trial. Ann Rheum Dis 2011; 70:1605–1612. [DOI] [PubMed] [Google Scholar]
- 10. Wallace CA, Huang B, Bandeira M, et al. Patterns of clinical remission in select categories of juvenile idiopathic arthritis. Arthritis & Rheumatism 2005; 52:3554–3562. [DOI] [PubMed] [Google Scholar]
- 11. Wallace CA, Giannini EH, Spalding SJ, et al. Trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis. Arthritis Rheum 2012; 64:2012–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wallace CA, Giannini EH, Huang B, et al. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2011; 63:929–936. [DOI] [PubMed] [Google Scholar]
- 13▪. Wallace CA, Giannini EH, Spalding SJ, et al. Clinically inactive disease in a cohort of children with new-onset polyarticular juvenile idiopathic arthritis treated with early aggressive therapy: time to achievement, total duration, and predictors. J Rheumatol 2014; 41:1163–1170. [DOI] [PubMed] [Google Scholar]; This analysis of the original TREAT study confirms that disease duration prior to treatment and early achievement of ACR 70 are strong predictors of achievement of clinically inactive disease.
- 14. Albers HM, Wessels JAM, van der Straaten RJHM, et al. Time to treatment as an important factor for the response to methotrexate in juvenile idiopathic arthritis. Arthritis Care Res 2009; 61:46–51. [DOI] [PubMed] [Google Scholar]
- 15. Consolaro A, Bracciolini G, Ruperto N, et al. Remission, minimal disease activity, and acceptable symptom state in juvenile idiopathic arthritis: defining criteria based on the juvenile arthritis disease activity score. Arthritis Rheum 2012; 64:2366–2374. [DOI] [PubMed] [Google Scholar]
- 16. McErlane F, Beresford MW, Baildam EM, et al. Validity of a three-variable Juvenile Arthritis Disease Activity Score in children with new-onset juvenile idiopathic arthritis. Ann Rheum Dis 2013; 72:1983–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hinze C, Gohar F, Foell D. Management of juvenile idiopathic arthritis: hitting the target. Nat Rev Rheumatol 2015; 11:290–300. [DOI] [PubMed] [Google Scholar]
- 18▪. England N. Interim Clinical Commissioning Policy Statement: Biologic Therapies for the treatment of Juvenile Idiopathic Arthritis (JIA). 2015. Available from: https://www.engage.england.nhs.uk/consultation/specialised-services-policies/user_uploads/biolgcs-juvenl-idiop-arthrs-pol.pdf. [Google Scholar]; New policy statement from NHS England that sets a clear goal of treatment and defined outcome measures. Also includes the use of TNFα inhibition at the start if sacroiliac disease present, and includes rituximab and tocilizumab for refractory JIA.
- 19▪. Ringold S, Weiss PF, Colbert RA, et al. Childhood Arthritis and Rheumatology Research Alliance consensus treatment plans for new-onset polyarticular juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2014; 66:1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]; Standardized consensus-driven treatment plans for treatment of polyarticular JIA released by the Childhood Arthritis and Rheumatology Research Alliance. Importantly, there are three treatment plans, to enable future comparisons of data for treatment approaches.
- 20. Beukelman T, Patkar NM, Saag KG, et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: Initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res 2011; 63:465–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wulffraat NM, Vastert B. Time to share. Pediatr Rheumatol Online J 2013; 11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22▪. Brunner HI, Ruperto N, Zuber Z, et al. Efficacy and safety of tocilizumab in patients with polyarticular-course juvenile idiopathic arthritis: results from a phase 3, randomised, double-blind withdrawal trial. Ann Rheum Dis 2014; 0:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first clinical trial in polyarticular JIA to confirm the safety and efficacy of tocilizumab.
- 23. Mease PJ, Cohen S, Gaylis NB, et al. Efficacy and safety of retreatment in patients with rheumatoid arthritis with previous inadequate response to tumor necrosis factor inhibitors: results from the SUNRISE trial. J Rheumatol 2010; 37:917–927. [DOI] [PubMed] [Google Scholar]
- 24. Lopez-Olivo MA, Amezaga Urruela M, McGahan L, et al. Rituximab for rheumatoid arthritis. Cochrane Database Syst Rev 2015; 1:Cd007356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to antitumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 2006; 54:2793–2806. [DOI] [PubMed] [Google Scholar]
- 26. Narváez J, Díaz-Torné C, Juanola X, et al. Rituximab therapy for refractory systemic-onset juvenile idiopathic arthritis. Ann Rheum Dis 2009; 68:607–608. [DOI] [PubMed] [Google Scholar]
- 27. Feito JG, Pereda CA. Rituximab therapy produced rapid and sustained clinical improvement in a patient with systemic onset juvenile idiopathic arthritis refractory to TNF alpha antagonists. J Clin Rheumatol 2009; 15:363–365. [DOI] [PubMed] [Google Scholar]
- 28. Alexeeva EI, Valieva SI, Bzarova TM, et al. Efficacy and safety of repeat courses of rituximab treatment in patients with severe refractory juvenile idiopathic arthritis. Clin Rheumatol 2011; 30:1163–1172. [DOI] [PubMed] [Google Scholar]
- 29. Cohen MD, Keystone EC. Intravenous golimumab in rheumatoid arthritis. Expert Rev Clin Immunol 2014; 10:823–830. [DOI] [PubMed] [Google Scholar]
- 30. Brunner H, Ruperto N, Tzaribachev N, et al. A148: a multi-center, double-blind, randomized-withdrawal trial of subcutaneous golimumab in pediatric patients with active polyarticular course juvenile idiopathic arthritis despite methotrexate therapy: week 48 results. Arthritis Rheum 2014; 66:S191–S192. [Google Scholar]
- 31. Keystone EC, Genovese MC, Klareskog L, et al. Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann Rheum Dis 2009; 68:789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smolen JS, Kay J, Doyle M, Landewe R, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor alpha inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther 2015; 17:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kavanaugh A, McInnes IB, Mease P, et al. Clinical efficacy, radiographic and safety findings through 5 years of subcutaneous golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of a randomised, placebo-controlled trial (the GO-REVEAL study). Ann Rheum Dis 2014; 73:1689–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deodhar A, Braun J, Inman RD, et al. Golimumab administered subcutaneously every 4 weeks in ankylosing spondylitis: 5-year results of the GO-RAISE study. Ann Rheum Dis 2015; 74:757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keystone E, Heijde DVD, Mason D, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: Findings of a fifty-two–week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 2008; 58:3319–3329. [DOI] [PubMed] [Google Scholar]
- 36. Smolen J, Landewé RB, Mease P, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis 2009; 68:797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fleischmann R, Vencovsky J, van Vollenhoven RF, et al. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann Rheum Dis 2009; 68:805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pediatric Arthritis Study of Certolizumab Pegol (PASCAL). Available from: https://clinicaltrials.gov/ct2/show/study/NCT01550003?show_locs=Y%23locn. [Google Scholar]
- 39. Nair RP, Duffin KC, Helms C, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-κB pathways. Nat Genet 2009; 41:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Karaderi T, Harvey D, Farrar C, et al. Association between the interleukin 23 receptor and ankylosing spondylitis is confirmed by a new UK case–control study and meta-analysis of published series. Rheumatology 2009; 48:386–389. [DOI] [PubMed] [Google Scholar]
- 41. Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008; 371:1665–1674. [DOI] [PubMed] [Google Scholar]
- 42. Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008; 371:1675–1684. [DOI] [PubMed] [Google Scholar]
- 43. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med 2010; 362:118–128. [DOI] [PubMed] [Google Scholar]
- 44. McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 2013; 382:780–789. [DOI] [PubMed] [Google Scholar]
- 45. Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional nonbiological and biological antitumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis 2014; 73:990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. A Study of the Safety and Efficacy of Ustekinumab in Adolescent Patients With Psoriasis (CADMUS) 2015. Available from: https://clinicaltrials.gov/ct2/show/study/NCT01090427. [Google Scholar]
- 47. Poddubnyy D, Hermann KG, Callhoff J, et al. Ustekinumab for the treatment of patients with active ankylosing spondylitis: results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann Rheum Dis 2014; 73:817–823. [DOI] [PubMed] [Google Scholar]
- 48. Kaur K, Kalra S, Kaushal S. Systematic review of tofacitinib: a new drug for the management of rheumatoid arthritis. Clin Ther 2014; 36:1074–1086. [DOI] [PubMed] [Google Scholar]
- 49. Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690 550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013; 381:451–460. [DOI] [PubMed] [Google Scholar]
- 50. Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014; 370:2377–2386. [DOI] [PubMed] [Google Scholar]
- 51. van der Heijde D, Tanaka Y, Fleischmann R, et al. Tofacitinib (CP-690 550) in patients with rheumatoid arthritis receiving methotrexate: Twelve-month data from a twenty-four–month phase III randomized radiographic study. Arthritis Rheum 2013; 65:559–570. [DOI] [PubMed] [Google Scholar]
- 52. van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. Engl J Med 2012; 367:508–519. [DOI] [PubMed] [Google Scholar]
- 53. Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014; 371:1163–1165. [DOI] [PubMed] [Google Scholar]
- 54. Kremer JM, Bloom BJ, Breedveld FC, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690 550 versus placebo. Arthritis Rheum 2009; 60:1895–1905. [DOI] [PubMed] [Google Scholar]
- 55. Pharmacokinetics Of CP-690 550 In Pediatric Patients With Juvenile Idiopathic Arthritis (JIA)NCT01513902 2015. Available from: https://clinicaltrials.gov/ct2/show/NCT01513902. [Google Scholar]
- 56. Foell D, Wulffraat N, Wedderburn LR, et al. Methotrexate withdrawal at 6 vs 12 months in juvenile idiopathic arthritis in remission: a randomized clinical trial. JAMA 2010; 303:1266–1273. [DOI] [PubMed] [Google Scholar]
- 57. Hinks A, Moncrieffe H, Martin P, et al. Association of the 5-aminoimidazole-4-carboxamide ribonucleotide transformylase gene with response to methotrexate in juvenile idiopathic arthritis. Ann Rheum Dis 2011; 70:1395–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moncrieffe H, Ursu S, Holzinger D, et al. A subgroup of juvenile idiopathic arthritis patients who respond well to methotrexate are identified by the serum biomarker MRP8/14 protein. Rheumatology (Oxford) 2013; 52:1467–1476. [DOI] [PubMed] [Google Scholar]
- 59. Cobb J, Cule E, Moncrieffe H, et al. Genome-wide data reveal novel genes for methotrexate response in a large cohort of juvenile idiopathic arthritis cases. Pharmacogenom J 2014; 14:356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bulatovic M, Heijstek MW, Van Dijkhuizen EH, et al. Prediction of clinical nonresponse to methotrexate treatment in juvenile idiopathic arthritis. Ann Rheum Dis 2012; 71:1484–1489. [DOI] [PubMed] [Google Scholar]
- 61. Moncrieffe H, Hinks A, Ursu S, et al. Generation of novel pharmacogenomic candidates in response to methotrexate in juvenile idiopathic arthritis: correlation between gene expression and genotype. Pharmacogenet Genom 2010; 20:665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]


