Abstract
Rapid reduction of suicidal thoughts is critical for treating suicidal patients. Clinical trials evaluating these treatments require appropriate measurement. Key methodological issues include: 1) the use of single or multi-item assessments, and 2) evaluating whether suicidal ideation measures can track rapid change over time. The current study presents data from two randomized, placebo-controlled, crossover clinical trials evaluating ketamine in individuals with treatment-resistant depression (n=60). Participants were assessed for suicidal thoughts using the Hamilton Depression Rating Scale (HAM-D), Montgomery-Asberg Depression Rating Scale (MADRS), Beck Depression Inventory (BDI), and Scale for Suicidal Ideation (SSI) at eight time points over three days. Assessments were compared using correlational analyses and effect sizes at 230 minutes and three days after ketamine infusion. Linear mixed models evaluated change in ideation across all time points. The HAM-D and MADRS suicide items demonstrated correlations of r > .80 with the first five items of the SSI (SSI5). On linear mixed models, an effect for ketamine was found for the HAM-D, MADRS, BDI items, and SSI5 (p<.001), but not for the full SSI (p=.88), which suggests a limited ability to assess change over time in patients with low levels of suicidal thoughts. Taken together, the results suggest that repeated suicidal assessments over minutes to days appear to detect improvement in suicidal thoughts after ketamine infusion compared to placebo. The MADRS suicide item, BDI suicide item, and SSI5 may be particularly sensitive to rapid changes in suicidal thoughts.
Keywords: suicidal ideation, assessment, ketamine, psychometrics, depression
Introduction
Recent research with rapid-acting antidepressants, such as the N-methyl-D-aspartate (NMDA) receptor antagonist ketamine, has shown that such agents are capable of reducing depressive symptoms within hours rather than weeks, as seen with traditional antidepressants (Zarate et al., 2012, Zarate et al., 2006). A burgeoning area of interest is the potential reduction in suicidal thoughts after such treatment, given that ketamine has been associated with decreased suicidal thoughts within two hours of administration (DiazGranados et al., 2010a, Larkin and Beautrais, 2011, Price et al., 2014, Zarate, Brutsche, 2012). Because just one medication (clozapine) is FDA-approved for suicide risk (and, indeed, clozapine is only indicated for individuals with schizophrenia and is not considered to be rapid-acting), effective treatments for suicidal thoughts are urgently needed. Notably, the development of fast-acting interventions for individuals at risk for suicide was recently highlighted by the US National Action Alliance for Suicide Prevention as a critical research priority (National Action Alliance for Suicide Prevention: Research Prioritization Task Force, 2014).
Because rapid-acting antidepressants such as ketamine are evaluated for their potential efficacy in alleviating suicidal thoughts, appropriate assessment and measurement of changes in suicide risk will be needed. Suicide measurements range from lifetime comprehensive evaluations of suicide risk factors to single items from a depression assessment (Brown, 2002, Goldston, 2000). In contrast to the way such assessments are typically administered for conventional antidepressant agents, rapid-acting treatments require repeated assessments over hours to days. Such a study design raises several questions about assessment. First, which type of assessment should be used? In the interest of time, would it be adequate to rely on a single item from a depression scale to assess suicide risk rather than a longer suicide-specific assessment (Desseilles et al., 2012)? Second, are current suicide assessments sensitive to change over relatively short time periods? For instance, in such evaluations, does repeated questioning about suicide over the course of a single day impact the validity of patient responses? These questions underscore the importance of evaluating the psychometrics of repeated suicide assessment instruments, both in direct comparisons as well as tracking responses over time.
Our ketamine clinical trials focused on evaluating ketamine’s antidepressant effects as the primary outcome; nevertheless, these trials also assessed suicidal thoughts using a number of different methods, including single items from the clinician-administered Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1960), the Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979), and the self-reported Beck Depression Inventory (BDI) (Beck et al., 1961), as well as a suicide-specific measure, the Scale for Suicide Ideation (SSI) (Beck et al., 1979). Over the course of several placebo-controlled clinical trials, these measures have been administered repeatedly within minutes, hours, and days after ketamine infusion. The variety of measures administered at each time point facilitates the comparison of correlations and correlated change between single items and longer versions of suicide assessments. In addition, data from double-blind, crossover, placebo-controlled studies permit us to investigate how responses to these suicide assessments may change over time. Such analyses can guide the design and interpretation of studies evaluating the potential efficacy of ketamine—among other rapid-acting antidepressants—as an anti-suicidal agent.
Material and Methods
Participants
Data in the present study were drawn from two randomized, crossover, placebo-controlled trials evaluating the efficacy of ketamine in the treatment of depression (both treatment-resistant major depressive disorder (MDD) and bipolar I or II (BD) depression); results of these studies have been published elsewhere (Diazgranados et al., 2010b, Ibrahim et al., 2012, Zarate, Brutsche, 2012, Zarate, Singh, 2006). Participants were admitted to the Experimental Therapeutics and Pathophysiology Branch of the NIMH as inpatients. Written informed consent was obtained from all patients in accordance with the NIH Combined Neuroscience (CNS) Institutional Review Board. Diagnoses of MDD and BD were confirmed by Structured Clinical Interview for Axis I Diagnostic and Statistical Manual (DSM)–IV-TR Disorders, patient version (SCID-I/P) (First et al., 2001). Patients were rated as experiencing a major depressive episode with moderate to severe levels of severity (objectively defined as ≥ 18 on the 17-item HAM-D (Zarate, Singh, 2006), or ≥ 20 or ≥ 22 on the MADRS (Diazgranados, Ibrahim, 2010b, Zarate, Brutsche, 2012)) at the time of screening and before each infusion. Participants ages 18–65 years currently experiencing a depressive episode without psychotic features, and with no diagnosis of substance use within the three months prior to consent (with the exception of nicotine or caffeine) were eligible for participation.
As part of these placebo-controlled trials, ketamine hydrochloride (0.5 mg/kg) was administered intravenously over 40 minutes. Saline infusion was administered as a placebo. As part of the clinical trial protocol, all participants were required to be medication-free for at least two weeks before ketamine infusion (five weeks for fluoxetine), with the exception of BD patients who were maintained on therapeutic doses of either lithium or valproate. Participants could be excluded in one of the trials if they had acute suicidal thoughts (defined by a score of over 4 on item 10 of the MADRS or by clinical judgment) at time of consent; this means that acutely suicidal patients were not consented into the study and then withdrawn from their medications. In addition, participants whose suicidal thoughts increased over the course of the medication taper or clinical trial were not systematically excluded from study participation. It should be noted that one patient was withdrawn from the study during the time period under analysis due to both worsening depression and suicidal thoughts.
Measurements
The assessment instruments used in the present study included the HAM-D, MADRS, BDI, and SSI. The HAM-D is a 17-item clinician-administered measure of depression severity (Hamilton, 1960). It includes one item assessing suicide risk, which is rated on a scale of 0 (“absent”) to 4 (“attempts at suicide”). The MADRS is a 10-item clinician-administered measure of depression severity (Montgomery and Asberg, 1979). The MADRS includes one item assessing suicide risk, which is rated on a scale of 0 (“enjoys life or takes it as it comes”) to 6 (“explicit plans for suicide when there is an opportunity; active preparations for suicide”). Odd-numbered ratings are not given specific definitions. The BDI is a widely used 21-item self-report measure of depression severity (Beck, Ward, 1961). It includes one item assessing suicidal thoughts on a scale from 0 (“I don’t have any thoughts of killing myself”) to 3 (“I would kill myself if I had the chance”). Finally, the SSI is a 19-item, clinician-administered assessment of suicidal thoughts (Beck, Kovacs, 1979). The first five items assess wish to live, wish to die, reasons for living or dying, desire to make an active suicide attempt, and passive suicidal thoughts. If the patient gives a positive response on the last two items, then the remaining 14 items are administered. These items include characteristics of suicidal thoughts, potential attempts, and any preparation towards making an actual attempt. For the purposes of this analysis, scores from the first five SSI items (SSI5) and all SSI items (SSITotal) were included as potential measures of suicidal thoughts.
Timing of Measurements
Assessments were administered 60 minutes before ketamine infusion. During this assessment, patients were asked to report their symptoms during the last 24 hours. Post-infusion assessments occurred at 40, 80, 120, and 230 minutes and at Days 1, 2, and 3. At each of these assessments, patients were asked to report their symptoms since the last assessment (i.e. at the 230-minute assessment, patients reported on their symptoms for the last two hours; at the Day 3 assessment, they described symptoms for the last 24 hours).
Statistical Analysis
Comparison of single- and multi-item suicide assessments
This analysis focused on the 230-minute and Day 3 assessment time points in order to capture one brief and one more distal time point in relation to a range of time points after ketamine infusion. The 230-minute time point is often used in ketamine analyses because it assesses the short-term effects of ketamine infusion after psychotomimetic effects have dissipated (Niciu et al., 2014, Zarate, Singh, 2006). The Day 3 assessment was used because it was further removed from the time of ketamine infusion. The presence or absence of baseline suicidal ideation was determined using the literature on appropriate cut-off scores (Brown, 2002, Weitz et al., 2014). A score of 1 or more on the HAM-D suicide item, 1 or more on the BDI suicide item, 2 or more on the MADRS suicide item, or 2 or more on the SSITotal or SSI5 were considered to be “any suicidal ideation” (as compared to acute suicidal ideation, which commonly requires higher cutoff scores). Pearson correlations of scales at static points were conducted to assess convergent validity.
Change in ideation across time
Pearson correlations of absolute change from baseline to 230 minutes and Day 3 after ketamine infusion were performed to assess sensitivity to change. Linear mixed models were used to evaluate changes in suicidal ideation across the seven time points after infusion. Included in the model were time and intervention status as fixed within-subjects factors, as well as a fixed intercept and the interaction between drug and time. Linear mixed models were limited to participants who reported any ideation at baseline on that specific measure (i.e. the HAM-D model included participants who scored 1 or more on the HAM-D suicide item at baseline) and also controlled for baseline suicidal ideation. A compound symmetry covariance structure and restricted maximum likelihood estimates were used. Cohen’s d effect sizes of the difference in ideation scores at 230 minutes and Day 3 between ketamine and placebo were calculated. Due to the use of linear mixed models, all figures include estimated marginal means and standard errors of these mean estimates. As a post-hoc analysis, the first timepoint at which suicidal ideation response to ketamine was determined to be significantly different from placebo was evaluated. IBM SPSS version 21 was used for statistical analyses and significance was considered at p<.05, two-tailed.
Results
Sixty participants were included in the analysis, 23 with MDD and 37 with BD. Of the sample, 37 were female (62%) with a mean age of 41.6 years (SD = 11.3). Lifetime suicide attempts were reported by 48% (n = 28) of the sample and 18% (n = 11) reported more than one lifetime suicide attempt. The average length of illness was 25.3 years (SD= 11.4). Differences between measures and the frequency of suicidal thoughts at baseline are presented in Table 1.
Table 1.
Description of Study Measures and Frequencies at Baseline
| Percentage Reporting Suicidal Ideation | ||||
|---|---|---|---|---|
| Rater | Number of Items | Any | Acute | |
| HAM-D Suicide | Clinician | 1 | 62% scored 1 or higher “Wishes to be dead or any thoughts of possible death to self” |
7% scored 3 “Suicidal ideas or gestures” |
| MADRS Suicide | Clinician | 1 | 70% scored 2 or higher “Weary of life. Only fleeting suicidal thoughts.” |
8% scored 4 “Probably better off dead, suicidal thoughts are common, and suicide is considered as a possible solution, but without specific plans or intention” |
| BDI Suicide | Self | 1 | 43% scored 1 or higher “I have thoughts of killing myself, but would not carry them out.” |
9% scored 3 “I would like to kill myself” |
| SSI5/SSITotal | Clinician | 5 or 19 Five items used as screen:
|
50% scored 2 or higher (SSITotal) | 5% scored 5 or higher (SSITotal) |
HAM-D: Hamilton Depression Rating Scale; MADRS: Montgomery Asberg Depression Rating Scale; BDI: Beck Depression Inventory; SSI5: First five items from Scale for Suicide Ideation; SSITotal: All items from Scale for Suicide Ideation
Correlations between each of the suicide assessments at 230 minutes and Day 3 are presented in Table 2. All correlations had r>.60, and correlations between the HAM-D suicide item, MADRS suicide item, and SSI5 were r>.80. The BDI and SSITotal demonstrated lower correlations overall.
Table 2.
Pearson Correlation Matrix of Suicidal Ideation Items at 230 Minutes and Three Days Post-Infusion
| 230 Minutes
| |||||
|---|---|---|---|---|---|
| HAM-D | MADRS | BDI | SSITotal | SSI5 | |
| HAM-D | -- | .86** | .69** | .73** | .87** |
| MADRS | -- | .65** | .63** | .81** | |
| BDI | -- | .75** | .77** | ||
| SSITotal | -- | .88* | |||
| SSI5 | -- | ||||
| Day 3
| |||||
|---|---|---|---|---|---|
| HAM-D | MADRS | BDI | SSITotal | SSI5 | |
| HAM-D | -- | .81** | .66** | .65** | .81** |
| MADRS | -- | .65** | .57** | .80** | |
| BDI | -- | .63** | .77** | ||
| SSITotal | -- | .81** | |||
| SSI5 | -- | ||||
HAM-D: Hamilton Depression Rating Scale; MADRS: Montgomery Asberg Depression Rating Scale; BDI: Beck Depression Inventory; SSI5: First five items from Scale for Suicide Ideation; SSITotal: All items from Scale for Suicide Ideation
p < .05
p < .01
Correlations of change from baseline between each of the suicide assessments are presented in Table 3. All correlations had r>.40. The strongest correlation across both time points was between the SSI5 and the BDI; correlations between the BDI and other single item measures were lower.
Table 3.
Pearson Correlation Matrix of Change in Suicidal Ideation Items at 230 Minutes and Three Days Post-Infusion
| 230 Minutes
| |||||
|---|---|---|---|---|---|
| HAM-D | MADRS | BDI | SSITotal | SSI5 | |
| HAM-D | -- | .71** | .54** | .55** | .59** |
| MADRS | -- | .45** | .53** | .62** | |
| BDI | -- | .58** | .78** | ||
| SSITotal | -- | .75** | |||
| SSI5 | -- | ||||
| Day 3
| |||||
|---|---|---|---|---|---|
| HAM-D | MADRS | BDI | SSITotal | SSI5 | |
| HAM-D | -- | .61** | .47** | .59** | .59** |
| MADRS | -- | .43** | .55** | .62** | |
| BDI | -- | .70** | .74** | ||
| SSITotal | -- | .82** | |||
| SSI5 | -- | ||||
HAM-D: Hamilton Depression Rating Scale; MADRS: Montgomery Asberg Depression Rating Scale; BDI: Beck Depression Inventory; SSI5: First five items from Scale for Suicide Ideation; SSITotal: All items from Scale for Suicide Ideation
p < .05.
p < .01.
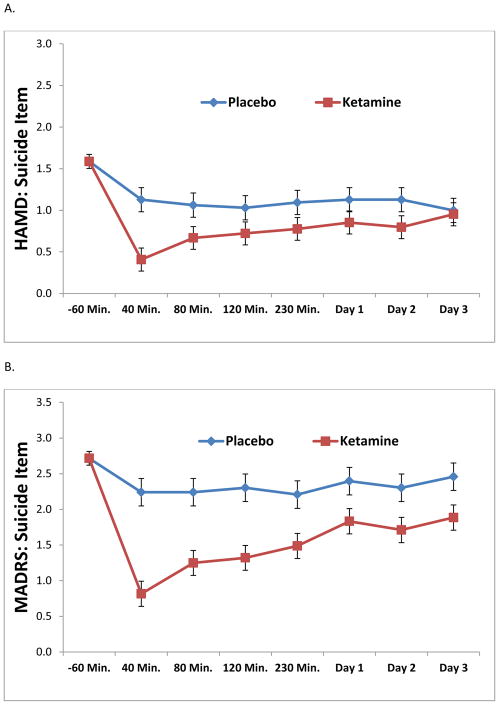
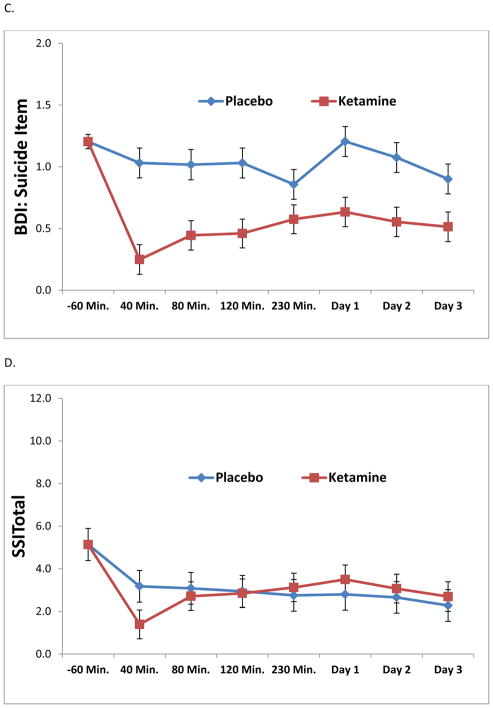
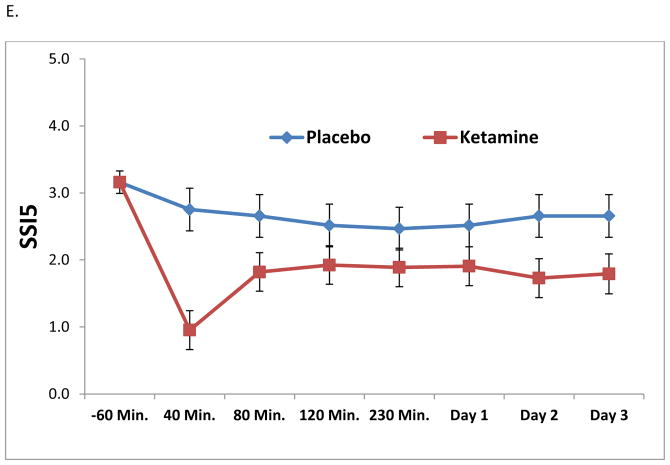
Results from the linear mixed models are presented in Table 4. All analyses, with the exception of SSITotal, demonstrated significant drug effects of ketamine on suicidal thoughts (p < .01). Figures 1A through 1E depict the change in ideation scores over time using each measure. Across 230 minutes and Day 3, the strongest effect sizes were demonstrated by the MADRS item, the BDI item, and the SSI5; taken together, these results suggest sensitivity to change. As a post-hoc analysis, the first time point of significant difference between ketamine and placebo was 40 minutes for all assessments (p < .05). It should be noted that, due to concerns about the potential psychotomimetic effects of ketamine at the 40-minute time point, the 80-minute time point was also evaluated. All assessments, with the exception of SSITotal, demonstrated a significant difference between ketamine and placebo at the 80-minute time point.
Table 4.
Linear Mixed Models of Main Effects, Interactions, and Effect Sizes for Suicidal Ideation Items
| Suicide item or assessment | Drug | Time by Drug | Cohen’s d for ketamine compared to placebo | |||
|---|---|---|---|---|---|---|
|
| ||||||
| F | p | F | p | 230 minutes post-infusion | Day 3 post-infusion | |
| HAM-D | 25.14 | <.001 | 1.62 | .14 | .20 | .03 |
| MADRS | 81.06 | <.001 | 2.33 | .03 | .32 | .25 |
| BDI | 99.11 | <.001 | 1.88 | .08 | .28 | .38 |
| SSITotal | .02 | .88 | 1.81 | .10 | .06 | .06 |
| SSI5 | 39.40 | <.001 | 1.25 | .28 | .20 | .30 |
Note: Each model included patients who had suicidal thoughts at baseline on that assessment (i.e. the HAM-D model included only patients who reported ideation at baseline). Models also adjusted for baseline suicidal thoughts.
HAM-D: Hamilton Depression Rating Scale; MADRS: Montgomery Asberg Depression Rating Scale; BDI: Beck Depression Inventory; SSI5: First five items from Scale for Suicide Ideation; SSITotal: All items from Scale for Suicide Ideation
Figure 1.
Figure 1A–E. Linear mixed models demonstrating the effects of ketamine on suicidal ideation compared to placebo by item or assessment. In order to compare across measures, all data were plotted across a y-axis of approximately three standard deviations of the data.
Because results from the SSITotal differed dramatically from results with the other items, an outlier analysis was conducted. One participant had a very high score on the SSITotal (score > 20) and was considered to be an “extreme” outlier (interquartile range*3). When the linear mixed model was run excluding this participant, results remained non-significant [drug effect: F(1,314) = 2.02, p = .14; interaction: F(6, 285) = 1.15, p = .33].
Discussion
This study evaluated the repeated assessment of several measures of suicidal ideation across three days post-ketamine infusion. In a sample of treatment-resistant depressed patients with either MDD or BD who received ketamine, we found that the HAM-D and MADRS suicide items were strongly correlated with the SSI5. The SSI5, MADRS item, and BDI item demonstrated particular sensitivity to rapid change as demonstrated by moderate effect sizes. In contrast, the longer version of the SSI (SSITotal) was not as sensitive to rapid changes in suicidal thoughts.
With regard to which type of assessment of suicidal ideation should be used, our findings suggest that single-item and multi-item measures appear to yield comparable assessments. Correlations demonstrated adequate agreement, particularly when comparing the HAM-D and MADRS suicide items and the SSI5 (r’s > .80). It should be noted that, in contrast to the HAM-D, MADRS, and SSI, the BDI is a self-reported measure, which may have led to differences across the measures. In addition, when comparing correlations between the single items, the SSITotal, and the SSI5, the SSITotal demonstrated reduced agreement. It is possible that the differing administration of the SSI (some patients are administered five items and some 19, depending on their level of severity) may have introduced unwanted variability into the correlations, effect sizes, and linear mixed models. Nevertheless, results suggest that the HAM-D and MADRS suicide items may have convergent validity with other clinician-administered measures in samples of depressed patients with relatively low levels of suicidal thoughts.
To address the second question posed by this analysis, we used repeated assessments capable of capturing changes in suicidal ideation over a short period of time. As demonstrated by the results from linear mixed models, the trajectory of suicide symptoms over the course of three days differed significantly from the trajectory on placebo for most scales. The MADRS, BDI, and SSI5 also detected a “small to moderate” effect for ketamine on suicidal ideation at 230 minutes and Day 3 post-infusion, as demonstrated by the Cohen’s d between drug and placebo. Again, the linear mixed model using SSITotal as the outcome measure found no significant drug effect (p = .88), which may have been due to the increased variability introduced by using the extra items. In contrast, the SSI5 was sensitive to rapid changes in suicidal thoughts over a short period of time. The time point at which a difference between ketamine and placebo was detected was 40 minutes for all measures, although this difference was not found at 80 minutes for the SSITotal. The 40-minute time point is clinically significant because ketamine’s dissociative effects may not have dissipated at this time point, which may limit the validity of patient response; this, in turn, led to our decision to focus on the 230-minute time point assessment for correlations and effect sizes. It is important to note that despite the repeated suicide assessment, we found no evidence of a iatrogenic increase in suicidal symptoms, which is consistent with other findings in the literature (Crawford et al., 2011, Gould et al., 2005, Mathias et al., 2012). In addition, overall suicidal ideation was reduced in the drug condition when compared to the placebo arm, suggesting that this improvement was not simply due to repeated assessments.
The most significant limitation of this post-hoc analysis is that patients were selected as part of a clinical trial for treatment-resistant depression and not for acute suicide risk. Further studies of patients selected for suicidal thoughts, with and without depressive symptoms, are indicated. Such investigations may also benefit from additional suicide-specific measurements such as the Suicide Status Form (SSF) (Jobes et al., 2004) or the Columbia Suicide Severity Rating Scale (C-SSRS) (Posner et al., 2011) in addition to those reported here. Moreover, the present study used several timeframes across the three days of assessment, which means that the assessment at the 230-minute time point is not directly comparable with the Day 3 time point. Similar analyses that lasted for seven days post-ketamine infusion also repeatedly assessed depressive symptoms (Luckenbaugh et al., 2014), although this time frame was not consistently available with the current dataset. Lastly, it is important to highlight that all suicidal thoughts were reported by the patient. No implicit measures (Nock et al., 2010) or suicide biomarkers were tracked over the same time period in this sample. Indeed, the lack of validated suicide biomarkers is a significant obstacle to suicide research (Lee and Kim, 2011), and suicidal thoughts, while clinically significant, are only a proxy for suicide risk (Klonsky and May, 2014). As research expands into the phenomenology and neurobiology of treatment for suicide, further understanding of suicidal ideation measures and implicit/neurobiological measures will be needed.
Conclusions
With the advent of rapid-acting interventions for suicide risk, instruments that can be quickly administered but are sensitive enough to detect rapid changes in suicidal thoughts over time will be critical. While single items from depression scales may correlate with longer suicide assessment measures, the results of the current investigation suggest that the MADRS suicide item, the BDI suicide item, and the first five items of the SSI may be particularly suited to assessing rapid changes in suicidal thoughts.
Highlights.
Clinical trials of ketamine and suicide will require appropriate measurement.
We compared several suicide assessment measures in ketamine clinical trials.
Single items and longer measures of suicidal thoughts were correlated.
Items from the MADRS, BDI, and SSI may be particularly sensitive to rapid changes.
Acknowledgments
The authors thank the 7SE research unit and staff for their support. Ioline Henter, MA (NIMH) provided invaluable editorial assistance.
Role of Funding Source
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; NCT00088699 and 04-M-0222), by a NARSAD Independent Investigator to CAZ, and by a Brain & Behavior Mood Disorders Research Award to CAZ. The NIMH, NARSAD, and the Brain & Behavior Research Foundation had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Author Contributions
Contributors: All authors contributed to manuscript writing or data analysis, and agreed to submit the final version for publication.
EDB: conceptualized the study design; completed and interpreted the statistical analysis; drafted the manuscript; edited the manuscript for intellectual content; and approved the final manuscript before submission.
DAL: helped conceptualize study design; assisted in the statistical design, analysis, and interpretation; edited the manuscript for intellectual content; and approved the final manuscript before submission.
EMR: assisted in interpreting the statistical analysis; edited the manuscript for intellectual content; and approved the final manuscript before submission.
TLW: assisted in interpreting the statistical analysis; edited the manuscript for intellectual content; and approved the final manuscript before submission.
NEB: assisted in interpreting the statistical analysis; edited the manuscript for intellectual content; and approved the final manuscript before submission.
RA: assisted in interpreting the statistical analysis; edited the manuscript for intellectual content; and approved the final manuscript before submission.
MJN: assisted in interpreting the statistical analysis; edited the manuscript for intellectual content; and approved the final manuscript before submission.
JLVV: assisted in interpreting the statistical analysis; edited the manuscript for intellectual content; and approved the final manuscript before submission.
CAZ: provided research supervision; assisted in conceptualizing the study design; assisted in interpreting the statistical analysis; edited the manuscript for intellectual content; and approved the final manuscript before submission.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth D. Ballard, Email: elizabeth.ballard@nih.gov.
David A. Luckenbaugh, Email: luckenbd@mail.nih.gov.
Erica M. Richards, Email: erica.richards@nih.gov.
Tessa L. Walls, Email: tessa.walls@nih.gov.
Nancy E. Brutsché, Email: brutschn@mail.nih.gov.
Rezvan Ameli, Email: amelir@mail.nih.gov.
Mark J. Niciu, Email: mark.niciu@nih.gov.
Jennifer L. Vande Voort, Email: VandeVoort.Jennifer@mayo.edu.
Carlos A. Zarate, Jr, Email: zaratec@mail.nih.gov.
References
- Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol. 1979;47:343–52. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Brown GK. A review of suicide assessment measures for intervention research with adults and older adults. National Institute of Mental Health; 2002. [Google Scholar]
- Desseilles M, Perroud N, Guillaume S, Jaussent I, Genty C, Malafosse A, et al. Is it valid to measure suicidal ideation by depression rating scales? J Affect Disord. 2012;136:398–404. doi: 10.1016/j.jad.2011.11.013. [DOI] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim L, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010a;71:1605–11. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010b;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV TR Axis I Disorders, Research Version, Patient Edition. New York: New York State Psychiatric Institute, Biometrics Research; 2001. [Google Scholar]
- Goldston D. Assessment of suicidal behaviors and risk among children and adolescents. Technical report submitted to NIMH under Contract No. 263-MD-909995. 2000 [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37:1526–33. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobes DA, Nelson KN, Peterson EM, Pentiuc D, Downing V, Francini K, et al. Describing suicidality: an investigation of qualitative SSF responses. Suicide Life Threat Behav. 2004;34:99–112. doi: 10.1521/suli.34.2.99.32788. [DOI] [PubMed] [Google Scholar]
- Klonsky ED, May AM. Differentiating suicide attempters from suicide ideators: a critical frontier for suicidology research. Suicide Life Threat Behav. 2014;44:1–5. doi: 10.1111/sltb.12068. [DOI] [PubMed] [Google Scholar]
- Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14:1127–31. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- Lee BH, Kim YK. Potential peripheral biological predictors of suicidal behavior in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:842–7. doi: 10.1016/j.pnpbp.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Luckenbaugh DA, Ameli R, Brutsche N, Zarate CA. Rating depression over brief time intervals with the Hamilton Depression Rating Scale: Standard vs. abbreviated scales. J Psychiatr Res. 2014 doi: 10.1016/j.jpsychires.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- National Action Alliance for Suicide Prevention: Research Prioritization Task Force. A prioritized research agenda for suicide prevention: An action plan to save lives. Rockville, MD: National Institute of Mental Health and the Research Prioritization Task Force; 2014. [Google Scholar]
- Niciu MJ, Luckenbaugh DA, Ionescu DF, Guevara S, Machado-Vieira R, Richards EM, et al. Clinical predictors of ketamine response in treatment-resistant major depression. J Clin Psychiatry. 2014;75:e417–23. doi: 10.4088/JCP.13m08698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Park JM, Finn CT, Deliberto TL, Dour HJ, Banaji MR. Measuring the suicidal mind: implicit cognition predicts suicidal behavior. Psychol Sci. 2010;21:511–7. doi: 10.1177/0956797610364762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–77. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ, et al. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety. 2014;31:335–43. doi: 10.1002/da.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz E, Hollon SD, Kerkhof A, Cuijpers P. Do depression treatments reduce suicidal ideation? The effects of CBT, IPT, pharmacotherapy, and placebo on suicidality. J Affect Disord. 2014;167:98–103. doi: 10.1016/j.jad.2014.05.036. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–46. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]