Abstract
Familial pancreatic cancer (FPC) designates kindreds that contain at least two first degree relatives with pancreatic ductal adenocarcinoma. Studies of FPC have focused on discovery of genetic etiology and on management of those at genetically high risk. Over a decade of research reveals that a half dozen known hereditary syndromes or genes are associated with increased risk of developing pancreatic cancer, the most prominent of which are BRCA2 and CDKN2A. The search for novel predisposition genes is ongoing, using traditional genetic epidemiology approaches as well as next generation sequencing technologies. These strategies have been successful, with the identification of FPC genes PALB2 and ATM. Genetic risk assessment and testing is already available. Effective, evidence-based criteria and recommendations for managing patients and at-risk family members in light of genetic status are nascent, but being disseminated. Research on personalized therapy for pancreatic cancer among mutation carriers is ongoing. Due to limited experience worldwide, guidance is often based upon expert opinion, though all agree that research is needed to improve the shaping of options.
Keywords: Genetic susceptibility, familial risk, genetic testing, risk assessment and management
Introduction
Pancreatic cancer is a devastating diagnosis for patients and their families, and is the fourth leading cause of cancer death. Among the major cancers, pancreatic cancer has the worst survival and historically has been the least studied. Approximately 95% of pancreatic neoplasms are ductal adenocarcinomas. The rapid mortality of patients with pancreatic adenocarcinoma makes this cancer challenging for research into basic, translational, and epidemiologic studies. For genetic or molecular investigations which require biospecimens for DNA studies, involving patients who are often too ill to participate and whose disease precludes surgical resection (with consequent lack of tumor tissue), has posed difficulties.
This longstanding dearth of knowledge has resulted in only minimal inroads to improve risk reduction or survival. In the United States, incidence and mortality rates have remained largely unchanged since 1973. During 2005-2009, the incidence rate for Whites was 11.6/105 and for African-Americans, 15.2/105. Mortality rates were comparably 10.7/105 for Whites and 13.8/105 for African-Americans.1 The 5-year survival has been 4-6% for decades.2 The low survival from pancreatic cancer is primarily due to the advanced stage at diagnosis in the majority of cases: by the time of diagnosis, 80% of pancreatic carcinomas are no longer localized to the pancreas. To date, no reliable screening tests or effective cures for pancreatic cancer are available; there are few long-term survivors.
It is crucial to advance our knowledge of etiology to enable evidence-based strategies to decrease incidence and mortality. For years, pancreatic cancer was thought to be a sporadic disease, due in part to the lack of systematic studies and the inherent challenges as described above. Over the past two decades, however, there has been sustained effort to elucidate its genetics. As demonstrated for a variety of cancers, genetic epidemiology and family-based approaches have led to important breakthroughs in a variety of diseases, and particularly cancer.3-5 Discerning familial patterns of cancer incidence, combined with detailed studies of clinical and DNA variation, has defined a variety of inherited cancer syndromes and their causal genes. We will review here the evidence for a genetic component of pancreatic cancer, studies of hereditary syndromes that feature increased risk of pancreatic cancer, and the current status of clinical translation of the findings.
Evidence for genetic basis of pancreatic cancer
Familial clustering
Early reports of familial clusters of pancreatic cancer provided the first suggestion that a least a hereditary, but rare form of pancreatic cancer might exist. Reports of clusters included families in which multiple siblings were affected (but not the parents)6-9 or one family where each of three generations contained an affected member.10
Familial aggregation studies and analysis of families
More formal study designs that apply epidemiologic and genetic segregation analysis methods are widely accepted standards to uncover existence of genetic basis for a cancer. One conventional approach to investigating potential host susceptibility is to perform case-control comparisons of family history of pancreatic cancer. A comprehensive summary of these studies and estimated risks are listed in Table 1. Seven case control studies, two cohort studies, one population-based genealogic analysis, and one case series that estimated incidence of pancreatic cancer in relatives have found that first degree relatives have at least a two-fold increased risk of developing pancreatic cancer. These findings are remarkably consistent, given that case ascertainment and data collection spanned thirty or more years, multiple countries and cultures, and different methods for estimating risk. A systematic review and meta-analysis by Permuth-Wey and Egan11 of a cohort study and seven case-control studies totaling 6,568 pancreatic cancer cases calculated an overall relative risk of 1.80 (95% CI: 1.48-2.12). They also found that 1.3% of pancreatic cancer in the population is attributable to family history. The risk was consistent for both males and females, and did not differ by early or late age at diagnosis. With respect to risk for second degree relatives (aunts, uncles, grandparents, grandchildren), both Hassan et al.12 and Shirts et al.13 reported risks comparable to those of first degree relatives (relatives risks of 2.9 (95% CI: 1.3-6.3) and 1.59 (95% CI; 1.31-2.91, respectively). In addition, a large multicenter cohort study examined risk by number of affected individuals and showed even high risk associated with having two or more first degree relatives with pancreatic cancer with odds ratio of 4.26 (95% CI: 0.48-37.79).16
Table 1.
Family history and estimated risks of pancreatic cancer in case-control and cohort studies. Case-control study designs reported unless otherwise specified.
| Location, Years of Study | Cases, N | Controls, N | Risk of pancreatic cancer in family members | Reference | |
|---|---|---|---|---|---|
| Risk | 95% CI | ||||
| Louisiana, 1979-83 | 362 | 1,408 | 5.25 | 2.1-13.2 | Falk et al.49 |
| Canada, 1984-88 | 174 | 136 | 5.0 | 1.2-24.5 | Ghadirian et al.50 |
| Italy, 1983-1992 | 363 | 1,234 | 2.8 | 1.3-6.3 | Fernandez et al.51 |
| United States, 1986-89 | 484 | 2,099 | 3.2 | 1.8-5.6 | Silverman et al.52 |
| Japan, Cohort, 1988-99 | 200 | 2,200 | 2.09 | 1.01-4.33 | Inoue et al.53 |
| United States, 1996-99 | 247 | 420 | 2.49 | 1.3-4.7 | Schenk et al.54 |
| Texas, 2000-2006 | 888 | 888 | 3.3 | 1.8-6.1 | Hassan et al.12 |
| United States, 2005-2009 | 654 | 697 | 2.79 | 1.44-4.08 | Austin et al.55 |
| International, PanScan Cohort Consortium (1 case-control and 10 cohort studies), 1985-2001 | 1,183 | 1,205 | 1.76 | 1.19-2.61 | Jacobs et al.16 |
| Utah, Genealogy database, 1966-2010 | 1,411 | -- | RR=1.84 | 1.47-2.29 | Shirts et al.13 |
| Minnesota, Case series, 2000-2004 | 426 | -- | SIR=1.88 | 1.27-2.68 | McWilliams et al.56 |
Abbreviations: CI, confidence interval; RR, Relative risk; SIR, Standardized incidence ratio
Our experience and that of others has shown that 8-10% of pancreatic adenocarcinoma patients report having had a first degree relative (parent, sibling, or child) with pancreatic cancer.14-15 This proportion is congruent with family history patterns observed in series of patients with colorectal cancer, breast cancer, lung cancer, and prostate cancer. In addition, a population-based twin study of cancer in Sweden by Lichtenstein et al.17 estimated pancreatic cancer heritability to be 36%, similar to colorectal cancer (35%), higher than breast cancer (27%), and slightly lower than prostate cancer (42%). Taken together, this implies that pancreatic adenocarcinoma susceptibility patterns would be consistent with those seen for the more common cancers and we would likewise expect that predisposition genes exist.
Segregation analysis is a statistical method that determines if a gene consistent with a Mendelian inheritance pattern could cause the observed familial aggregation of a trait. Klein et al.18 analyzed family histories 287 pancreatic cancer patients seen from 1994-1999 at Johns Hopkins Hospital in Baltimore, Maryland. The analysis rejected non-genetic transmission models. The data best fit a major gene model that was predicted to follow an autosomal dominant pattern of a rare allele; 0.7% of the population would carry a high risk of developing pancreatic cancer due to this putative gene. A smaller study of 70 families by Banke et al.19 arrived at a similar conclusion.
Familial pancreatic cancer (FPC) defined to advance research
Increased attention on the genetic analysis of pancreatic cancer required that a standard definition be applied so that research on risk factors and gene discovery in the familial setting would be consistent. In 1998, Hruban et al.20 proposed that FPC would be defined as kindreds containing at least a pair of individuals who were affected with pancreatic adenocarcinoma and who were first degree relatives. This definition was simple, yet provided a sufficient boundary and is now widely used and facilitates a variety of studies. In particular, the multicenter Pancreatic Cancer Genetic Epidemiology (PACGENE) Consortium was formed to systematically collect risk factor and family history data plus germline DNA from blood or saliva from members of FPC kindreds. The resources would be used for gene discovery and genetic epidemiologic characterization.14 Many of the advances described here were enabled by the ongoing activities of the PACGENE Consortium members. To date, 44,183 patients at seven sites have been screened for family history, of whom 3,190 (7.2%) with positive family history have been enrolled, along with 7,012 of their adult (99% unaffected) relatives.
Characteristics of FPC: Sex, incident risk, age at onset, smoking, other cancers
Based on the PACGENE data, approximately half of FPC patients are male, which is consistent with the proportion observed in sporadic pancreatic cancer. With respect to incident risk, family history studies described earlier clearly document the risk. Klein et al.21 analyzed 5,179 individuals in 838 Johns Hopkins FPC kindreds and quantified risk using standardized incidence ratios (SIR) that compared the number of incident pancreatic cancers observed with those expected using Surveillance, Epidemiology and End Results (SEER)22 rates. During the followup period from time of enrollment, 19 pancreatic cancers developed among the relatives. The observed-to-expected rate of pancreatic cancer was 9.0 (95% CI: 4.5-16.1), significantly increased compared to members of sporadic kindreds. It was also noted that with increasing number of affected individuals in the pedigree, the risk increased: three affected first degree relatives in the kindred had a SIR=32.0 (95% CI:10.2-74.7), two affected had a SIR=6.4 (95% CI: 1.8-16.4), versus one affected had a SIR=4.6 (95% CI, 0.5-16.4). Compared to the general population incidence of 9 per 100,000, FPC relatives with three affected individuals in the pedigree have an estimated incidence of 288 per 100,000, and for individuals with two affected individuals in the kindred, the incidence is 57.6 per 100,000, and 41.1 per 100,000 if an individual has one affected relative.
Risk of developing pancreatic cancer in the FPC setting was higher in smokers than in nonsmokers. Individuals with a strong family history of pancreatic cancer have a significantly increased risk of developing pancreatic cancer. Unlike hereditary breast cancer or hereditary colorectal cancer syndromes, where the age of onset can be much younger by 10 to 20 years compared to sporadic cases, the difference in median age at diagnosis in FPC is approximately five years. Compared to the general pancreatic cancer population (from SEER data) where the mean age at diagnosis was 70.0 ±12.1 years, the mean age among FPC cases was 65.4 ±11.6. Among the PACGENE kindreds, mean ages at diagnosis did not significantly differ when stratified by number of affected individuals in the pedigree. With respect to smoking history, 37% are never smokers, 47.1% are ever smokers, with smoking status is unknown on 14.9%. In an Australian sample of 68 FPC patients, 60.3% were never smokers.15 We and others have observed increased risk of other cancers in FPC kindreds, particularly breast cancer, melanoma, and colorectal cancer. However, these risks have not been systematically disentangled from analyses that also include germline mutations in cancer susceptibility genes. In addition, much of the focus is on cancer among at-risk relatives.
Genetic studies of familial pancreatic cancer
Hereditary syndromes with increased risk pancreatic cancer
In 1996, Lynch et al.23 asserted that genetic factors were estimated to play a significant role in 5% of the total pancreatic cancer burden. Much research has been accomplished in the intervening time. While novel genes that predispose to FPC remain to be discovered, increased risk of pancreatic cancer is now known to be associated with half a dozen inherited syndromes with known germline mutations, including BRCA1, BRCA2, CDKN2A, PALB2, ATM, mismatch repair genes, and PRSS1 and SPINK2 of hereditary pancreatitis.24 These syndromes are summarized in Table 2, along with associated malignancies, and estimates of pancreatic risk in these syndromic settings. The most prominent syndromes are hereditary breast-ovarian cancer syndrome, particularly due to germline mutations in BRCA2, and familial atypical mole and melanoma syndrome, due to mutations in CDKN2A.
Table 2.
Genes and syndromes associated and estimates of risk of developing pancreatic adenocarcinoma. The probabilities of detecting a deleterious mutation in the predisposition genes shown were based upon studies that sequenced the entire gene in series of familial pancreatic cancer (FPC) patients.
| Gene | Chromosome | Predisposition syndrome | Associated Malignancies | Risk of pancreatic cancer | FPC patients with deleterious mutations | ||
|---|---|---|---|---|---|---|---|
| Proportion | % | Reference | |||||
|
| |||||||
| ATM | 11q23 | Familial breast cancer | Breast | Increased risk: not well defined | 2/168 | 1.2 | Roberts et al.36 |
| 1/39 | 2.6 | Grant et al.38 | |||||
|
| |||||||
| BRCA1 | 17q21.31 | Hereditary breast and ovarian cancer | Breast (particularly premenopausal), ovary, male breast, prostate | No effect up to OR=2.26 (95% CI 1.26-4.06); SIR=2.55 (95% CI 1.03-5.31) 57 |
6/516 | 1.2 | Zhen et al.37 |
|
|
|
||||||
| BRCA2 | 13q13.1 | Breast (particularly premenopausal), ovary, male breast, prostate, melanoma | OR=3.5 (95% CI 1.87-6.58); SIR=2.13 (95% CI 0.36-7.03)57 |
19/516 | 3.7 | Zhen et al.37 | |
|
| |||||||
| CDKN2A | 9p21.3 | Familial atypical mole and melanoma | Melanoma | SIR= 13-38 | 14/519 | 2.7 | Zhen et al.37 |
|
| |||||||
| Mismatch repair: | Hereditary nonpolyposis colorectal cancer (Lynch syndrome) | Colorectum, endometrial, ovary, stomach, small bowel, urinary tract (ureter, renal pelvis) biliary, glioblastoma, skin (sebaceous) | No effect up to SIR=8.6 (95% CI 4.7-15.7) | -- | -- | -- | |
| MLH1 | 3p22.2 | ||||||
| MSH2 | 2p21 | ||||||
| MSH6 | 2p16.3 | ||||||
| PMS2 | 7p22.1 | ||||||
|
| |||||||
| PALB2 | 16p12.2 | Familial breast cancer | Fanconi anemia, breast, esophagus, prostate, stomach | Increased risk: not well defined | 3/96 | 3.1 | Jones et al.35 |
| 3/521 | 0.6 | Zhen et al.37 | |||||
|
| |||||||
| PRSS1 | 7q34 | Hereditary pancreatitis | -- | SIR=67 (95% CI 8-80) | -- | -- | -- |
| SPINK1 | 5q32 | ||||||
|
| |||||||
| STK11 (LKB1) | 19p13.3 | Peutz Jeghers syndrome | Colorectum, small bowel, stomach, breast, gynecologic | SIR=132 | -- | -- | -- |
It is also important to note that this review does not discuss low penetrance common genetic polymorphisms which confer modest risk of pancreatic cancer (odds ratio <1.3). The susceptibility variants were identified by genomewide association studies (GWAS) involving large samples of sporadic pancreatic cancer cases and healthy controls. The variants offer opportunities to study gene pathways and genetic-environment interactions.24 However, translation of these findings to the clinic is unlikely for some time.
Gene discovery studies in FPC
Family based gene discovery studies focused on linkage studies and candidate gene approaches. Genetic linkage analysis requires a panel of hundreds to thousands of genetic markers spaced across the genome, which are then used in conjunction with the family structure and cancer phenotypes to assess the probability that an allele in a specific marker is co-transmitted through the pedigree with the cancer phenotype. A lod score (log of the odds that the allele is transmitted with the phenotype versus independently of the phenotype) is calculated for each family and examined in aggregate. To date, no formal linkage analysis of a large number of families has been published. The only linkage analysis of a single FPC kindred in the literature examined a linkage region on chromosome 4p25 erroneously concluded that the PALLD gene encoding the palladin protein was the predisposition gene.26 PALLD mutation analysis of 48 FPC patients was unable to support the original linkage finding.27
An alternative approach to FPC gene discovery is candidate gene analysis. Studies that use this approach are based on a plausible biological or clinical rationale for examining a candidate gene in patients with pancreatic cancer. van der Heijden et al.28 identified gene mutations in FANCC and FANCG, in the Fanconi anemia among young-onset pancreatic cance. Rogers et al.29 examined 38 FPC kindreds for mutations but was not able to attribute mutations to FPC. Couch et al.30 performed a mutation screen of the FANCC and FANCG genes in 421 unselected Mayo Clinic cases, and found two mutations of FANCC in sporadic young onset patients, but none in FANCG.
In another candidate gene study, McWilliams et al.31 analyzed for 39 mutations in the cystic fibrosis transmembrane regulator (CFTR) gene in 949 unselected White Mayo Clinic pancreatic cancer cases and used data on 13,340 White controls from a clinical laboratory database. They found that 5.3 carried a common CFTR mutation versus 3.8% of controls, giving an OR=1.40 (95% CI: 1.04-1.89). Among patients who were younger when their disease was diagnosed (<60 years), the carrier frequency was higher than in controls (OR=1.82 (95% CI: 1.14-2.94).
Analogously, Murphy et al.32reported 17% prevalence of BRCA2 mutations among affected individuals from 26 European FPC kindreds containing three or more affected members with pancreatic cancer. Subsequent studies of individuals with pancreatic cancer from families meeting FPC criteria estimated BRCA2 prevalence ranging between 6–10%.33 Among Ashkenazi Jews, similar mutation prevalences were observed for both BRCA1 and BRCA2 34
Novel gene discovery in FPC patients using next generation sequencing
Advances in sequencing technology, bioinformatics, and computing capacity have moved genomic researchers considerably forward in discovery of susceptibility genes for FPC. High throughput sequencing of FPC kindreds results in discovery of two genes that were not previously known to increase risk of pancreatic cancer: PALB2 and ATM. In both cases, functional roles were supported by the loss of heterozygosity of the wild-type allele in the pancreatic tumor of the patients. In the course of complete exome sequencing of unselected pancreatic cancer patients, Jones et al.35 identified a germline truncating mutation in PALB2 that co-segregrated in an FPC patient. This led to screening the DNA of 96 more FPC patients specifically for PALB2 mutations. Truncating mutations were detected in three additional patients, however no difference was observed in age at diagnosis of the mutation carriers. No PALB2 mutations were found in 1,084 normal controls. Similarly, mutations in the ataxia telangiectasia mutated (ATM) gene were discovered to segregate with the pancreatic cancer phenotype in two FPC kindreds by Roberts et al.36 The investigators screened the ATM gene for mutations in DNA of 166 FPC patients, and identified four carriers of deleterious mutations. No similar mutations were seen in 190 controls.
Taken together, the candidate gene approach and the unbiased genomic sequencing approach are revealing, gene by gene, the extensive genetic heterogeneity of the FPC phenotype. In addition to adding to the catalog of genes, they provide an opportunity to study the potential effect of genetic mutations on age at diagnosis and risk of developing other cancers.
Genetic analysis of cancer syndrome genes
With the identification of susceptibility genes, particularly in the context of hereditary cancer syndromes, genetic testing for multiple susceptibility genes is readily feasible. The approach used with this opportunity is to characterize the genetic variation in FPC patients tested across genes. In a PACGENE Consortium study, Zhen et al.37 collected and performed mutation analysis of germline DNA samples from 727 unrelated probands with positive family history (521 met criteria for FPC). All patient samples were tested for mutations in BRCA1, BRCA2, PALB2, and CDKN2A. Prevalence of mutations among FPC probands was estimated. They found that prevalence of deleterious mutations among FPC probands was: BRCA1, 1.2%; BRCA2, 3.7%; PALB2, 0.6%; and CDKN2A, 2.5%. The probability of testing positive for deleterious mutations in any of the four genes ranges up to 10.4%, depending upon family history of cancers. BRCA2 and CDKN2A account for the majority of mutations in FPC. These results are summarized in Table 2.
In the Ontario Pancreas Cancer Registry study,38 germline DNA from 290 patients with varying degrees of family history sequenced a panel of 13 genes (APC, ATM, BRCA1, BRCA2, CDKN2A, MLH1, MSH2, MSH6, PALB2, PMS2, PRSS1, STK11, and TP53). While 11 total deleterious mutations were found (three in ATM, one in BRCA1, two in BRCA2, one in MLH1, two in MSH2, one in MSH6, and one in TP53, the only mutation detected among 39 FPC patients was one in ATM. Due to the variation in family history and FPC status, the aggregate probability of having a positive gene test is 3.8% in the Ontario study compared to over 10% in familial subsets in the PACGENE study.
The emerging trend of multiple genetic testing of patients regardless of family history indications is a concern. Commercial testing is already offered through genetic testing companies, but recommendations regarding who is appropriate for genetic testing are nascent. While there is a need for more genetic epidemiologic evidence, the aggregate prevalence of mutations in current genetic panels is such that useful information may be learned. Several whole genome and whole exome sequencing studies of large numbers of pancreatic cancer and FPC patients series are ongoing. They are expected to inform risk assessment and genetic testing using multi-gene panels.
Clinical translation of familial pancreatic cancer research
As has occurred with other cancer genes, the transfer of discoveries to the clinical laboratory and bedside is occurring rapidly. Regrettably, there still remain gaps in our knowledge so that strategies cannot be fully shaped in order to guide patients and their families. Multiple fronts have been opened: personalized therapy, risk assessment and genetic testing, screening and surveillance.
Personalized therapy
When a novel germline mutation is identified, its relevance and contribution to the cancer phenotype is investigated. As described for PALB2 and ATM, a functional role is sought, such as examining the matched tumor for additional mutations and loss of heterozygosity. Work then proceeds to identify mechanisms to establish whether existing therapies could be re-directed to address effect of the genetic alteration. In particular, several investigations of PARPi [poly (ADP-ribose) polymerase inhibitor] in patients with BRCA1 or BRCA2 mutations in germline and/or tumor have been initiated39-40, and several pancreatic cancer specific trials are enrolling41. It is still too early to know whether the clinical trial outcomes will lend credence to personalized therapy for mutation carriers.
Genetic testing and cancer risk assessment
By far, the most rapidly translational activity following discovery of a susceptibility gene is determining the gene/mutation utility and suitability for genetic testing, risk assessment, and counseling. Family history alone may trigger a referral to a cancer genetic counselor. With the array of genetic tests available, it is unclear whether health care providers or patients will seek to take up these new options. Genetic testing of at-risk individuals, particularly in cancer high risk clinics, may be routinely offered. As discussed above, the cancer multigene testing panels are a double-edged sword. Genetic testing can providing more information than was previously available, and the yield may be higher, but interpretation may be hampered for lack of evidence upon which to develop next steps.42-43
Screening options for high risk individuals
Clinicians are being pushed by the pace of genetic discoveries and testing for FPC. It has been a challenge to develop commensurate screening, surveillance and management guidelines for genetically high-risk individuals. Clinical experiences of 49 experts were shared in the Cancer of the Pancreas Screening (CAPS) Consortium to develop consensus on a number of clinical scenarios.45 There was excellent agreement on goals of a screening program (should detect and treat T1N0M0 margin-negative PC and high-grade dysplastic precursor lesions). Candidates for screening include first degree relatives of an affected patient in an FPC kindred, patients with Peutz-Jeghers syndrome, and mutation carriers of FPC hereditary cancer syndrome genes with an affected first degree relative. While initial screening should include endoscopic ultrasonography (EUS) and/or MRI/magnetic resonance cholangiopancreatography, the CAPS Consortium could not reach consensus on ages to initiate or stop surveillance, nor on longer term management of problematic scenarios.45
The American College of Gastroenterology (ACG) has published its clinical guidelines on genetic testing and management of hereditary gastrointestinal cancer syndromes, including hereditary pancreatic cancer43. Agreeing with the CAPS Consortium, the ACG states that surveillance of genetically high risk individuals should be performed at experienced centers with a multidisciplinary approach. The ACG guidelines include a conditional recommendation with low quality of evidence that surveillance for pancreatic cancer should be with EUS and/or MRI annually starting at age 50 years, or 10 years younger than the earliest age of pancreatic cancer in the family. The quality of the supporting data for the guidelines is low and, combined with the experience and caution of others47-48, it is important to maintain perspective when counseling high risk relatives. Taken together, the experts most familial with screening and surveillance of at-risk individuals in the setting of FPC are taking careful steps forward but the challenges to achieve consensus underscore the need for more research and collaboration.
Figure 1.
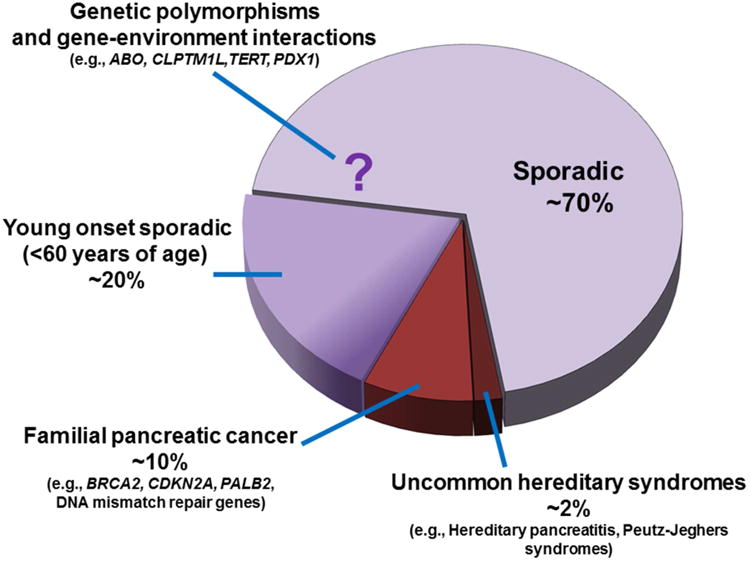
Subsets of pancreatic adenocarcinoma patients who carry gene mutations or variants that increase susceptibility. The majority of pancreatic adenocarcinoma is considered sporadic without any known associated genetic or hereditary factor. Low penetrance genetic polymorphisms which confer modest risks (odds ratio <1.3) were identified in genomewide association studies. A modest proportion of young onset sporadic pancreatic cancer patients carry deleterious germline mutations in known cancer genes or polymorphisms associated with pancreatic cancer risk. The genetic basis of familial pancreatic cancer is only partly explained by known cancer genes. Uncommon hereditary syndromes confer increased risk of pancreatic cancer.
Key Points.
Familial pancreatic cancer (FPC) designates kindreds that contain two or more first degree relatives ever diagnosed with pancreatic ductal adenocarcinoma.
Patients with FPC constitute 8-10 percent of all pancreatic cancer patients. Positive family history of pancreatic cancer is a consistent risk factor, with two-fold increased risk to first degree relatives.
While novel genes that predispose to FPC remain to be discovered, increased risk of pancreatic cancer is now known to be associated with half a dozen inherited syndromes with known germline mutations, including BRCA1, BRCA2, CDKN2A, PALB2, ATM, mismatch repair genes, and PRSS1 and SPINK2 of hereditary pancreatitis.
Predisposition genetic testing for individuals in FPC kindreds is feasible, and typically will consist of sequencing a panel of multiple genes. Cancer risk assessment is less precise and research into prevention and screening is nascent.
Guidelines for management of family members at risk for FPC are being developed or disseminated. Due to limited experience worldwide, guidance is often based upon expert opinion. All agree that more research is needed to improve the shaping of options.
Summary.
-FPC kindreds contain at least two first degree relatives with pancreatic ductal adenocarcinoma.
-Genetic studies of FPC have uncovered important new genes and insights about the genetics of pancreatic cancer.
-Over a decade of research reveals that a half dozen known hereditary syndromes or genes are associated with increased risk of developing pancreatic cancer, the most prominent of which are BRCA2 and CDKN2A.
-Next generation sequencing technologies successfully identified new FPC genes, PALB2 and ATM. At the same time, with rapid dissemination of the knowledge to the clinical setting, many challenges have been generated.
-Genetic testing, risk assessment, and management of those at risk have proven the need for effective, evidence-based criteria.
-Guidelines are based upon expert opinion, though all agree that research is needed to improve management.
Acknowledgments
Supported in part by National Cancer Institute grants R01 CA97075 and P50 CA102701.
Footnotes
Disclosures: The author has no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Atlanta: American Cancer Society; 2013. Cancer Facts & Figures 2013. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf. [Google Scholar]
- 2.American Cancer Society. Atlanta: American Cancer Society; 2015. Cancer Facts & Figures 2015. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. [Google Scholar]
- 3.de la Chapelle A. Genetic predisposition to colorectal cancer. Nat Rev Cancer. 2004 Oct;4(10):769–80. doi: 10.1038/nrc1453. [DOI] [PubMed] [Google Scholar]
- 4.Hemminki K, Rawal R, Chen B, Bermejo JL. Genetic epidemiology of cancer: from families to heritable genes. Int J Cancer. 2004 Oct 10;111(6):944–50. doi: 10.1002/ijc.20355. [DOI] [PubMed] [Google Scholar]
- 5.Antoniou AC, Easton DF. Models of genetic susceptibility to breast cancer. Oncogene. 2006 Sep 25;25(43):5898–905. doi: 10.1038/sj.onc.1209879. [DOI] [PubMed] [Google Scholar]
- 6.Ghadirian P, Simard A, Baillargeon J. Cancer of the pancreas in two brothers and one sister. Int J Pancreatol. 1987 Oct-Dec;2(5-6):383–91. doi: 10.1007/BF02788437. [DOI] [PubMed] [Google Scholar]
- 7.Dat NM, Sontag SJ. Pancreatic carcinoma in brothers. Ann Intern Med. 1982 Aug;97(2):282. doi: 10.7326/0003-4819-97-2-282_1. [DOI] [PubMed] [Google Scholar]
- 8.MacDermott RP, Kramer P. Adenocarcinoma of the pancreas in four siblings. Gastroenterology. 1973 Jul;65(1):137–9. [PubMed] [Google Scholar]
- 9.Friedman JM, Fialkow PJ. Familial carcinoma of the pancreas. Clin Genet. 1976 May;9(5):463–9. doi: 10.1111/j.1399-0004.1976.tb01598.x. [DOI] [PubMed] [Google Scholar]
- 10.Ehrenthal D, Haeger L, Griffin T, Compton C. Familial pancreatic adenocarcinoma in three generations. A case report and a review of the literature. Cancer. 1987 May 1;59(9):1661–4. doi: 10.1002/1097-0142(19870501)59:9<1661::aid-cncr2820590923>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 11.Permuth-Wey J, Egan KM. Family history is a significant risk factor for pancreatic cancer: results from a systematic review and meta-analysis. Fam Cancer. 2009;8(2):109–17. doi: 10.1007/s10689-008-9214-8. Epub 2008 Sep 2. [DOI] [PubMed] [Google Scholar]
- 12.Hassan MM, Bondy ML, Wolff RA, Abbruzzese JL, Vauthey JN, Pisters PW, Evans DB, Khan R, Chou TH, Lenzi R, Jiao L, Li D. Risk factors for pancreatic cancer: case-control stud. Am J Gastroenterol. 2007 Dec;102(12):2696–707. doi: 10.1111/j.1572-0241.2007.01510.x. Epub 2007 Aug 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirts BH, Burt RW, Mulvihill SJ, Cannon-Albright LA. A population-based description of familial clustering of pancreatic cancer. Clin Gastroenterol Hepatol. 2010 Sep;8(9):812–6. doi: 10.1016/j.cgh.2010.05.012. Epub 2010 May 23. [DOI] [PubMed] [Google Scholar]
- 14.Petersen GM, de Andrade M, Goggins M, Hruban RH, Bondy M, Korczak JF, Gallinger S, Lynch HT, Syngal S, Rabe KG, Seminara D, Klein AP. Pancreatic cancer genetic epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2006 Apr;15(4):704–10. doi: 10.1158/1055-9965.EPI-05-0734. [DOI] [PubMed] [Google Scholar]
- 15.Humphris JL, Johns AL, Simpson SH, Cowley MJ, Pajic M, Chang DK, Nagrial AM, Chin VT, Chantrill LA, Pinese M, Mead RS, Gill AJ, Samra JS, Kench JG, Musgrove EA, Tucker KM, Spigelman AD, Waddell N, Grimmond SM, Biankin AV. Australian Pancreatic Cancer Genome Initiative. Clinical and pathologic features of familial pancreatic cancer. Cancer. 2014 Dec 1;120(23):3669–75. doi: 10.1002/cncr.28863. Epub 2014 Oct 14. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs EJ, Chanock SJ, Fuchs CS, Lacroix A, McWilliams RR, Steplowski E, Stolzenberg-Solomon RZ, Arslan AA, Bueno-de-Mesquita HB, Gross M, Helzlsouer K, Petersen G, Zheng W, Agalliu I, Allen NE, Amundadottir L, Boutron-Ruault MC, Buring JE, Canzian F, Clipp S, Dorronsoro M, Gaziano JM, Giovannucci EL, Hankinson SE, Hartge P, Hoover RN, Hunter DJ, Jacobs KB, Jenab M, Kraft P, Kooperberg C, Lynch SM, Sund M, Mendelsohn JB, Mouw T, Newton CC, Overvad K, Palli D, Peeters PH, Rajkovic A, Shu XO, Thomas G, Tobias GS, Trichopoulos D, Virtamo J, Wactawski-Wende J, Wolpin BM, Yu K, Zeleniuch-Jacquotte A. Family history of cancer and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan) Int J Cancer. 2010 Sep 1;127(6):1421–8. doi: 10.1002/ijc.25148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000 Jul 13;343(2):78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 18.Klein AP, Beaty TH, Bailey-Wilson JE, Brune KA, Hruban RH, Petersen GM. Evidence for a major gene influencing risk of pancreatic cancer. Genet Epidemiol. 2002 Aug;23(2):133–49. doi: 10.1002/gepi.1102. [DOI] [PubMed] [Google Scholar]
- 19.Banke MG, Mulvihill JJ, Aston CE. Inheritance of pancreatic cancer in pancreatic cancer-prone families. Med Clin North Am. 2000 May;84(3):677–90. doi: 10.1016/s0025-7125(05)70250-9. [DOI] [PubMed] [Google Scholar]
- 20.Hruban RH, Petersen GM, Kern S, Ha P. Genetics of cancer of the pancreas: from genes to families. In: Pitt HA, editor. Surgical Oncology Clinics of North America. 1. Vol. 7. New York: W. B. Saunders; 1998. pp. 1–23. Pancreatic Cancer. [PubMed] [Google Scholar]
- 21.Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, Griffin C, Cameron JL, Yeo CJ, Kern S, Hruban RH. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004 Apr 1;64(7):2634–8. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute Surveillance, Epidemiology, and End Results Program. http://seer.cancer.gov/
- 23.Lynch HT, Smyrk T, Kern SE, Hruban RH, Lightdale CJ, Lemon SJ, Lynch JF, Fusaro LR, Fusaro RM, Ghadirian P. Familial pancreatic cancer: a review. Semin Oncol. 1996 Apr;23(2):251–75. [PubMed] [Google Scholar]
- 24.Klein AP. Genetic susceptibility to pancreatic cancer. Mol Carcinog. 2012 Jan;51(1):14–24. doi: 10.1002/mc.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eberle MA, Pfutzer R, Pogue-Geile KL, et al. A new susceptibility locus for autosomal dominant pancreatic cancer maps to chromosome 4q32-34. Am J Hum Genet. 2002;70:1044–8. doi: 10.1086/339692. Epub 2002 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pogue-Geile KL, Chen R, Bronner MP, et al. Palladin mutation causes familial pancreatic cancer and suggests a new cancer mechanism. PLoS Med. 2006;3:e516. doi: 10.1371/journal.pmed.0030516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein AP, Borges M, Griffith M, Brune K, Hong SM, Omura N, Hruban RH, Goggins M. Absence of deleterious palladin mutations in patients with familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2009 Apr;18(4):1328–30. doi: 10.1158/1055-9965.EPI-09-0056. Epub 2009 Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Heijden MS, Yeo CJ, Hruban RH, Kern SE. Fanconi anemia gene mutations in young-onset pancreatic cancer. Cancer Res. 2003 May 15;63(10):2585–8. [PubMed] [Google Scholar]
- 29.Rogers CD, van der Heijden MS, Brune K, Yeo CJ, Hruban RH, Kern SE, Goggins M. The genetics of FANCC and FANCG in familial pancreatic cancer. Cancer Biol Ther. 2004 Feb;3(2):167–9. doi: 10.4161/cbt.3.2.609. Epub 2004 Feb 1. [DOI] [PubMed] [Google Scholar]
- 30.Couch FJ, Johnson MR, Rabe K, Boardman L, McWilliams R, de Andrade M, Petersen G. Germ line Fanconi anemia complementation group C mutations and pancreatic cancer. Cancer Res. 2005 Jan 15;65(2):383–6. [PubMed] [Google Scholar]
- 31.McWilliams RR, Petersen GM, Rabe KG, Holtegaard LM, Lynch PJ, Bishop MD, Highsmith WE., Jr Cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations and risk for pancreatic adenocarcinoma. Cancer. 2010 Jan 1;116(1):203–9. doi: 10.1002/cncr.24697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy KM, Brune KA, Griffin C, et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17% Cancer Res. 2002 Jul 1;62(13):3789–3793. PubMed: 12097290. [PubMed] [Google Scholar]
- 33.Couch FJ, Johnson MR, Rabe KG, et al. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2007 Feb;16(2):342–346. doi: 10.1158/1055-9965.EPI-06-0783. PubMed: 17301269. [DOI] [PubMed] [Google Scholar]
- 34.Stadler ZK, Salo-Mullen E, Patil SM, et al. Prevalence of BRCA1 and BRCA2 mutations in Ashkenazi Jewish families with breast and pancreatic cancer. Cancer. 2012 Jan 15;118(2):493–499. doi: 10.1002/cncr.26191. PubMed: 21598239. [DOI] [PubMed] [Google Scholar]
- 35.Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, Parsons DW, Lin JC, Palmisano E, Brune K, Jaffee EM, Iacobuzio-Donahue CA, Maitra A, Parmigiani G, Kern SE, Velculescu VE, Kinzler KW, Vogelstein B, Eshleman JR, Goggins M, Klein AP. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009 Apr 10;324(5924):217. doi: 10.1126/science.1171202. Epub 2009 Mar 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts NJ, 1, Jiao Y, Yu J, Kopelovich L, Petersen GM, Bondy ML, Gallinger S, Schwartz AG, Syngal S, Cote ML, Axilbund J, Schulick R, Ali SZ, Eshleman JR, Velculescu VE, Goggins M, Vogelstein B, Papadopoulos N, Hruban RH, Kinzler KW, Klein AP. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov. 2012 Jan;2(1):41–6. doi: 10.1158/2159-8290.CD-11-0194. Epub 2011 Dec 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhen DB, Rabe KG, Gallinger S, Syngal S, Schwartz AG, Goggins MG, Hruban RH, Cote ML, McWilliams RR, Roberts NJ, Cannon-Albright LA, Li D, Moyes K, Wenstrup RJ, Hartman AR, Seminara S, Klein AP, Petersen GM. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer (FPC): A PACGENE study. Genet Med. 2014 Nov 20; doi: 10.1038/gim.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grant RC, Selander I, Connor AA, Selvarajah S, Borgida A, Briollais L, Petersen GM, Lerner-Ellis J, Holter S, Gallinger S. prevalence of germline mutations in cancer predisposition genes in patients with pancreatic cancer. Gastroenterology. 2014 Dec 2; doi: 10.1053/j.gastro.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lowery MA, Kelsen DP, Stadler ZK, Yu KH, Janjigian YY, Ludwig E, D'Adamo DR, Salo-Mullen E, Robson ME, Allen PJ, Kurtz RC, O'Reilly EM. An emerging entity: pancreatic adenocarcinoma associated with a known BRCA mutation: clinical descriptors, treatment implications, and future directions. Oncologist. 2011;16(10):1397–402. doi: 10.1634/theoncologist.2011-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vyas O, Leung K, Ledbetter L, Kaley K, Rodriguez T, Garcon MC, Saif MW. Clinical outcomes in pancreatic adenocarcinoma associated with BRCA-2 mutation. Anticancer Drugs. 2015 Feb;26(2):224–6. doi: 10.1097/CAD.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 41.National Institutes of Health. http://ClinicalTrials.gov.
- 42.Verna EC, Hwang C, Stevens PD, Rotterdam H, Stavropoulos SN, Sy CD, Prince MA, Chung WK, Fine RL, Chabot JA, Frucht H. Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res. 2010 Oct 15;16(20):5028–37. doi: 10.1158/1078-0432.CCR-09-3209.. Epub 2010 Sep 28. [DOI] [PubMed] [Google Scholar]
- 43.Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW. ACG Clinical Guideline: Genetic Testing and Management of Hereditary Gastrointestinal Cancer Syndromes. Am J Gastroenterol. 2015 Feb;110(2):223–62. doi: 10.1038/ajg.2014.435. Epub 2015 Feb 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fendrich V, Langer P, Bartsch DK. Familial pancreatic cancer--status quo. Int J Colorectal Dis. 2014 Feb;29(2):139–45. doi: 10.1007/s00384-013-1760-3. Epub 2013 Aug 16. [DOI] [PubMed] [Google Scholar]
- 45.Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I, Nio Y, Schulick RS, Bassi C, Kluijt I, Levy MJ, Chak A, Fockens P, Goggins M, Bruno M International Cancer of Pancreas Screening (CAPS) Consortium. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013 Mar;62(3):339–47. doi: 10.1136/gutjnl-2012-303108. Epub 2012 Nov 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartsch DK, Gress TM, Langer P. Familial pancreatic cancer--current knowledge. Nat Rev Gastroenterol Hepatol. 2012 Aug;9(8):445–53. doi: 10.1038/nrgastro.2012.111. [DOI] [PubMed] [Google Scholar]
- 47.Langer P, Kann PH, Fendrich V, Habbe N, Schneider M, Sina M, Slater EP, Heverhagen JT, Gress TM, Rothmund M, Bartsch DK. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut. 2009 Oct;58(10):1410–8. doi: 10.1136/gut.2008.171611. Epub 2009 May 25. [DOI] [PubMed] [Google Scholar]
- 48.Templeton AW, 1, Brentnall TA. Screening and surgical outcomes of familial pancreatic cancer. Surg Clin North Am. 2013 Jun;93(3):629–45. doi: 10.1016/j.suc.2013.02.002. Epub 2013 Apr 13. [DOI] [PubMed] [Google Scholar]
- 49.Falk RT, Pickle LW, Fontham ET, Correa P, Fraumeni JF., Jr Life-style risk factors for pancreatic cancer in Louisiana: a case–control study. Am J Epidemiol. 1988;128(2):324–336. doi: 10.1093/oxfordjournals.aje.a114972. [DOI] [PubMed] [Google Scholar]
- 50.Ghadirian P, Liu G, Gallinger S, Schmocker B, Paradis AJ, Lal G, Brunet JS, Foulkes WD, Narod SA. Risk of pancreatic cancer among individuals with a family history of cancer of the pancreas. Int J Cancer. 2002 Feb 20;97(6):807–10. doi: 10.1002/ijc.10123. [DOI] [PubMed] [Google Scholar]
- 51.Fernandez E, La Vecchia C, D'Avanzo B, Negri E, Franceschi S. Family history and the risk of liver, gallbladder, and pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 1994 Apr-May;3(3):209–12. [PubMed] [Google Scholar]
- 52.Silverman DT, Schiffman M, Everhart J, Goldstein A, Lillemoe KD, Swanson GM, Schwartz AG, Brown LM, Greenberg RS, Schoenberg JB, Pottern LM, Hoover RN, Fraumeni JF., Jr Diabetes mellitus, other medical conditions and familial history of cancer as risk factors for pancreatic cancer. Br J Cancer. 1999 Aug;80(11):1830–7. doi: 10.1038/sj.bjc.6690607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inoue M, Tajima K, Takezaki T, Hamajima N, Hirose K, Ito H, Tominaga S. Epidemiology of pancreatic cancer in Japan: a nested case-control study from the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC) Int J Epidemiol. 2003 Apr;32(2):257–62. doi: 10.1093/ije/dyg062. [DOI] [PubMed] [Google Scholar]
- 54.Schenk M, Schwartz AG, O'Neal E, Kinnard M, Greenson JK, Fryzek JP, Ying GS, Garabrant DH. Familial risk of pancreatic cancer. J Natl Cancer Inst. 2001 Apr 18;93(8):640–4. doi: 10.1093/jnci/93.8.640. [DOI] [PubMed] [Google Scholar]
- 55.Austin MA, Kuo E, Van Den Eeden SK, Mandelson MT, Brentnall TA, Kamineni A, Potter JD. Family history of diabetes and pancreatic cancer as risk factors for pancreatic cancer: the PACIFIC study. Cancer Epidemiol Biomarkers Prev. 2013 Oct;22(10):1913–7. doi: 10.1158/1055-9965.EPI-13-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McWilliams RR, Rabe KG, Olswold C, De Andrade M, Petersen GM. Risk of malignancy in first-degree relatives of patients with pancreatic carcinoma. Cancer. 2005 Jul 15;104(2):388–94. doi: 10.1002/cncr.21166. [DOI] [PubMed] [Google Scholar]
- 57.Iqbal J, Ragone A, Lubinski J, Lynch HT, Moller P, Ghadirian P, Foulkes WD, Armel S, Eisen A, Neuhausen SL, Senter L, Singer CF, Ainsworth P, Kim-Sing C, Tung N, Friedman E, Llacuachaqui M, Ping S, Narod SA Hereditary Breast Cancer Study Group. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2012 Dec 4;107(12):2005–9. doi: 10.1038/bjc.2012.483. Epub 2012 Oct 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Axilbund J, Wiley E. Genetic Testing by Cancer Site: Pancreas. Cancer Journal. 18(4):350-354, 2012. Semin Oncol. 1996 Apr;23(2):251–75. doi: 10.1097/PPO.0b013e3182624694. [DOI] [PubMed] [Google Scholar]


