Abstract
Purpose:
To report the safety and the effectiveness of deep sclerectomy (DS) with a new nonabsorbable uveoscleral hema implant (Esnoper-Clip) designed to increase trabecular and uveoscleral outflow and to achieve higher intrascleral blebs.
Materials and Methods:
Twenty-seven eyes of 27 patients with open-angle glaucoma, who underwent DS with an Esnoper-Clip implant, were included in this study. All patients were followed up after 12 months.
Results:
A significant decrease in intraocular pressure was observed after surgery, changing from a preoperative mean of 26.6±5.2 mm Hg to a postoperative mean of 15.3±5 mm Hg (P<0.001) at 12 months. There was also a significant reduction in the number of glaucoma drugs needed, varying from 2.5 per patient to 0.3 (P<0.001) 1 year after surgery. The main intrascleral lake height and volume at 12 months was 0.7±0.1 mm and 3.9±1.3 mm3, respectively. No intraoperative complications occurred. The main postoperative complications were a positive Seidel test result at 24 hours in 2 eyes (7.4%), hyphema in 2 eyes (7.4%), and choroidal detachment in 1 eye (3.7%). All these complications resolved successfully. The need for additional mitomycin-C injections was recorded in 4 eyes (14.8%), twice in 2 of them. Twelve eyes (44.4%) underwent postsurgical Nd:YAG laser goniopuncture with a mean time between surgery and this procedure of 4.3 months. Mean intraocular pressure after Nd:YAG laser goniopuncture decreased from 19.2 to 15.5 mm Hg (P<0.001).
Conclusion:
DS with an uveoscleral hema implant (Esnoper-Clip) is a safe and effective technique for the management of open-angle glaucoma.
Key Words: deep sclerectomy, open-angle glaucoma, Esnoper-Clip
Glaucoma is a complex eye disease which is the second cause of blindness in the world. It consists of the progressive deterioration of the layer of retinal nerve fibers and of the optic nerve, which lead to the appearance of defects in the visual field and of atrophy of the optic nerve. In the majority of cases, this pathology is associated with increased intraocular pressure (IOP) due to the inhibition of correct drainage of the aqueous humor of the eye. The management of glaucoma requires chronic treatment with a spectrum of therapeutic options including drugs, laser treatment, incisional filtration surgery, drainage devices, and surgical implants.
Deep sclerectomy (DS) is a nonpenetrating procedure for the treatment of open-angle glaucoma (OAG). This can be enhanced with intraoperative mitomycin-C (MMC) or with the use of implants designed to avoid secondary collapse by maintaining the space created after removing the deep scleral flap. These devices can be implanted intrasclerally or in the supraciliary space, with a view to facilitating trabecular and uveoscleral outflow. Its implantation in the scleral bed has been more widely practiced, but there are also a few reports of the placement of the implant in the supraciliary space1,2 which indicate that it may be a promising alternative. It is still unknown whether placing an implant in the supraciliary space produces a higher reduction in IOP than in the scleral space, but the maintenance of both spaces seems to be related to satisfactory IOP control. One problem of supraciliary implantation is that the size of the intrascleral lake could be reduced and this could lead to less IOP reduction.1 Esnoper-Clip is a new nonreabsorbable foldable hema implant with 2 ft, one for the intrascleral bed and the other for the supraciliary space. Its shape seeks to increase the trabecular and uveoscleral outflow, maintaining both spaces and achieve higher intrascleral blebs. The aim of this study was to evaluate the safety and effectiveness of this implant after a 12-month follow-up and if it could be an alternative to existing implants.
MATERIALS AND METHODS
The implant used for all patients was the Esnoper-Clip (AJL Ophthalmics, Álava, Spain), a new uveoscleral hema implant developed at the Glaucoma Unit of the Hospital Universitari Germans Trias i Pujol, Barcelona (Universitat Autonoma of Barcelona, Spain). It is a nonreabsorbable and foldable hema implant (2-hydroxyethyl methacrylate) with 2 ft designed to maintain supraciliary and intrascleral spaces and to achieve higher intrascleral blebs (Fig. 1).
FIGURE 1.

The new foldable uveoscleral hema implant (Esnoper-Clip) designed and developed at the Glaucoma Unit of the Hospital Universitari Germans Trias i Pujol, Barcelona (Universitat Autonoma of Barcelona), Spain.
Patients
This study, to evaluate the safety and effectiveness of Esnoper-Clip implants, involved a prospective analysis of 27 eyes from 27 patients with OAG who underwent DS with an uveoscleral implant (Esnoper-Clip) implant between October 2011 and January 2013 followed up 1 year after surgery. Their surgical history included 16 uneventful phacoemulsifications, performed at least 6 months before surgery. All patients were over 18 years of age and presented with uncontrollable primary or secondary OAG IOP (≥21 mm Hg), or were under maximal tolerable medical treatment. They signed the consent form approved by the Department of Ophthalmology of the Hospital Germans Trias i Pujol (Barcelona, Spain) and the study was conducted in accordance with the principles established in the Declaration of Helsinki and was approved by the Institutional Review Board of the Hospital Germans Trias I Pujol (Barcelona, Spain). Exclusion criteria were prior antiglaucoma surgery, or any other surgery which could have affected the conjunctiva in the region of the intervention, including cataract surgery 6 months before the present intervention, moderate or severe diabetic retinopathy, and other causes of ocular neovascularization. We also excluded glaucoma patients with a high risk of failure, that is those presenting with neovascularization, aphakia, inflammation, juvenile cases, and cases of posttrauma and postsurgery glaucoma.
In the preoperative assessment, patients underwent a complete examination including best-corrected visual acuity (BCVA) on a decimal scale, biomicroscopy, Goldmann tonometry, pachymetry (UP-1000; Nidek, Japan) gonioscopy, fundoscopy, and the glaucoma therapy was recorded.
Follow-up visits were scheduled at 24 hours, 1, 3, 6, and 12 months after surgery, and included BCVA, slit-lamp examination, tonometry, gonioscopy, and fundoscopy. Anterior segment optical coherence tomography (AS-OCT) (Visante OCT; Carl Zeiss Meditec, Jena, Germany) which provides an objective method to assess the anterior segment of the eye and the surgical area, was performed at months 1, 3, 6, and 12, to the intrascleral bleb height at 90 and 180 degrees and the highest was chosen. To determinate the volume of the intrascleral lake, the semiellipsoid has been considered to be the most similar geometric shape. We calculated its value from the height (H), the vertical diameter or length (L), and the horizontal diameter or width (W) using the formula Volume=1/6×π×H×L×W. AS-OCT measurements and analysis were performed by the same investigator (J.C.M.).
Surgical Technique
All surgical operations were performed by the same surgeon (J.L.A.). A superior 4/0 nylon traction suture was passed through the superior cornea and a fornix-based conjunctival flap was dissected (Fig. 2A), followed by cauterization of bleeding vessels and dissection of the superficial scleral flap (5×5 mm2) of one third of the scleral depth, extending 2 mm into clear cornea. Afterwards, a MMC impregnated sheet was laid between the scleral flap and the remaining sclera as well as over the flap, and left there for 2 minutes and then irrigated thoroughly with balanced salt solution. Subsequently, a deeper 4×4 mm2 scleral flap was dissected and removed, and Schlemm canal was deroofed with a capsulorhexis forceps. The implant has 2 plates; 1 was placed in a full-thickness suprachoroidal bag 2 mm behind the scleral spur, after the technique firstly described by Muñoz1 (Fig. 2B). After folding the implant the other foot was placed into the intrascleral lake (Fig. 2C). It can be fixed without suturing because it has 2 lateral notches that do not allow anterior displacement. To achieve higher intrascleral blebs, an intrascleral pocket was created at the posterior limit of the intrascleral bed to fix the posterior edge of the implant, thereby helping to keep the scleral lips apart (Fig. 2D). No suture or a loose one, was used for the superficial scleral flap and the conjunctiva was sutured with a nylon 10/0. Postoperative treatment consisted of topical ofloxacin (Exocin; Alcon Cusí SA, El Masnou, Barcelona, Spain) 4 times a day during 1 week and prednisolone acetate (10 mg/mL) (Predforte; Allergan Pharmaceuticals Ireland, Westport, Ireland) 6 times a day, the latter in a descending dosage over 6 weeks. Goniopuncture with the YAG laser (Visulas YAG III Combi; Carl Zeiss Meditec, Dublin, CA) was performed when percolation of aqueous humor was considered insufficient.
FIGURE 2.
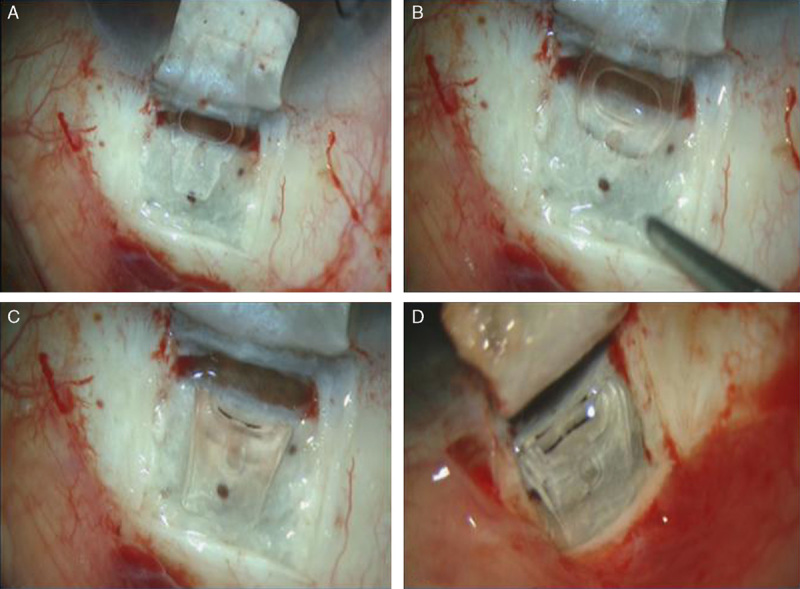
Surgical technique. A, Superficial scleral flap (5×5 mm2) of one third of the scleral depth, reaching 2 mm of clear cornea. B, Deep 4×4 mm2 scleral flap is dissected and removed, and Schlemm canal was deroofed with a capsulorhexis forceps. The implant has 2 plates; one was placed in a full-thickness suprachoroidal bag 2 mm behind the scleral spur. C, After folding the implant the other foot was placed into the intrascleral lake. It can be fixed without suturing because it has 2 lateral notches that do not allow anterior displacement. D, To achieve higher intrascleral blebs, an intrascleral pocket was created at the posterior limit of the intrascleral bed to fix the posterior edge of the implant, thereby helping to keep the scleral lips apart.
Statistical Analysis
Statistical analyses were performed using SPSS software for Windows (version 15.0; SPSS Inc., Chicago, IL). All data were reported as means with SD. The differences in continuous variables before and after surgery were compared statistically using the nonparametric Friedman test. Differences in presurgery IOP values and those measured at each follow-up were analyzed using the Wilcoxon matched-pairs signed-rank test. Probability values <0.05 were considered to be statistically significant.
RESULTS
Demographics and characteristics of patients included in this study are shown in Table 1. Mean preoperative IOP was 26.6±5.2 mm Hg. Twelve months later, we found a statistically significant reduction in IOP which had reached a mean of 15.3±5 mm Hg (P<0.001). This change represents a 42.5% reduction in IOP reduction (Table 2). There was also a statistically significant reduction in the number of medications after surgery, starting with a mean of 2.5 and arriving at 0.3 at the end of the study (P<0.001) (Fig. 3). The most common type of glaucoma that was included in the study was primary open-angle glaucoma (88.9% of patients). The mean volume and height of the intrascleral bleb 12 months after surgery were 3.9±1.3 mm3 and 0.7±0.1 mm, respectively (Fig. 3).
TABLE 1.
Clinical and Demographic Characteristics of Patients Included in the Study
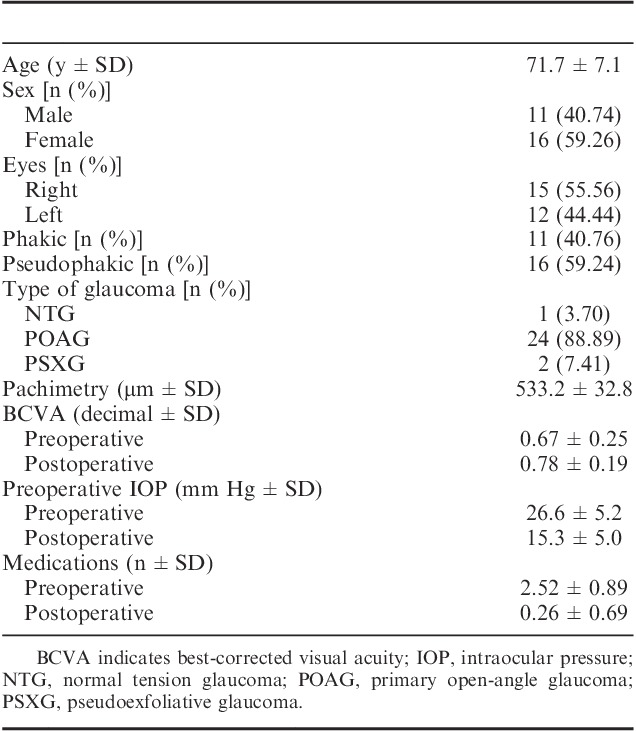
TABLE 2.
Intraocular Pressure Evolution and Numbers of Medications
FIGURE 3.
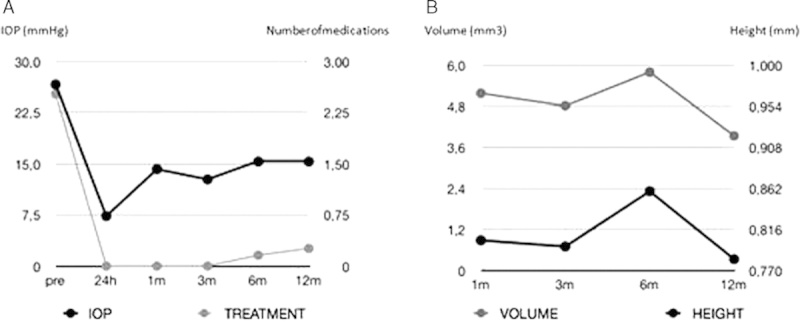
A, Mean changes in intraocular pressure (IOP) and number of medications (treatment). B, Mean changes in volume and height of the intrascleral lake.
No intraoperative complications occurred. The main postoperative complications were Seidel at 24 hours in 2 eyes (7.4%), hyphema in 2 eyes (7.4%), choroidal detachment in 1 eye (3.7%), all of which resolved successfully and the need for additional MMC injections in 4 eyes (14.8%) (in 2 eyes, on 2 occasions). No statistically significant variation was found between preoperative and postoperative BCVA (P=0.507). Twelve eyes (44.4%) underwent postsurgical Nd:YAG goniopuncture (Nd:YAG GP) with a mean time between surgery and this procedure of 4.3 months. Complications related to goniopuncture were limited to choroidal detachment in 1 eye that resolved uneventfully. In this case, IOP dropped from 20 to 2 mm Hg. The mean IOP after Nd:YAG GP decreased from 19.2 to 15.5 mm Hg (P<0.001).
DISCUSSION
DS with a supraciliary implant is an alternative that has been shown to be safe and effective with different types of implants.1,2 The main problem with this class of implants is that they are not specifically designed for this space, and so their effectiveness can be limited. The intrascleral lake can lose height or even collapse over time and probably lose efficacy, because intrascleral bleb height plays an important role in lowering IOP.3–5 In contrast, intrascleral implants do not facilitate uveoscleral outflow as well as supraciliary implants.
Reports of supraciliary implantation are somewhat scarce and until now no studies with ultrasound biomicroscopy (UBM) or AS-OCT have been published. Muñoz1 reported isolated DS and Bonilla et al2 described phaco-DS resulting in IOP reductions from 26.4±6.9 to 14±3.3 mm Hg and 23±5 to 18±3 mm Hg, respectively, after 1 year. In these series, the number of medications was reduced from 2.8 to 0.3 and from 2.5 to 0.7, respectively. In our series, IOP decreased from 26.6 to 15.3 mm Hg and the number of drugs to be taken was reduced from 2.5 to 0.26 at 12 months. The incidence of complications is similar to that associated with intrascleral or supraciliary implantation described by other authors. We can conclude that the present technique has no more additional complications for DS than other alternatives.
The importance and significance of the intrascleral and suprachoroidal spaces is a controversial issue, but currently it is widely accepted that both are good prognostic factors, but not the ones. This controversy may be due to discrepancies in findings using AS-OCT or UBM. UBM measurements have limited resolution and with AS-OCT, it is very difficult to determine the presence or absence of uveoscleral outflow.
Mavrakanas et al3 and Fernandez-Buenaga et al4 using AS-OCT, Perez-Rico without impant and Marchini et al5 using UBM reported a positive inverse correlation between postoperative IOP and intrascleral bleb height. Mavrakanas et al3 only investigated eyes having DS with a collagen implant and postoperatively clinically flat blebs, whereas Fernández-Buenaga et al4 investigated a heterogeneous group of eyes after DS using 3 different implants (Aquaflow, Esnoper, SK-Gel). Both authors found bleb heights between 0.58 and 0.65 mm, respectively, and found a significant correlation between height and IOP with Esnoper and Aquaflow implants. Here, we have not studied the correlation between IOP and intrascleral volume or height, but we did calculate the mean median bleb height (0.78±0.1 mm) and volume (3.9±1.3 mm3) 12 months after operation. These values in volume (P=0.706) or height (P=0.586) were not significantly different to those before operation.
Another point to be considered is that the shape of our implant tries to keep the sclera lips apart, thereby facilitating transcleral outflow (Fig. 4). This could decrease the height and volume of the intrascleral lake but in contrast, transcleral outflow represents a link between the intrascleral lake and the conjunctival filtering blebs and it had been found withUBM6 to be a positive prognostic factor. Perez-Rico et al5 using AS-OCT reported transcleral outflow to be a positive factor in 89.4% of eyes, 5 years after DS without implant. This finding brings into question the mandatory use of implants in DS. The same author reported the association between lower IOP and maximum anteroposterior length, height, and intrascleral volume.
FIGURE 4.
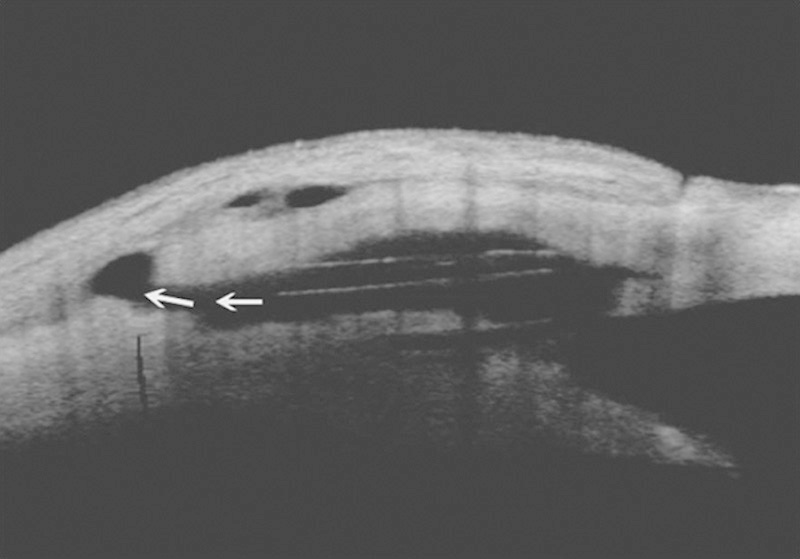
Anterior segment optical coherence tomography. The intrascleral lake and supraciliary space after the implantation with transcleral outflow (arrows) can be observed.
It had been published, in studies with UBM, as the tissue heals with scarring occupying the intrascleral lake6 over time in almost 10% of eyes with7 or without implants.8 Using AS-OCT in eyes which have undergone DS without implant, the presence of the intrascleral lake had been revealed in 100% of the eyes after 5 years.5 Discrepancies in these results are probably related to the trabeculo-Descemet membrane (TDM) thickness and to the fact that if there is insufficient peeling of the inner wall of Schlemm canal, enough outflow will not be achieved to maintain the intrascleral lake.
Uveoscleral outflow in different series has a prevalence from 7% to 60%.9,10 However, these series are hardly comparable because they used different implants with varying follow-up times. Using UBM7,11 it has been found that eyes with suprachoroidal filtration have lower IOP than those without suprachoroidal filtration, whereas other authors8 could find no evidence of suprachoroidal filtration in any case, 1 year after surgery. Ravinet et al12 also found a similar rate of IOP and postoperative treatments in eyes with or without hypoechoic areas after DS with T-Flux and Healon GV. Supraciliary implantation probably favors either a ciliary body detachment with subsequent decrease in aqueous production, or choroidal resorption leading to low postoperative IOP. Although it seems logical to think that this could encourage late chronic ocular hypotony, there is no evidence of this in the literature or in the present series. Nevertheless, it should not be forgotten that young myopics are probably more likely to suffer from hypotony with supraciliary implantation.
The reported incidence of goniopuncture varies drastically from 4.7% to 63%. This wide variation is likely due to the application of different criteria, implants, and IOP targets. The type of implant is likely a very significant factor in Nd:YAG GP as some implants may enhance more fibrosis over the TDM, or displacement above the TDM. This new device which we report here has 2 lateral notches that prevent this from happening, and even in cases in which it does not remain fixed in the suprachoroidal bag, its shape does not collapse the TDM which remains always free without any filtration limitations.
On the basis of the present findings, the uveoscleral implant (Esnoper-Clip) can be considered to be a promising alternative because it ensures the maintenance of both spaces helping to avoid collapse over time. Further clinical trials with long-term follow-up and a larger numbers of patients will be needed to assess the effectiveness of this new uveoscleral implant, the morphology of the surgical area, the real relevance of the intrascleral lake characteristics, and their correlation with reduced IOP.
Footnotes
AJL Ophthalmics supports the Health Sciences Research Institute “Germans Trias i Pujol” Foundation.
Disclosure: The authors declare no conflict of interest
REFERENCES
- 1.Muñoz G. Nonstitch suprachoroidal technique for T-flux® implantation in deep sclerectomy. J Glaucoma. . 2009;18:262–264. [DOI] [PubMed] [Google Scholar]
- 2.Bonilla R, Loscos J, Valldeperas X, et al. Supraciliary hema implant in combined deep sclerectomy and phacoemulsification: one year results. Open Ophthalmol J. . 2012;6:59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mavrakanas N, Mendrinos E, Shaarawy T. Postoperative IOP is related to intrascleral bleb height in eyes with clinically flat blebs following deep sclerectomy with collagen implant and mitomycin. Br J Ophthalmol. . 2010;94:410–413. [DOI] [PubMed] [Google Scholar]
- 4.Fernández-Buenaga R, Rebolleda G, Casas-Llera P, et al. A comparison of intrascleral bleb height by anterior segment OCT using three different implants in deep sclerectomy. Eye (Lond). . 2012;26:552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pérez-Rico C, Gutiérrez-Ortíz C, Moreno-Salgueiro A, et al. Visante anterior segment optical coherence tomography analysis of morphologic changes after deep sclerectomy with intraoperative mitomycin-C and no implant use. J Glaucoma. . 2014;23:e86–e90. [DOI] [PubMed] [Google Scholar]
- 6.Marchini G, Marraffa M, Brunelli C, et al. Ultrasound biomicroscopy and intraocular-pressure-lowering mechanisms of deep sclerectomy with reticulated hyaluronic acid implant. J Cataract Refract Surg. . 2001;27:507–517. [DOI] [PubMed] [Google Scholar]
- 7.Kazakova D, Roters S, Schnyder CC, et al. Ultrasound biomicroscopy images: long-term results after deep sclerectomy with collagen implant. Graefes Arch Clin Exp Ophthalmol. . 2002;240:918–923. [DOI] [PubMed] [Google Scholar]
- 8.Khairy HA, Atta HR, Green FD, et al. Ultrasound biomicroscopy in deep sclerectomy. Eye (Lond). . 2005;19:555–560. [DOI] [PubMed] [Google Scholar]
- 9.Chiou AG, Mermoud A, Underdahl JP, et al. An ultrasound biomicroscopic study of eyes after deep sclerectomy with collagen implant. Ophthalmology. . 1998;105:746–750. [DOI] [PubMed] [Google Scholar]
- 10.Contreras I, Noval S, Muñoz-Negrete FJ, et al. Ultrasound biomicroscopy in deep sclerectomy with a new acrylic implant. Arch Soc Esp Oftalmol. . 2006;81:445–450. [DOI] [PubMed] [Google Scholar]
- 11.Cabrejas L, Rebolleda G, Muñoz-Negrete FJ, et al. An ultrasound biomicroscopy study of filtering blebs after deep sclerectomy with a new acrylic implant. Eur J Ophthalmol. . 2010;22:391–399. [DOI] [PubMed] [Google Scholar]
- 12.Ravinet E, Bovey E, Mermoud A. T-Flux® implant versus Healon® GV in deep sclerectomy. J Glaucoma. . 2004;13:46–50. [DOI] [PubMed] [Google Scholar]


