Abstract
Introduction
Fingolimod 0.5 mg is an orally active sphingosine 1-phosphate receptor modulator approved for use in adults with relapsing multiple sclerosis (MS). The efficacy and safety profile of fingolimod has been well characterized in a large clinical development program. Here, we report the safety and tolerability of fingolimod in relapsing-remitting MS (RRMS) patients from Latin America.
Methods
A total of 162 patients with RRMS, predominantly from Latin American countries (138/162), were enrolled in this 16-week, single treatment arm, open-label, multi-center study. Unlike the phase III pivotal studies, this study permitted enrollment of patients with controlled diabetes, certain cardiac and pulmonary conditions, older age, and higher baseline Expanded Disability Status Scale. All patients were monitored clinically for a minimum of 6 hours after the first dose. Safety and tolerability assessments were based on adverse events, clinically notable laboratory abnormalities, vital signs, ophthalmic examinations, and electrocardiograms.
Results
Overall, the safety and tolerability profile was consistent with that reported previously in phase 3 studies and the FIRST study. Adverse events (AEs) were predominantly mild (n = 49, 35.5%) or moderate (n = 27, 19.6%). Three patients (2.2%) discontinued fingolimod due to AEs. Infections were reported in 33 patients (23.9%) and were predominantly mild in nature (n = 28, 20.3%). Increases in alanine aminotransferase enzymes of ≥3, ≥5 and ≥10 upper limit of normal were reported in five (3.7%), three (2.2%) and one (0.7%) patients, respectively. Hypertension cases (n = 3; 2.2%) did not result in treatment discontinuation and were controlled with antihypertensive therapy. Following first-dose administration, the majority of patients (90.6%) were discharged at 6 h. During the first-dose monitoring, 5 cases of bradycardia were reported; none required extended monitoring or treatment for symptomatic bradycardia.
Conclusion
The first dose of fingolimod 0.5 mg was well tolerated in RRMS patients from Latin America. The overall safety profile was clinically manageable and consistent with previous fingolimod studies.
Funding
Novartis.
Trial registration: ClinicalTrials.gov #NCT01497262.
Electronic supplementary material
The online version of this article (doi:10.1007/s12325-015-0224-2) contains supplementary material, which is available to authorized users.
Keywords: Fingolimod, First-dose observations, FIRST LATAM, Latin American, Relapsing-remitting multiple sclerosis, Safety
Introduction
Multiple sclerosis (MS) is one of the most common, autoimmune, inflammatory, demyelinating diseases of the central nervous system in young adults, affecting approximately 2.3 million people worldwide [1, 2]. Differences in the risk of MS and the disease course have been reported earlier in the Latin American population as compared to the well-studied Caucasian population [3–8]. For example, the Latin American MS population has higher age at disease onset and slower rate of progression of disability over time [4]. Furthermore, there is growing evidence suggestive of an increased frequency of MS incidence in this region, attributable mainly to the improved awareness of the disease [4, 8]. The expansion of treatment options for relapsing-remitting MS (RRMS), including high efficacy agents, offers the possibility of controlling suboptimal disease activity [8]. The availability of ethnically relevant clinical evidence is important for clinical management at the individual patient level [8].
Once-daily oral fingolimod 0.5 mg (Gilenya™, Novartis Pharma AG, Basel, Switzerland) is a first-in class sphingosine 1-phosphate receptor modulator approved for the treatment of relapsing forms of MS with a well-characterized efficacy and safety profile in a large clinical development program [9–12]. The safety and tolerability in the Hispanic patients were consistent with the overall study population in the fingolimod clinical trial program [13]. Smaller studies at country level in the region have provided further evidence of the safety and tolerability of fingolimod use in MS patients from Latin America; however, more comprehensive data on first-dose observation are still lacking [13–16].
The present study was aimed to provide additional data on the short-term safety and tolerability of fingolimod in more ethnically relevant MS patient population in Latin American countries.
Methods
Study Population and Study Design
FIRST LATAM (ClinicalTrials.gov Identifier: NCT01497262) was a 16-week, open-label, multinational, single treatment arm, safety study conducted in eight countries (Argentina, Brazil, Colombia, Mexico, Panama, Peru, Jordan and Malaysia). Data reported here include patients recruited at 26 centers across the Latin American countries (excluding Jordan and Malaysia). The study included 1–2 weeks of screening phase to determine eligibility. Standard 12-lead electrocardiograms (ECG) were performed at screening and pre-dose as part of the eligibility assessment. Eligible patients were males and females diagnosed with RRMS using the revised 2005 McDonald’s criteria [17]. Expanded eligibility criteria were applied to include patients with older age (age limit 18–65 years) and high baseline Expanded Disability Status Scale (EDSS) score range of 0–6.5. Patients with controlled diabetes mellitus (hemoglobin A1c [HbA1c] ≤7%) and certain cardiac and pulmonary conditions, excluded from pivotal studies, were also included. Patients with a manifestation of MS other than RRMS, active infection, macular edema, uncontrolled diabetes (HbA1c >7%), immunosuppression (either drug- or disease-induced), or had received treatment with corticosteroids or immunoglobulins within the previous month, or treatment with immunosuppressive medications or monoclonal antibodies within the previous 3 months were not eligible for enrollment in the study. Patients previously treated with fingolimod were not eligible to participate. Other exclusion criteria included any clinically significant cardiac, pulmonary, or hepatic disease, or any immunologic laboratory abnormalities.
All eligible patients were treated with fingolimod 0.5 mg for 16 weeks. Upon completion of the open-label treatment phase, the patients had the option of entering an extension study.
The FIRST LATAM study was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice [18] and the Declaration of Helsinki 1964, as revised in 2013 [19]. The protocol was approved by the Independent Ethics Committee or Institutional Review Board for each center. Each patient provided written informed consent before enrollment.
Assessments
The primary variables of the study included the nature and frequency of adverse events (AEs). The safety assessments included reporting of AEs, serious AEs (SAEs), vital signs, physical/neurological examinations, ECG, and laboratory examinations, and were carried out at weeks 2, 8, and 16 (end of study visit), with an ophthalmic examination at week 16. The severity of AEs was defined according to the Common Terminology Criteria (CTC) for AEs (mild, moderate, and severe or CTC grade 1–4).
All patients received the first dose of fingolimod and were monitored for at least 6 h post-dose. First-dose monitoring included an ECG at baseline and at 6 h as well as hourly sitting heart rate (HR) and blood pressure (BP) monitoring. Patients were asked to inform the investigator about any cardiac symptoms or other AEs. The initial discharge criteria after 6 h of cardiac monitoring were: HR ≥51 bpm, HR >80% of baseline value and was not the lowest hourly value, absence of symptoms associated with decreased HR, and ECG at 6 h showing no new significant ECG abnormalities. During the course of the study, following a regulatory authority review, revisions were made to fingolimod labeling and prescribing guidance; consequently, amendments were made to cardiac exclusion and first-dose discharge criteria in the study (extended monitoring required for HR <45 bpm, symptomatic or received treatment for bradycardia, lowest hourly HR, new-onset second-degree or higher atrioventricular block (AVB), QTc ≥500 ms at 6 h).
Statistical Analysis
Primary variables were summarized using descriptive statistics. Categorical variables were presented as numbers and percentages. Continuous variables were summarized as number, mean, standard deviation, and minimum and maximum values. The ECG parameters, vital signs and laboratory data were summarized as mean change from baseline values by visit. All the analyses were performed using SAS 9.3 (The SAS Institute, Cary, NC, USA).
Results
Subject Disposition and Demographic and Clinical Characteristics
The FIRST LATAM study enrolled 162 patients, including the 138 patients presented here from the Latin American countries [Argentina (n = 31), Brazil (n = 31), Colombia (n = 19), Mexico (n = 22), Panama (n = 10), Peru (n = 25)]. Of these, 130 (94.2%) completed the study and 8 (5.8%) discontinued the treatment. The reasons for discontinuation were abnormal laboratory values (n = 2; 1.4%), AEs (n = 1; 0.7%), abnormal test procedure results (n = 1; 0.7%), withdrawal of consent (n = 1; 0.7%), and protocol deviation (n = 1; 0.7%). Two patients discontinued the treatment because of pregnancy.
The mean (±SD) age of the study population was 37.7 (±9.4) years, 66.7% (n = 92) were females, and 96.4% (n = 133) were of Hispanic/Latino ethnicity. The majority of the patients (71.0%) had received DMTs previously which included any interferon β (55.8%), glatiramer acetate (17.4%), azathioprine (2.9%), natalizumab (1.4%) and other MS medications (5.8%). The mean duration of MS since diagnosis was 5.6 (±4.8) years, the mean EDSS score at baseline was 2.5 (±2.0), and the mean number of relapses in the year prior to baseline was 1.1 (±1.0). The mean exposure to the study drug was 117.1 (±29.1) days.
The most commonly reported medical history and continuing medical conditions of the enrolled patients at baseline were nervous system disorders (32.1%), psychiatric disorders (19.1%), surgical and medical procedures (19.1%), infections and infestations (14.2%), gastrointestinal disorders (11.1%), metabolism and nutrition disorders (11.1%), eye disorders (10.5%), and musculoskeletal and connective tissue disorders (10.5%).
A majority of patients (66%) were on medications or non-drug therapies prior to the start of study drug. The most common prior concomitant medications were gabapentin (9.9%), paracetamol (9.9%), omeprazole (8.0%), and cholecalciferol (5.6%). Concomitant medications administered after the start of study medication were taken by 80.9% of patients. The most common concomitant medications were paracetamol (18.5%), omeprazole (11.7%), gabapentin (10.5%), and ibuprofen (9.9%). Corticosteroids, like methylprednisolone and ethylprednisolone sodium, were permitted by the protocol for MS relapses and were received by 3.7% and 3.1% of the patients, respectively. Of the patients who had received prior MS treatment (70%), most common were the interferon beta therapies (55.6%) and glatiramer acetate (14.8%).
Overview of AEs and SAEs
Overall, AEs were predominantly mild (n = 49, 35.5%) or moderate (n = 27, 19.6%) in severity. Headache and fatigue were the most commonly reported AEs in this study. A total of 82 (59.4%) patients experienced an AE of which seven (5.1%) were classified as SAEs (Table 1). Of these seven patients, treatment was continued in six patients; one patient discontinued the treatment because of pregnancy and reported a spontaneous abortion in the follow-up period after discontinuation. All SAEs were considered unrelated to the study drug by the investigators. Infections (overall) were reported in 33 (23.9%) patients and were predominantly mild (n = 28, 20.3%). Herpes zoster infection was reported in one (0.7%) patient; no case of disseminated herpes was reported. Incidences of elevated alanine aminotransferase of ≥3, ≥5 and ≥10 upper limit of normal (ULN) were reported in five (3.7%), three (2.2%) and one (0.7%) patients, respectively. Study drug was permanently discontinued due to persistently elevated bilirubin (n = 1) and gamma glutamyl transferase (GGT) levels ≥2 ULN (n = 1). However, no case of Hy’s law was reported during the study. Overall, bradyarrhythmia AEs were reported in 12 (8.7%) patients of which 8 events were reported post first dose (days 23–142, Table 1). Of the six cases of bradycardia reported during the study, only one event was reported on post first dose (day 142). This event occurred after the patient had discontinued the study and no further records on vital signs are available. All these events were predominantly mild in nature and none were serious or led to study drug discontinuation. Mean (±SD) changes from baseline in systolic and diastolic BP were 3.4 (±10.3) and 2.5 (±7.5) mmHg. All three cases of hypertension (n = 3; 2.2%) were managed with antihypertensive therapy and did not result in treatment discontinuation. No case of macular edema was reported in the study.
Table 1.
Overall safety profile of fingolimod in the LATAM FIRST study
| Outcomes | Fingolimod 0.5 mg, N = 138 n (%) |
|---|---|
| SAEsa | 7 (5.1) |
| Any AEs | 82 (59.4) |
| AEs (≥2.5%) | |
| Headache | 22 (15.9) |
| Fatigue | 9 (6.5) |
| Bradycardia | 6 (4.3) |
| Diarrhea | 6 (4.3) |
| Anxiety | 5 (3.6) |
| Nausea | 5 (3.6) |
| Back Pain | 4 (2.9) |
| Depression | 4 (2.9) |
| Dizziness | 4 (2.9) |
| Influenza | 4 (2.9) |
| Lymphopenia | 4 (2.9) |
| Urinary tract infection | 4 (2.9) |
| Safety areas of special interest | |
| Bradyarrhythmiab | 12 (8.7) |
| Bradycardia | 6 (4.3) |
| Dizziness | 4 (2.9) |
| Hypotension | 2 (1.4) |
| Presyncope | 1 (0.7) |
| Malaise | 1 (0.7) |
| Infections (>1%) | |
| Infections (total) | 33 (23.9) |
| Influenza | 4 (2.9) |
| Urinary tract infection | 4 (2.9) |
| Bronchitis | 3 (2.2) |
| Gastroenteritis | 3 (2.2) |
| Nasopharyngitis | 3 (2.2) |
| Cervicitis | 2 (1.4) |
| Pharyngitis | 2 (1.4) |
| Rhinitis | 2 (1.4) |
| Upper respiratory tract infections | 2 (1.4) |
| Herpes infections | |
| Oral Herpes | 2 (1.4) |
| Genital herpes simplex | 1 (0.7) |
| Herpes simplex | 1 (0.7) |
| Herpes zoster | 1 (0.7) |
| Leucopenia and lymphopenia | 4 (2.9) |
| Thromboembolic eventsc | 2 (1.4) |
| Malignancies (Thyroid neoplasm) | 1 (0.7) |
| Hypertension (Mean change in BP from baseline, mmHg (±SD) n = 130d) | 3 (2.2) |
| Systolic | 3.4 (±10.3) |
| Diastolic | 2.5 (±7.5) |
| Liver transaminase elevation (including AST, ALT, Bi, GGT, AP) | 5 (3.6) |
AEs adverse events, ALT alanine transaminase, AP alkaline phosphatase, AST aspartate transaminase, Bi bilirubin, BP blood pressure, GGT gamma glutamyl transferase, N no. of patients, SAEs serious adverse events, SD standard deviation
aSAEs: MS relapses (n = 3; severity: 2 mild, 1 moderate); d-fibrin increased (n = 1; no associated clinical abnormality); gastroenteritis (n = 2; severity: 1 mild, 1 severe)
bA minority of bradyarrhythmia AEs were reported after first dose (days 23–142): dizziness 3; bradycardia 1; presyncope 1; malaise 2; hypotension 1
cThromboembolic AEs: 1 (0.7%) case each of monoparesis and angina pectoris. Monoparesis was of moderate severity and was not suspected by the investigator to be related to the study medication. Angina pectoris was of mild severity, suspected to be related to study drug medication. Neither AE resulted in study drug discontinuation
dNo. of patients for whom measurements were available
First-Dose Observations
The majority of patients (90.6%) were discharged at 6 h (Table 2). Of the six cases of bradycardia reported during the study, five events were reported during the first-dose observation period. However, none of the patients reported symptoms of bradycardia or required treatment for symptomatic bradycardia. The decrease and recovery in HR after first dose was consistent with the known pattern for fingolimod. No new onset of second degree or higher AVBs or prolongations in QTc interval were detected with ECG monitoring (protocol mandated requirements for extended monitoring; Table 2). With continued treatment, the mean HR returned to baseline levels (Fig. 1) and by week 16, the decrease in mean (±SD) HR was −1.08 (±8.66) bpm relative to the pre-dose baseline mean value. A small decrease in BP was observed which returned to baseline by 6 h: peak time/mean changes [mmHg (±SD): systolic (between 3 and 5 h) −2.49 (±8.63) and diastolic (between 4 and 5 h) −2.94 (±7.90)]. During the first-dose observation, mild hypotension was reported in one patient (mean sitting systolic/diastolic BP was lowest at 3 h post-dose: 82.0/64.0 mmHg), whose BP recovered to baseline by the end of first-dose observation period (mean sitting systolic/diastolic BP at 7 h post-dose: 100.0/70.0 mmHg) and continued treatment. No patient underwent extended monitoring due to an abnormal ECG or prolonged QT interval.
Table 2.
First-dose observations
| N = 138 (%) | |
|---|---|
| Patients discharged at 6 h | 125 (90.6) |
| Required extended monitoring after 6 ha | 13 (9.4) |
| Reasons for extended monitoring | |
| Lowest HR at 6 h | 11 (84.6) |
| HR <80% of baseline value | 1 (7.7) |
| Symptomatic/required treatment | 0 |
| ECG abnormality | 0 |
| QTc ≥500 ms | 0 |
| Addition monitoring duration (range, hours) | 1–6 hours |
| Bradycardiab | |
| All | 5 (3.6) |
| Mild | 4 (2.9) |
| Moderate | 1 (0.7) |
| Severe | 0 |
AEs adverse events, AV atrioventricular, ECG electrocardiogram, HR heart rate, N no. of patients
aFirst-dose monitoring guidance was updated during the course of the study, removing criteria for HR at discharge must be >80% of the baseline value and for repeat monitoring on day 2 if HR decreases by >30% from baseline at any time during the 6-h monitoring on day 1, and adding criteria on requirement of extended first dose monitoring if QTc ≥ 500 msec at 6 h
bOne patient reported dizziness alone without bradycardia. One patient reported bradycardia and hypotension on day 1
Fig. 1.
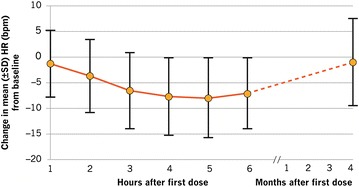
Hourly mean change in heart rate (HR) in 6 h following first dose and at 4 months. SD standard deviation
Safety data from the overall study population (n = 162) were comparable to the findings presented here for the LATAM population (n = 138) in the study (Data not shown).
Discussion
In the FIRST LATAM study, we investigated the short-term safety and tolerability of fingolimod 0.5 mg dose in a Latin American MS patient population. The overall safety profile was consistent with previous clinical trial experience, particularly the larger FIRST study (n = 2415) of comparable duration, with no new or unexpected safety signals [20, 21].
Overall, fingolimod was well tolerated in the study population. Only three patients discontinued treatment due to AEs, of whom two discontinued due to persistently elevated bilirubin and GGT levels (≥2 ULN) and one discontinued due to an AE (erythema nodosum). AEs were generally reported as mild to moderate in nature, with the most common AEs being headache and fatigue. The overall nature and frequency of the AEs, including the specific safety areas of interest (namely infections, liver enzyme elevation, BP changes, and macular edema) were consistent with the previous experience [20, 21]. The majority of infections were mild with upper respiratory tract infections being the most common. The incidence of herpes zoster infection was low; no disseminated cases were reported. The reported case of herpes zoster infection was considered mild in severity and suspected to be related to study drug. Patient continued on the fingolimod and received valaciclovir concomitantly. The infection was resolved within 5 weeks. Liver enzyme elevations and BP changes were clinically manageable and did not result in treatment discontinuation. No cases of macular edema were reported.
The first dose of fingolimod, in this current study, was well tolerated with transient, mostly asymptomatic decreases in HR, which were consistent with previous clinical trial experience and are attributed to the pharmacodynamic properties of fingolimod [22]. The majority of patients were discharged at 6 h, while a minority of patients underwent extended monitoring to ensure that protocol mandated HR recovery criteria were met. No abnormal ECG findings were reported and no patient discontinued treatment following the first-dose procedure. Although, in contrast to the larger FIRST study, bradycardia was reported in proportionally higher number of patients in this study (3.6% versus 0.6%) [20, 22], all of these AEs resolved without medical treatment on the same day.
A few other post-marketing studies have reported the fingolimod safety experience in Latin American populations [13–15]. A post hoc analysis of Hispanic/Latino (n = 89) patients from the fingolimod clinical trial program reported similar HR changes with no reports of symptomatic bradycardia. No patient discontinued the treatment following the first dose [13]. Similar to our current study, first-dose fingolimod experience from a single Chilean center (n = 78) was reported to be well tolerated; there were no reports of symptomatic bradycardia and all patients were discharged at 6 h [15]. Similarly, the preliminary analysis of a multi-center, prospective study in Argentina (n = 92) demonstrated a well-tolerated cardiac safety profile of fingolimod. Extended monitoring due to prolonged bradycardia was required in three patients; however, none of the patients developed symptomatic bradycardia [16]. In a retrospective chart review of first-dose experience from several Brazilian clinics (n = 180 RRMS patients), 99.4% of patients continued fingolimod treatment after the initial dose, and extended monitoring due to symptomatic bradycardia was required for 12 patients (6.7%) [14].
The results of the short-term FIRST LATAM study provide ethnically relevant Latin American patient data, which are consistent with the results from the overall fingolimod clinical trial program. Further longer term follow-up in such patient cohorts will provide more information to further aid clinical decision making in Latin America.
Conclusion
Overall, the FIRST LATAM study demonstrates that the first dose of fingolimod 0.5 mg is well tolerated, the pharmacodynamic effects are consistent with previous findings, and fingolimod has a clinically manageable safety profile in Latin American RRMS patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Sponsorship, article processing charges, and the open access charge for this study were funded by Novartis.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, from inception to publication and have given final approval for the version to be published.
The authors would like to acknowledge Rahul Birari (Novartis Healthcare Pvt. Ltd.) for providing medical writing support, which encompassed preparation of a first draft, formatting, referencing, preparing tables and figures, incorporating the authors’ revisions, and submission, all under the direction of the authors, and Hashem Salloukh (Medical Communications, Novartis Pharma AG, Basel) for editorial assistance and manuscript coordination. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Conflict of interest
L. Ordoñez-Boschetti received compensation as a consultant and speaker as well as research support from Novartis, Stendhal, Merck Serono, and Teva. R. Rey received compensation as a consultant and speaker as well as research support from Novartis, Pfizer, Biogen Idec, Valeant, and Ivax. A. Cruz received compensation as a consultant and speaker as well as research support from Abbott (for Biogen), Bayer, and Novartis. A. Cruz is a member of the advisory board for Genzyme. A. Sinha and N. Frider are employees of Novartis. T. Reynolds was an employee of Novartis during the conduct of the study and preparation of the manuscript, but is presently an employee of EMEA medical affairs manager, Eisai Europe Ltd. R. Alvarenga received consulting fees, research support or speaker’s honoraria from Genzyme Novartis, Teva and Biogen Idec.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was provided by all patients before being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- 2.Multiple Sclerosis International Federation. Atlas of MS. 2013. http://www.msif.org/wp-content/uploads/2014/09/Atlas-of-MS.pdf. Accessed 21 May 2015.
- 3.Correale J. MS in Latin America. Int MS J. 2008;15:3–5. [PubMed] [Google Scholar]
- 4.Luetic G. MS in Latin America. Int MS J. 2008;15:6–11. [PubMed] [Google Scholar]
- 5.Ojeda E, Diaz-Cortes D, Rosales D, Duarte-Rey C, Anaya JM, Rojas-Villarraga A. Prevalence and clinical features of multiple sclerosis in Latin America. Clin Neurol Neurosurg. 2013;115:381–387. doi: 10.1016/j.clineuro.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 6.Amezcua L, Lund BT, Weiner LP, Islam T. Multiple sclerosis in Hispanics: a study of clinical disease expression. Mult Scler. 2011;17:1010–1016. doi: 10.1177/1352458511403025. [DOI] [PubMed] [Google Scholar]
- 7.Corona T, Roman GC. Multiple sclerosis in Latin America. Neuroepidemiology. 2006;26:1–3. doi: 10.1159/000089230. [DOI] [PubMed] [Google Scholar]
- 8.Correale J, Abad P, Alvarenga R, et al. Management of relapsing-remitting multiple sclerosis in Latin America: practical recommendations for treatment optimization. J Neurol Sci. 2014;339:196–206. doi: 10.1016/j.jns.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Kappos L, Antel J, Comi G, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 10.Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:545–556. doi: 10.1016/S1474-4422(14)70049-3. [DOI] [PubMed] [Google Scholar]
- 11.Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 12.Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 13.Chinea Martinez AR, Correale J, Coyle PK, Meng X, Tenenbaum N. Efficacy and safety of fingolimod in Hispanic patients with multiple sclerosis: pooled clinical trial analyses. Adv Ther. 2014;31:1072–1081. doi: 10.1007/s12325-014-0154-4. [DOI] [PubMed] [Google Scholar]
- 14.Fragoso YD, Arruda CC, Arruda WO, et al. The real-life experience with cardiovascular complications in the first dose of fingolimod for multiple sclerosis. Arq Neuropsiquiatr. 2014;72:712–714. doi: 10.1590/0004-282X20140102. [DOI] [PubMed] [Google Scholar]
- 15.Vergara Soto E, Uribe San Martín R, Ciampi Díaz E, et al. Seguridad De La Primera Dosis De Fingolimod Administrado Bajo Vigilancia Ambulatoria En El Centro De Esclerosis Múltiple De La Pontifi cia Universidad Católica De Chile. VIII Latin American Congress of Multiple Sclerosis (LACTRIMS); 2014.
- 16.Kuperman G, Nunez V, Mattiazi M, et al. Cardiac safety profile during first-dose monitoring period of fingolimod (Gilenya) treatment in patients with relapsing remiting MS: data from the Argentinean registry (REAL). Interim Results (P3.250). Neurology. 2015;84(14 Supplement).
- 17.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 18.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH harmonised tripartite guideline. Guideline for good clinical practice. 1996. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed 21 May 2015.
- 19.World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. 2015. http://www.wma.net/en/30publications/10policies/b3/index.html. Accessed 30 June 2015.
- 20.Gold R, Comi G, Palace J, et al. Assessment of cardiac safety during fingolimod treatment initiation in a real-world relapsing multiple sclerosis population: a phase 3b, open-label study. J Neurol. 2014;261:267–276. doi: 10.1007/s00415-013-7115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kappos L, Comi G, Palace J, et al. Safety and tolerability of fingolimod 0.5 mg during the first 4 months of administration in patients with relapsing forms of multiple sclerosis. Results from the open-label, multicentre FIRST study. (P336). European Neurologial Society (ENS). 2012.
- 22.Camm J, Hla T, Bakshi R, Brinkmann V. Cardiac and vascular effects of fingolimod: mechanistic basis and clinical implications. Am Heart J. 2014;168:632–644. doi: 10.1016/j.ahj.2014.06.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


