Abstract
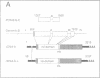
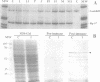
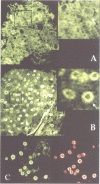
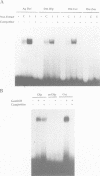
A novel rel family member, Gambif1 (gambiae immune factor 1), has been cloned from the human malaria vector, Anopheles gambiae, and shown to be most similar to Drosophila Dorsal and Dif. Gambif1 protein is translocated to the nucleus in fat body cells in response to bacterial challenge, although the mRNA is present at low levels at all developmental stages and is not induced by infection. DNA binding activity to the kappaB-like sites in the A.gambiae Defensin and the Drosophila Diptericin and Cecropin promoters is also induced in larval nuclear extracts following infection. Gambif1 has the ability to bind to kappaB-like sites in vitro. Co-transfection assays in Drosophila mbn-2 cells show that Gambif1 can activate transcription by interacting with the Drosophila Diptericin regulatory elements, but is not functionally equivalent to Dorsal in this assay. Gambif1 protein translocation to the nucleus and the appearance of kappaB-like DNA binding activity can serve as molecular markers of activation of the immune system and open up the possibility of studying the role of defence reactions in determining mosquito susceptibility/refractoriness to malaria infection.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abastado J. P., Miller P. F., Hinnebusch A. G. A quantitative model for translational control of the GCN4 gene of Saccharomyces cerevisiae. New Biol. 1991 May;3(5):511–524. [PubMed] [Google Scholar]
- Beg A. A., Sha W. C., Bronson R. T., Ghosh S., Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995 Jul 13;376(6536):167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- Böhnlein E., Lowenthal J. W., Siekevitz M., Ballard D. W., Franza B. R., Greene W. C. The same inducible nuclear proteins regulates mitogen activation of both the interleukin-2 receptor-alpha gene and type 1 HIV. Cell. 1988 Jun 3;53(5):827–836. doi: 10.1016/0092-8674(88)90099-2. [DOI] [PubMed] [Google Scholar]
- Chalk R., Townson H., Natori S., Desmond H., Ham P. J. Purification of an insect defensin from the mosquito, Aedes aegypti. Insect Biochem Mol Biol. 1994 Apr;24(4):403–410. doi: 10.1016/0965-1748(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Deryckere F., Gannon F. A one-hour minipreparation technique for extraction of DNA-binding proteins from animal tissues. Biotechniques. 1994 Mar;16(3):405–405. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström Y., Kadalayil L., Sun S. C., Samakovlis C., Hultmark D., Faye I. kappa B-like motifs regulate the induction of immune genes in Drosophila. J Mol Biol. 1993 Jul 20;232(2):327–333. doi: 10.1006/jmbi.1993.1392. [DOI] [PubMed] [Google Scholar]
- Gay N. J., Keith F. J. Drosophila Toll and IL-1 receptor. Nature. 1991 May 30;351(6325):355–356. doi: 10.1038/351355b0. [DOI] [PubMed] [Google Scholar]
- Geisler R., Bergmann A., Hiromi Y., Nüsslein-Volhard C. cactus, a gene involved in dorsoventral pattern formation of Drosophila, is related to the I kappa B gene family of vertebrates. Cell. 1992 Nov 13;71(4):613–621. doi: 10.1016/0092-8674(92)90595-4. [DOI] [PubMed] [Google Scholar]
- Georgel P., Kappler C., Langley E., Gross I., Nicolas E., Reichhart J. M., Hoffmann J. A. Drosophila immunity. A sequence homologous to mammalian interferon consensus response element enhances the activity of the diptericin promoter. Nucleic Acids Res. 1995 Apr 11;23(7):1140–1145. doi: 10.1093/nar/23.7.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgel P., Meister M., Kappler C., Lemaitre B., Reichhart J. M., Hoffmann J. A. Insect immunity: the diptericin promoter contains multiple functional regulatory sequences homologous to mammalian acute-phase response elements. Biochem Biophys Res Commun. 1993 Dec 15;197(2):508–517. doi: 10.1006/bbrc.1993.2508. [DOI] [PubMed] [Google Scholar]
- Ghosh G., van Duyne G., Ghosh S., Sigler P. B. Structure of NF-kappa B p50 homodimer bound to a kappa B site. Nature. 1995 Jan 26;373(6512):303–310. doi: 10.1038/373303a0. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990 Apr 12;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Gifford A. M., Riviere L. R., Tempst P., Nolan G. P., Baltimore D. Cloning of the p50 DNA binding subunit of NF-kappa B: homology to rel and dorsal. Cell. 1990 Sep 7;62(5):1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- Gilmore T. D. NF-kappa B, KBF1, dorsal, and related matters. Cell. 1990 Sep 7;62(5):841–843. doi: 10.1016/0092-8674(90)90257-f. [DOI] [PubMed] [Google Scholar]
- Gwadz R. W., Kaslow D., Lee J. Y., Maloy W. L., Zasloff M., Miller L. H. Effects of magainins and cecropins on the sporogonic development of malaria parasites in mosquitoes. Infect Immun. 1989 Sep;57(9):2628–2633. doi: 10.1128/iai.57.9.2628-2633.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann J. A. Innate immunity of insects. Curr Opin Immunol. 1995 Feb;7(1):4–10. doi: 10.1016/0952-7915(95)80022-0. [DOI] [PubMed] [Google Scholar]
- Hoyos B., Ballard D. W., Böhnlein E., Siekevitz M., Greene W. C. Kappa B-specific DNA binding proteins: role in the regulation of human interleukin-2 gene expression. Science. 1989 Apr 28;244(4903):457–460. doi: 10.1126/science.2497518. [DOI] [PubMed] [Google Scholar]
- Hultmark D. Immune reactions in Drosophila and other insects: a model for innate immunity. Trends Genet. 1993 May;9(5):178–183. doi: 10.1016/0168-9525(93)90165-e. [DOI] [PubMed] [Google Scholar]
- Ip Y. T., Reach M., Engstrom Y., Kadalayil L., Cai H., González-Crespo S., Tatei K., Levine M. Dif, a dorsal-related gene that mediates an immune response in Drosophila. Cell. 1993 Nov 19;75(4):753–763. doi: 10.1016/0092-8674(93)90495-c. [DOI] [PubMed] [Google Scholar]
- Kappler C., Meister M., Lagueux M., Gateff E., Hoffmann J. A., Reichhart J. M. Insect immunity. Two 17 bp repeats nesting a kappa B-related sequence confer inducibility to the diptericin gene and bind a polypeptide in bacteria-challenged Drosophila. EMBO J. 1993 Apr;12(4):1561–1568. doi: 10.1002/j.1460-2075.1993.tb05800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Matsui M., Kubo T., Natori S. Purification and characterization of a 59-kilodalton protein that specifically binds to NF-kappa B-binding motifs of the defense protein genes of Sarcophaga peregrina (the flesh fly). Mol Cell Biol. 1993 Jul;13(7):4049–4056. doi: 10.1128/mcb.13.7.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B., Kromer-Metzger E., Michaut L., Nicolas E., Meister M., Georgel P., Reichhart J. M., Hoffmann J. A. A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9465–9469. doi: 10.1073/pnas.92.21.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Lowenberger C., Bulet P., Charlet M., Hetru C., Hodgeman B., Christensen B. M., Hoffmann J. A. Insect immunity: isolation of three novel inducible antibacterial defensins from the vector mosquito, Aedes aegypti. Insect Biochem Mol Biol. 1995 Jul;25(7):867–873. doi: 10.1016/0965-1748(95)00043-u. [DOI] [PubMed] [Google Scholar]
- Meister M., Braun A., Kappler C., Reichhart J. M., Hoffmann J. A. Insect immunity. A transgenic analysis in Drosophila defines several functional domains in the diptericin promoter. EMBO J. 1994 Dec 15;13(24):5958–5966. doi: 10.1002/j.1460-2075.1994.tb06941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Müller C. W., Rey F. A., Sodeoka M., Verdine G. L., Harrison S. C. Structure of the NF-kappa B p50 homodimer bound to DNA. Nature. 1995 Jan 26;373(6512):311–317. doi: 10.1038/373311a0. [DOI] [PubMed] [Google Scholar]
- Nolan G. P., Ghosh S., Liou H. C., Tempst P., Baltimore D. DNA binding and I kappa B inhibition of the cloned p65 subunit of NF-kappa B, a rel-related polypeptide. Cell. 1991 Mar 8;64(5):961–969. doi: 10.1016/0092-8674(91)90320-x. [DOI] [PubMed] [Google Scholar]
- Petersen U. M., Björklund G., Ip Y. T., Engström Y. The dorsal-related immunity factor, Dif, is a sequence-specific trans-activator of Drosophila Cecropin gene expression. EMBO J. 1995 Jul 3;14(13):3146–3158. doi: 10.1002/j.1460-2075.1995.tb07317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichhart J. M., Georgel P., Meister M., Lemaitre B., Kappler C., Hoffmann J. A. Expression and nuclear translocation of the rel/NF-kappa B-related morphogen dorsal during the immune response of Drosophila. C R Acad Sci III. 1993 Oct;316(10):1218–1224. [PubMed] [Google Scholar]
- Richman A. M., Bulet P., Hetru C., Barillas-Mury C., Hoffmann J. A., Kafalos F. C. Inducible immune factors of the vector mosquito Anopheles gambiae: biochemical purification of a defensin antibacterial peptide and molecular cloning of preprodefensin cDNA. Insect Mol Biol. 1996 Aug;5(3):203–210. doi: 10.1111/j.1365-2583.1996.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez M. C., Zamudio F., Torres J. A., Gonzalez-Ceron L., Possani L. D., Rodriguez M. H. Effect of a cecropin-like synthetic peptide (Shiva-3) on the sporogonic development of Plasmodium berghei. Exp Parasitol. 1995 Jun;80(4):596–604. doi: 10.1006/expr.1995.1075. [DOI] [PubMed] [Google Scholar]
- Rushlow C., Frasch M., Doyle H., Levine M. Maternal regulation of zerknüllt: a homoeobox gene controlling differentiation of dorsal tissues in Drosophila. Nature. 1987 Dec 10;330(6148):583–586. doi: 10.1038/330583a0. [DOI] [PubMed] [Google Scholar]
- Salazar C. E., Mills-Hamm D., Kumar V., Collins F. H. Sequence of a cDNA from the mosquito Anopheles gambiae encoding a homologue of human ribosomal protein S7. Nucleic Acids Res. 1993 Aug 25;21(17):4147–4147. doi: 10.1093/nar/21.17.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Sha W. C., Liou H. C., Tuomanen E. I., Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995 Jan 27;80(2):321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Franzoso G., Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- Sinden R. E. The biology of Plasmodium in the mosquito. Experientia. 1984 Dec 15;40(12):1330–1343. doi: 10.1007/BF01951886. [DOI] [PubMed] [Google Scholar]
- Steward R. Dorsal, an embryonic polarity gene in Drosophila, is homologous to the vertebrate proto-oncogene, c-rel. Science. 1987 Oct 30;238(4827):692–694. doi: 10.1126/science.3118464. [DOI] [PubMed] [Google Scholar]
- Steward R., Zusman S. B., Huang L. H., Schedl P. The dorsal protein is distributed in a gradient in early Drosophila embryos. Cell. 1988 Nov 4;55(3):487–495. doi: 10.1016/0092-8674(88)90035-9. [DOI] [PubMed] [Google Scholar]
- Sun S. C., Faye I. Cecropia immunoresponsive factor, an insect immunoresponsive factor with DNA-binding properties similar to nuclear-factor kappa B. Eur J Biochem. 1992 Mar 1;204(2):885–892. doi: 10.1111/j.1432-1033.1992.tb16708.x. [DOI] [PubMed] [Google Scholar]
- Thisse C., Perrin-Schmitt F., Stoetzel C., Thisse B. Sequence-specific transactivation of the Drosophila twist gene by the dorsal gene product. Cell. 1991 Jun 28;65(7):1191–1201. doi: 10.1016/0092-8674(91)90014-p. [DOI] [PubMed] [Google Scholar]
- Thompson J. D., Higgins D. G., Gibson T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994 Nov 11;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg A., Miller L. H. Critical stages in the development of Plasmodium in mosquitoes. Parasitol Today. 1991 Jul;7(7):179–181. doi: 10.1016/0169-4758(91)90127-a. [DOI] [PubMed] [Google Scholar]
- Weih F., Carrasco D., Durham S. K., Barton D. S., Rizzo C. A., Ryseck R. P., Lira S. A., Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 1995 Jan 27;80(2):331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]