Abstract
We have shown that bipolar individuals have reduced quality diets, including lower intake of polyunsaturated fatty acids (PUFA). We have also reported reduced plasma levels of the n-6 PUFA, linoleic acid (LA), and the n-3 PUFA, eicosapentaenoic acid (EPA) in bipolar subjects. In the current analysis we hypothesized that LA and EPA plasma levels would mediate lower self-reported mental health and life functioning scores in bipolar subjects. In a cross-sectional study, we collected a 7-day diet record in bipolar (n=56) and control subjects (n=46) followed by a fasted blood draw. We used structured equation modeling path analysis to test for mediating effects of dietary intake and plasma levels of LA and EPA on self-reported mental health questionnaire scores, including the Life Functioning Questionnaire (LFQ), the Patient Health Questionnaire (PHQ9), and the Short Form Health Survey (SF12), extracting the mental health component summary score (SF12-MH). We adjusted for age, gender, psychiatric medication use, body mass index (BMI), and total caloric intake as covariates with bipolar disorder as the primary predictor. We found a significant path association from bipolar disorder to lower plasma LA levels (p=0.03) and significant paths from plasma LA to PHQ9 (p=0.05), LFQ (p=0.01) and SF12-MH (p=0.05) scores, such that lower plasma LA predicted worse outcomes. We found no significant paths from plasma EPA levels to any of the outcome measures. These findings suggest that plasma LA levels partially mediate the effect of bipolar disorder on self-reported measures of mental health and life functioning.
Keywords: linoleic acid, LA, eicosapentaenoic acid, EPA, life functioning questionnaire, LFQ, short form health questionnaire, SF12, patient health questionnaire, PHQ9, structural equation modeling, path analysis, bipolar disorder
Introduction
Several studies have evaluated nutrient intakes as risk factors for mood disorders. Among the most studied nutrients in the risk of depressive disorders are the dietary polyunsaturated fatty acids (PUFA), especially the n-3 PUFA. Global studies report that dietary intakes of the n-3 PUFA, eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3), high in fatty fish, are negatively associated with incidence of depression and bipolar disorder (Abhinav et al., 2007; Hibbeln, Nieminen, Blasbalg, Riggs, & Lands, 2006; Noaghiul & Hibbeln, 2003). Studies of post-mortem brain tissue have found anomalous PUFA composition in bipolar and depressed subjects. Expression of the rate limiting enzymes responsible for synthesis of long chain n-6 and n-3 PUFA, including EPA and DHA, were lower in pre-frontal cortex of subjects with bipolar disorder (Liu & McNamara, 2011), and a related study showed variant PUFA composition in the orbital frontal cortex of bipolar subjects (McNamara et al., 2008). Furthermore, several studies have shown an association between n-3 PUFA tissue levels and suicide. In a Belgian study seasonal variation in n-3 intake associated with the rate of violent suicides (De Vriese, Christophe, & Maes, 2004); in a Chinese study blood cell levels of EPA were lower in suicide attempters (Huan et al., 2004); both EPA and arachidonic acid (AA; 20:4n-6) were lower in plasma from U.S. bipolar subjects with a history of suicide attempt (Evans et al., 2012); and a study of suicidality in American military personal showed an inverse association between serum DHA levels and suicide risk (Lewis et al., 2011). However a large Japanese study found that only very low fish intake in women predicted suicidality (Poudel-Tandukar et al., 2011) and analysis of existing data for large U.S. cohorts found no associations between fish intake and completed suicide, thus the data remain equivocal. Supplementation trials of omega-3 PUFA have improved depression scores in patients with recurrent self-harm (Hallahan, Hibbeln, Davis, & Garland, 2007), menopausal women with psychological distress (Lucas, Asselin, Merette, Poulin, & Dodin, 2009), elderly depressed women (Rondanelli et al., 2010), elderly patients with mild cognitive impairment (Sinn et al., 2012), and children with juvenile bipolar disorder (Clayton et al., 2009). However, recent meta-analyses have failed to conclude strong support for use of n-3 PUFA as anti-depressive agents in depression (Bloch & Hannestad, 2012) or bipolar disorder (Montgomery & Richardson, 2008), citing methodological heterogeneity and publication bias.
Less attention has been given to potential roles of the n-6 PUFA in regulating mood disorders, although they have been intensively studied in cardiovascular disease (CVD), which itself is a risk factor for depressive illness (Hare, Toukhsati, Johansson, & Jaarsma, 2014). The western diet is relatively high in the essential n-6, linoleic acid (LA; 18:2n-6), which comprises approximately 8% of total dietary energy in the United States (Hibbeln et al., 2006), but its role in health and disease is also controversial. A secondary prevention study that replaced saturated fat with LA in an Australian cohort of men with recent coronary events showed an increase of all-cause and cardiovascular related mortality in the treatment group (Ramsden et al., 2013b), and a recent review argues that high dietary LA associates with increased inflammatory markers (Choque, Catheline, Rioux, & Legrand, 2013). Contrarily, two recent reviews by Willett (Willett, 2007) and Czernichow et al. (Czernichow, Thomas, & Bruckert, 2010) conclude that increased dietary LA associates with reduced inflammatory markers and reduced CVD risk. These paradoxical studies further underscore the complexity of dietary PUFA metabolism in risk of disease.
The n-6 PUFA, LA, and the n-3 PUFA, alpha linolenic acid (LNA; 18:3n-3), are essential fatty acids that we must obtain from the diet, since we cannot synthesize them de novo. The longer chain n-6 and n-3 fatty acids, respectively, can be synthesized from these essential PUFA, although there are suggestions that the n-3s, EPA and DHA, are semi-essential as the rate of conversion from LNA is not efficient in humans (Plourde & Cunnane, 2007). The 18-carbon essential fatty acids, LA and LNA, compete for desaturation and elongation; and the 20-carbon n-6, dihomogamma linolenic acid (DGLA; 20:3n-6), the 20-carbon n-6, AA, and the 20-carbon n-3, EPA, compete as cyclooxygenase substrates for production of series 1, series 2 and series 3 prostaglandins, respectively, which have opposing inflammatory activities. PUFA metabolism is also central to production of oxidized metabolites with peroxisome proliferator activating receptor activity, endocannabinoids and a host of other bioactive metabolites (figure 1) in a highly complex system controlled by genetic (Tanaka et al., 2009), dietary (Rodriguez-Cruz, Sanchez Gonzalez, Sanchez Garcia, & Lopez-Alarcon, 2012), and other environmental factors (for a comprehensive review of lipid metabolism and human health see Leray, 2015 (Leray, 2015)). Furthermore, studies suggest that atypical antipsychotics (AAP) and mood stabilizer medications, commonly prescribed for treatment of bipolar disorder, influence PUFA metabolism. Mechanistic studies in rodents show inhibition of AA turnover and processing in brain phospholipids by the AAP, olanzapine and clozapine, (Cheon et al., 2011; Kim, Cheon, Modi, Rapoport, & Rao, 2012; Modi et al., 2013) and common mood stabilizer medications (Rapoport, Basselin, Kim, & Rao, 2009); and the AAP, risperidone and paliperidone, increase liver biosynthesis and erythrocyte membrane composition of PUFA (McNamara et al., 2011a). In humans, AAP and mood stabilizer use by bipolar subjects associates with n-3 and n-6 metabolite levels in plasma (Evans et al., 2014)
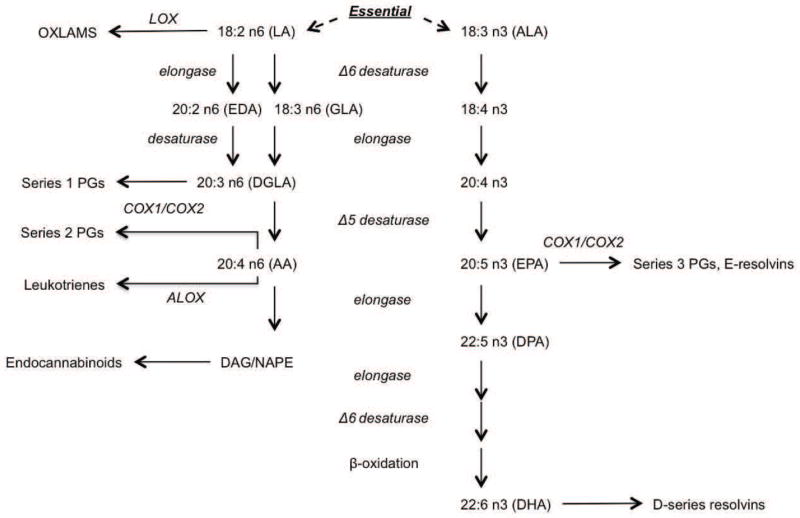
Figure 1.
Schematic of PUFA metabolism showing several metabolic fates of the essential n-6 fatty acid, LA and the essential n-3 fatty acid, LNA. Metabolism of LA leads to bioactive metabolites with immune, endothelial, and energy homeostasis functions. Abbreviations: OXLAMS, oxidized linoleic acid metabolites; LOX, lipoxygenase; LA, linoleic acid; EDA, eicosadienoic acid; GLA, gamma-linolenic acid; DGLA, dihomo-gamma-linolenic acid; PGs, prostaglandins; COX, cyclooxygenase; AA, arachidonic acid; DAG, diacyl glycerol; NAPE, N-acyl-phosphatidylethanolamine; ALA, alpha-linolenic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid; DHA, docosahexaenoic acid.
We have recently reported reduced n-6 and n-3 PUFA intake in a cross-sectional study of individuals with bipolar disorder (Evans et al., 2014). We found significantly reduced dietary intake of EPA, DHA and AA in bipolar individuals. Furthermore, we found reduced levels of EPA and LA, as well as several LA metabolites in plasma from bipolar individuals after correcting for age, gender, dietary intake, BMI, and psychiatric medication use; suggesting potential dysregulation of LA metabolism in bipolar disorder (Evans et al., 2014). Taken together the literature on the role of PUFA in regulating mood disorders suggests that dietary intake as well as factors regulating PUFA metabolism may independently associate with mood disorders and interact to yield their effects. Thus, studies would benefit from combining dietary and metabolic studies with clinical psychiatric outcome measures to understand the complex interactions.
In the current manuscript we use path analysis to evaluate dietary intake and plasma levels of LA and EPA, which we have previously reported to be lower in bipolar individuals, as mediators of the effect of bipolar illness on lower self-reported clinical measures of mental health and functionality. Specifically, we tested if the path from dietary intake to plasma levels of these PUFA mediate the effect of bipolar disorder on depression (assayed by the 9-item Patient Health Questionnaire, PHQ9 (Kroenke, Spitzer, & Williams, 2001)), life functionality (assayed by the Life Functioning Questionnaire, LFQ (Altshuler, Mintz, & Leight, 2002)) or self-reported mental health (assayed by the Short Form Healthy Survey, mental health component summary score, SF12-MH (Ware, Kosinski, & Keller, 1996)).
Subjects, Materials and Methods
Human Subjects
All subjects in the current study were recruited from the Heinz C. Prechter Longitudinal Study of Bipolar Disorder at the University of Michigan Depression Center (Langenecker, Saunders, Kade, Ransom, & McInnis, 2010). All individuals were diagnosed using the Diagnostic Interview for Genetic Studies (DIGS) (Nurnberger et al., 1994) and included in this current study are bipolar I individuals adhering to the DSM IV diagnostic criteria (American Psychiatric Association., 2000), with no current substance abuse and healthy unaffected controls who were willing to complete daily dietary log records for 7 days. All but two bipolar subjects were euthymic at the time of blood sampling, as determined by the Young Mania Rating Scale and the Hamilton Depression Scale. Most bipolar subjects were taking more than one psychiatric medication, which was adjusted for as described in the Statistical Methods section. All protocols were approved by the Internal Review Board for Human Studies at the University of Michigan.
Dietary Analysis
One hundred fifty four nutrients were extracted from seven-day diet records using the Nutrition Data System for Research (NDSR) software, 2011 version. All study subjects met with a dietician prior to the seven-day recording period for instruction on how to track foods and estimate portion sizes. Upon returning the food record, all subjects again met with a dietician who personally curated the seven-day record in the presence of the subject to clarify any misunderstood or under-detailed entries. For the purpose of the current analysis, nutrient data were collapsed to represent average daily intake and statistically analyzed as detailed below. One subject was omitted from the analysis with an excessive average daily caloric intake of approximately 6,000 kCal that was skewing the regression models and 6 subjects were omitted due to insufficient dietary data (e.g. unrealistically low caloric intake or missing diet records).
Lipomic Analysis
Total lipids were extracted from 75ul of fasted plasma according to the method of Bligh and Dyer (Bligh & Dyer, 1959). Heptadecanoic acid internal standards for lipid sub-classes were added to each sample prior to extraction. After hydrolysis, lipids were methylated and analyzed on an Omega Wax 250 capillary column (Supelco) using an Agilent 6890 gas chromatograph. Relative abundance of 22 different naturally occurring fatty acid species were done by comparison of retention times with known standards. Fatty acids were reported as nmol concentration terms and normalized to percent of total for statistical analysis of each of the 22 species.
Statistical Methods
To improve normality of the data, dietary intakes, including total caloric intake was natural log transformed, as were plasma LA and EPA levels (as percent total fatty acids). Psychiatric medication uses were coded as binary variables (taking or not taking), independently for three major classes of medications: 1) SSRI or SNRI anti-depressants (duloxetine, fluoxetine, sertraline, escitalopram, desvenlafaxine, venlafaxine), 2) mood stabilizers or anticonvulsants (lithium, lamotrigine, carbamazepine, topiramate, divalproex, gabapentin) or 3) atypical antipsychotics (clozapine, olanzapine, aripiprazole, risperidone, paliperidone, quetiapine, ziprasidone). These classes are abbreviated AD, AE and AAP for antidepressant use, anti-epileptic/mood stabilizer use and atypical antipsychotic use, respectively. No other classes of psychiatric medications were represented in more than 2 research subjects.
Data Analyses
SPSS Amos (Version 22, IBM Corp) was used to perform all statistical analyses. Models were set up to test paths from bipolar disorder to self-reported mental health scores through serial mediation of dietary LA intake and plasma LA, or dietary EPA intake and plasma EPA (figure 2). Covariates with bipolar diagnosis included age, gender, psychiatric medication, total caloric intake, and BMI. Models were designed beginning with all paths. Psychiatric medications to dietary intake paths were eliminated due to lack of significance and absence of justification for these relationships. Total caloric intakes to plasma levels of PUFA were also eliminated due to lack of significance after accounting for dietary intakes. Finally, direct paths from dietary intake to clinical outcomes were eliminated, as they were not significant independent of plasma PUFA concentrations. All other paths were left either due to significance or logical justification. Path analysis results were output as standardized beta coefficients for each hypothesized path and associated p-values. Models were run separately for each of the 3 self-reported clinical outcome measures and evaluated for quality.
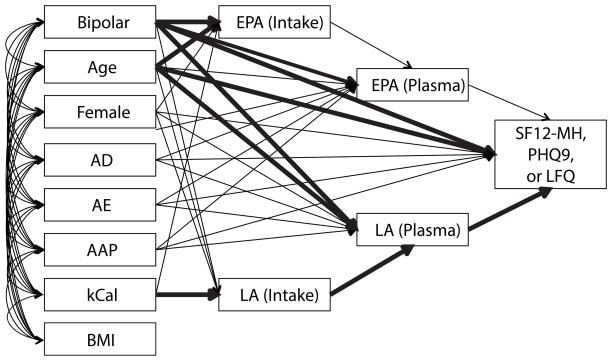
Figure 2.
Schematic of Path Analysis models. The left row of variates gives all the co-variates in the models and shows the allowed associations with the serial mediators on a path toward the clinical outcome measures on the far right. Three separate models were run; one for each of the self-reported scores shown. Table 2 gives the standardized beta coefficients and significance level for each of the associations schematically shown in the figure.
Results
Table 1 gives demographic characteristics of the study subjects as well as statistics for psychiatric medication use, self-report questionnaire scores, dietary intakes and relative plasma PUFA concentrations (as percent total fatty acids). We have previously detailed case-control dietary intake and plasma level differences, correcting for relevant covariates (Evans et al., 2014), so do not discuss that further here. Instead, we focus on associations with self-reported mental health measures. Using path analysis we tested for mediating effect of dietary and plasma LA and EPA levels in associations with mental health outcome measures derived from three self-reported questionnaires from bipolar and healthy control individuals. The PUFA, LA and EPA, were specifically analyzed because we previously found them to be decreased in bipolar subjects relative to controls after correcting for age, gender, psychiatric medication use, BMI, and dietary intake (Evans et al., 2014). The models for the current analyses are given in figure 2.
Table 1.
Subject Descriptives.
| Bipolar (n=56) | Control (n=46) | p-value | |
|---|---|---|---|
| Female (%) | 71.4 | 50 | 0.039 |
| Age in yrs (sd) | 44.2 (12.3) | 43.0 (15.4) | 0.656 |
| BMI (sd) | 30.0 (7.2) | 26.2 (4.7) | <0.001 |
| AD (% users) | 42.9 | 0 | - |
| AE (% users) | 62.5 | 0 | - |
| AAP (% users) | 48.2 | 0 | - |
| LFQ (sd) | 20.6 (8.9) | 14.9 (5.1) | <0.001 |
| PHQ9 (sd) | 7.16 (6.05) | 1.13 (1.86) | <0.001 |
| SF12-MH (sd) | 30.4 (12.4) | 44.2 (8.1) | <0.001 |
|
| |||
| Plasma PUFA as % total fatty acids, mean (sd) | |||
| LA | 31.4 (4.54) | 33.5 (4.57) | 0.027 |
| DGLA | 1.98 (0.46) | 1.72 (0.48) | 0.003 |
| AA | 8.67 (2.23) | 8.74 (2.30) | 0.908 |
| ALA | 0.52 (0.22) | 0.53 (0.21) | 0.719 |
| EPA | 0.34 (0.37) | 0.45 (0.39) | 0.066 |
| DHA | 1.23 (0.87) | 1.45 (0.99) | 0.254 |
|
| |||
| Dietary intakes as % total energy, mean (sd) | |||
| total Protein | 15.6 (3.0) | 16.5 (2.8) | 0.144 |
| total Carb | 49.2 (8.3) | 47.2 (9.0) | 0.390 |
| total Fat | 35.7 (6.9) | 35.4 (7.1) | 0.769 |
| SFA | 11.4 (3.2) | 11.5 (2.9) | 0.368 |
| MUFA | 12.7 (2.7) | 12.6 (3.2) | 0.783 |
| PUFA | 8.65 (2.31) | 8.22 (1.95) | 0.749 |
| LA | 7.66 (2.13) | 7.19 (1.77) | 0.609 |
| AA | 0.05 (0.03) | 0.07 (0.04) | 0.021 |
| ALA | 0.84 (0.32) | 0.76 (0.32) | 0.662 |
| EPA | 0.01 (0.02) | 0.04 (0.05) | 0.007 |
| DHA | 0.03 (0.05) | 0.09 (0.13) | 0.007 |
| Alcohol | 2.15 (4.46) | 2.87 (4.64) | 0.925 |
All models showed good fits with p > 0.1, ChiSq/df < 2.0, CFI > 0.95 and RMSEA < 0.06. After controlling for age, gender, psychiatric medication use, total caloric intake, and BMI, plasma LA but not plasma EPA partially mediated the effect of bipolar disorder on composite mental health and functionality scores from the LFQ, PHQ-9 and SF12-MH (Table 2). Thus, there were significant paths from bipolar disorder to plasma LA level (p = 0.03) and plasma LA level to mental health measures with poorer outcomes for the LFQ (p = 0.01), the PHQ9 (p = 0.05) and the SF12-MH (p = 0.05). There was also a significant path from bipolar to plasma EPA levels (p = 0.01), however this did not extend to a significant association with any mental health outcomes measured. Direct associations between dietary intake of either LA or EPA are not included in the final model because they decreased the model fit. However, when tested, neither was significantly associated with any of the mental health outcomes (all p > 0.05). All tested paths and those found significant are schematically shown in figure 2 and all tabular results are given in table 2.
Table 2.
Path analysis standardized regression coefficients and significance levels
| Dependent | Independent | std β | p | |
|---|---|---|---|---|
| LA (Intake) | <--- | BP | 0.03 | 0.69 |
| LA (Intake) | <--- | Age | 0.03 | 0.59 |
| LA (Intake) | <--- | Female | 0.14 | 0.06 |
| LA (Intake) | <--- | kCal | 0.81 | *** |
| EPA (Intake) | <--- | BP | −0.34 | *** |
| EPA (Intake) | <--- | Age | 0.26 | 0.00 |
| EPA (Intake) | <--- | Female | −0.01 | 0.94 |
| EPA (Intake) | <--- | kCal | 0.16 | 0.10 |
| LA (Plasma) | <--- | LA (Intake) | 0.42 | *** |
| LA (Plasma) | <--- | BP | −0.31 | 0.03 |
| LA (Plasma) | <--- | Age | −0.24 | 0.01 |
| LA (Plasma) | <--- | Female | 0.10 | 0.29 |
| LA (Plasma) | <--- | AD | 0.15 | 0.15 |
| LA (Plasma) | <--- | AAP | 0.23 | 0.03 |
| LA (Plasma) | <--- | AE | −0.17 | 0.15 |
| LA (Plasma) | <--- | BMI | −0.19 | 0.04 |
| EPA (Plasma) | <--- | EP (Intake) | 0.18 | 0.07 |
| EPA (Plasma) | <--- | BP | −0.40 | 0.01 |
| EPA (Plasma) | <--- | Age | 0.07 | 0.48 |
| EPA (Plasma) | <--- | Female | 0.16 | 0.09 |
| EPA (Plasma) | <--- | AD | 0.11 | 0.32 |
| EPA (Plasma) | <--- | AAP | 0.26 | 0.02 |
| EPA (Plasma) | <--- | AE | 0.10 | 0.44 |
| EPA (Plasma) | <--- | BMI | −0.08 | 0.45 |
|
| ||||
| LFQ | <--- | LA (Plasma) | −0.25 | 0.01 |
| LFQ | <--- | EPA (Plasma) | −0.08 | 0.42 |
| LFQ | <--- | BP | 0.28 | 0.06 |
| LFQ | <--- | Age | −0.09 | 0.37 |
| LFQ | <--- | Female | 0.13 | 0.17 |
| LFQ | <--- | BMI | 0.01 | 0.92 |
|
| ||||
| PHQ9 | <--- | LA (Plasma) | −0.16 | 0.05 |
| PHQ9 | <--- | EPA (Plasma) | 0.00 | 0.98 |
| PHQ9 | <--- | BP | 0.35 | 0.01 |
| PHQ9 | <--- | Age | −0.12 | 0.16 |
| PHQ9 | <--- | Female | 0.02 | 0.80 |
| PHQ9 | <--- | BMI | 0.17 | 0.05 |
|
| ||||
| SF12-MH | <--- | LA (Plasma) | 0.17 | 0.05 |
| SF12-MH | <--- | EPA (Plasma) | 0.00 | 0.99 |
| SF12-MH | <--- | BP | −0.42 | 0.00 |
| SF12-MH | <--- | Age | 0.16 | 0.07 |
| SF12-MH | <--- | Female | −0.09 | 0.31 |
| SF12-MH | <--- | BMI | −0.01 | 0.88 |
Discussion
In the current manuscript we report the testing of a statistical mediation model whereby we hypothesized that dietary intake of either LA or EPA would mediate the level of plasma LA or EPA, respectively, which would mediate response to self-reported mental health questionnaires. We found that plasma levels of both LA and EPA were significantly lower in bipolar subjects after controlling for age, gender, psychiatric medication use, dietary intake and BMI (consistent with our previous report (Evans et al., 2014)) and that LA plasma levels significantly associated with PHQ9, LFQ and SF12-MH scores such that lower LA plasma levels predicted clinically worse scores. We found no association between plasma EPA and any of the self-reported measures. Dietary intake of LA strongly predicted LA plasma levels, whereas dietary intake of EPA moderately predicted EPA plasma levels; and dietary intake of EPA but not LA was significantly lower in bipolar individuals. However, dietary intake EPA did not predict the clinical outcome measures. These data suggest that dietary intake of EPA explains the lower plasma levels of EPA observed in bipolar individuals, but that this does not associate with the clinical outcomes in the models tested. Conversely, lower plasma levels of LA in bipolar individuals significantly associate with mental and functional clinical outcome measures, but are not completely explained by dietary intake; suggesting that metabolism of LA varies in bipolar individuals and associates with the tested clinical outcomes. To our knowledge, this is the first report of direct associations between LA plasma levels and psychiatric clinical outcome measures.
Although higher plasma LA levels associated with better mental health and life functioning clinical outcome measures, we are cautions to interpret these data in a way to mean that increasing LA plasma (through increased dietary intake) would be beneficial in this context. Dietary LA intake did not associate with tested clinical measures and was not lower in bipolar subjects relative to controls, yet plasma LA levels were lower in bipolar subjects. These data suggest that metabolism of LA may be variant in bipolar disorder and that reduced plasma levels may be a marker of downstream metabolic processes that are responsible for the association with the clinical outcome measures.
Dietary LA is a direct or indirect source of a plethora of bioactive metabolites, including pro- and anti-inflammatory prostaglandins, endocannabinoids, and oxidized linoleic acid metabolites (OXLAMs) with endothelial function (figure 1). Reduced plasma LA in bipolar subjects after adjusting for dietary intake may be indicative of increased activity of any of these or other LA metabolic pathways, potentially compensating for the observed reduced intake of AA. In this scenario, increasing dietary LA to increase plasma levels may further drive variant metabolism and have unintended clinical consequences. Whereas, increasing dietary n-3s could compete for enzymatic processing and biosynthesis of downstream metabolites (see figure 1) and normalize the activity of LA metabolism. This hypothesis requires further testing of controlled dietary n-3 and n-6 intake studies coupled with systematic analysis of the various metabolic fates of PUFA and their associations with mental health clinical endpoints.
The benefit of decreased dietary LA intake with increased dietary n-3 has been shown in chronic headache suffers for both reduction of headache symptoms (Ramsden et al., 2013a), which are comorbid in bipolar disorder (Saunders et al., 2014) and reduction of metabolic endophenotypes, specifically LA-derived OXLAMS (Ramsden et al., 2012). The study controlled dietary intake of LA at a target of 2% total energy (relative to approximately 8% total energy in the average western diet), with or without n-3 supplementation (MacIntosh et al., 2013). A low n-6 diet without supplementation of n-3 lowered LA and increased n-3 PUFA but had no effect on AA levels. However, adding n-3 PUFA supplementation to the low LA diet effectively lowered AA as well (Taha et al., 2014). Another study showed increased EPA in low-compared to high-LA diets, with constant LNA intake (Liou, King, Zibrik, & Innis, 2007). These studies highlight the cross-influence that manipulating n-3 and n-6 PUFA intake levels have on biosynthesis of each other. Thus, diets low in the n-6, LA, increased the n-3 EPA, even under constant n-3 intake; and diets supplementing n-3s were able to reduce the n-6, AA, under low n-6 intake conditions. Other studies have shown that tissue AA levels are fairly resilient to dietary manipulation and are largely controlled genetically (Baylin, Ruiz-Narvaez, Kraft, & Campos, 2007) and it make take both reduced n-6 intake and increased in n-3 intake to effect a change, as seen in headache study above. In our subjects we have similar dietary intake of LA but reduced plasma LA levels and reduced intake of AA but similar plasma AA levels in bipolar subjects. This might be explained by increased use of LA to maintain AA levels in conditions of reduced AA intake. Furthermore, couple this with reduced EPA intake by bipolar subjects, which could relieve competition with AA for the production of inflammatory metabolites, and this may tip the scales toward inflammation, providing a possible explanation for the association of low LA levels with worse clinical outcomes.
In summary, we showed for the first time that low plasma LA associates with higher self-reported depression scores, lower self reported mental health scores and reduced self reported life functioning. However, a few limitations to this study warrant caution in the interpretation of these results. Reduced LA plasma levels in bipolar subjects with similar dietary intakes to controls suggests variant metabolism of LA, but we have not identified the metabolic pathway that explains the different plasma levels. We are currently pursuing cytokine profiles in the same samples and intend to collect urinary samples for prostaglandin metabolite profiling in future studies, to better understand inflammatory signatures that may mediate the effect of low plasma LA on clinical outcomes. Also, we only assayed total fatty acids in plasma so cannot comment on the potential important distribution of free to esterified species or phospholipid composition, however we have assays in progress to examine these profiles. The complexity of PUFA metabolism, interactions and competition between n-3 and n-6 species and the mechanism by which these may influence mental health requires continued study.
Highlights.
We used structured equation modeling path analysis
We hypothesized that plasma linoleic acid and eicosapentaenoic acid would mediate association of bipolar disorder with self-reported mental health
We found plasma linoleic acid but not eicosapentaenoic acid mediated association of bipolar disorder with SF12, PHQ9 and LFQ self-reported questionnaire scores.
Acknowledgments
The authors would like to thank Anh Trong for database management and Dominic Misiak for human subject recruitment; the staff at the Michigan Clinical Research Unit for their assistance in dietary data analysis and the staff at the Michigan Regional Comprehensive Metabolomics Resource Core for their assistance with metabolomics data interpretation.
Funding Sources
All components of the questionnaire collection and project infrastructure were supported by The Heinz C. Prechter Fund for Bipolar Research, dietary analysis was supported by NIH Grant # 5-K01-MH-093708-04 (Evans), lipomic assays were supported by the Michigan Nutrition Obesity Research Center (NIH grant #DK089503), and core services were supported by grant DK097153 of NIH to the University of Michigan.
Footnotes
Contributors:
SJE designed the experiments, aided in analysis of the data and wrote the manuscript; YWC aided in writing the revision of the manuscript; SA provided expertise in equation modeling and path analysis; GJH oversaw all recruitment and subject selection; CFB oversaw all lipomic assays and provided expertise in interpretation of lipomics data; MGM oversaw all clinical questionnaire data collection and provided expertise in analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abhinav K, Stanton B, Johnston C, Hardstaff J, Orrell RW, Howard R, Clarke J, Sakel M, Ampong MA, Shaw CE, Leigh PN, Al-Chalabi A. Amyotrophic lateral sclerosis in South-East England: a population-based study. The South-East England register for amyotrophic lateral sclerosis (SEALS Registry) Neuroepidemiology. 2007;29:44–48. doi: 10.1159/000108917. [DOI] [PubMed] [Google Scholar]
- Altshuler L, Mintz J, Leight K. The Life Functioning Questionnaire (LFQ): a brief, gender-neutral scale assessing functional outcome. Psychiatry Res. 2002;112:161–182. doi: 10.1016/s0165-1781(02)00180-4. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic criteria from DSM-IV-TR. Washington, D.C: American Psychiatric Association; 2000. [Google Scholar]
- Baylin A, Ruiz-Narvaez E, Kraft P, Campos H. alpha-Linolenic acid, Delta6-desaturase gene polymorphism, and the risk of nonfatal myocardial infarction. Am J Clin Nutr. 2007;85:554–560. doi: 10.1093/ajcn/85.2.554. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Hannestad J. Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis. Mol Psychiatry. 2012;17:1272–1282. doi: 10.1038/mp.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon Y, Park JY, Modi HR, Kim HW, Lee HJ, Chang L, Rao JS, Rapoport SI. Chronic olanzapine treatment decreases arachidonic acid turnover and prostaglandin E(2) concentration in rat brain. J Neurochem. 2011;119:364–376. doi: 10.1111/j.1471-4159.2011.07410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choque B, Catheline D, Rioux V, Legrand P. Linoleic acid: Between doubts and certainties. Biochimie. 2013 doi: 10.1016/j.biochi.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Eur J Clin Nutr. 2009;63:1037–1040. doi: 10.1038/ejcn.2008.81. [DOI] [PubMed] [Google Scholar]
- Czernichow S, Thomas D, Bruckert E. n-6 Fatty acids and cardiovascular health: a review of the evidence for dietary intake recommendations. Br J Nutr. 2010;104:788–796. doi: 10.1017/S0007114510002096. [DOI] [PubMed] [Google Scholar]
- De Vriese SR, Christophe AB, Maes M. In humans, the seasonal variation in polyunsaturated fatty acids is related to the seasonal variation in violent suicide and serotonergic markers of violent suicide. Prostaglandins Leukot Essent Fatty Acids. 2004;71:13–18. doi: 10.1016/j.plefa.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Evans SJ, Prossin AR, Harrington GJ, Kamali M, Ellingrod VL, Burant CF, McInnis MG. Fats and Factors: Lipid Profiles Associate with Personality Factors and Suicidal History in Bipolar Subjects. PLoS One. 2012;7:e29297. doi: 10.1371/journal.pone.0029297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SJ, Ringrose RN, Harrington GJ, Mancuso P, Burant CF, McInnis MG. Dietary intake and plasma metabolomic analysis of polyunsaturated fatty acids in bipolar subjects reveal dysregulation of linoleic acid metabolism. J Psychiatr Res. 2014;57:58–64. doi: 10.1016/j.jpsychires.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan B, Hibbeln JR, Davis JM, Garland MR. Omega-3 fatty acid supplementation in patients with recurrent self-harm. Single-centre double-blind randomised controlled trial. Br J Psychiatry. 2007;190:118–122. doi: 10.1192/bjp.bp.106.022707. [DOI] [PubMed] [Google Scholar]
- Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J. 2014;35:1365–1372. doi: 10.1093/eurheartj/eht462. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Nieminen LR, Blasbalg TL, Riggs JA, Lands WE. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutr. 2006;83:1483S–1493S. doi: 10.1093/ajcn/83.6.1483S. [DOI] [PubMed] [Google Scholar]
- Huan M, Hamazaki K, Sun Y, Itomura M, Liu H, Kang W, Watanabe S, Terasawa K, Hamazaki T. Suicide attempt and n-3 fatty acid levels in red blood cells: a case control study in China. Biol Psychiatry. 2004;56:490–496. doi: 10.1016/j.biopsych.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Kim HW, Cheon Y, Modi HR, Rapoport SI, Rao JS. Effects of chronic clozapine administration on markers of arachidonic acid cascade and synaptic integrity in rat brain. Psychopharmacology (Berl) 2012;222:663–674. doi: 10.1007/s00213-012-2671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Saunders EF, Kade AM, Ransom MT, McInnis MG. Intermediate: cognitive phenotypes in bipolar disorder. J Affect Disord. 2010;122:285–293. doi: 10.1016/j.jad.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leray C. Lipids : nutrition and health. Boca Raton: Taylor & Francis; 2015. [Google Scholar]
- Lewis MD, Hibbeln JR, Johnson JE, Lin YH, Hyun DY, Loewke JD. Suicide deaths of active-duty US military and omega-3 fatty-acid status: a case-control comparison. J Clin Psychiatry. 2011;72:1585–1590. doi: 10.4088/JCP.11m06879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou YA, King DJ, Zibrik D, Innis SM. Decreasing linoleic acid with constant alpha-linolenic acid in dietary fats increases (n-3) eicosapentaenoic acid in plasma phospholipids in healthy men. J Nutr. 2007;137:945–952. doi: 10.1093/jn/137.4.945. [DOI] [PubMed] [Google Scholar]
- Liu Y, McNamara RK. Elevated Delta-6 desaturase (FADS2) gene expression in the prefrontal cortex of patients with bipolar disorder. J Psychiatr Res. 2011;45:269–272. doi: 10.1016/j.jpsychires.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Asselin G, Merette C, Poulin MJ, Dodin S. Ethyl-eicosapentaenoic acid for the treatment of psychological distress and depressive symptoms in middle-aged women: a double-blind, placebo-controlled, randomized clinical trial. Am J Clin Nutr. 2009;89:641–651. doi: 10.3945/ajcn.2008.26749. [DOI] [PubMed] [Google Scholar]
- MacIntosh BA, Ramsden CE, Faurot KR, Zamora D, Mangan M, Hibbeln JR, Mann JD. Low-n-6 and low-n-6 plus high-n-3 diets for use in clinical research. Br J Nutr. 2013;110:559–568. doi: 10.1017/S0007114512005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Cole-Strauss A, Lipton JW. Differential effects of antipsychotic medications on polyunsaturated fatty acid biosynthesis in rats: Relationship with liver delta6-desaturase expression. Schizophr Res. 2011a;129:57–65. doi: 10.1016/j.schres.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Stanford KE, Hahn CG, Richtand NM. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatry Res. 2008;160:285–299. doi: 10.1016/j.psychres.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Liu Y. Reduced expression of fatty acid biosynthesis genes in the prefrontal cortex of patients with major depressive disorder. J Affect Disord. 2011b;129:359–363. doi: 10.1016/j.jad.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi HR, Taha AY, Kim HW, Chang L, Rapoport SI, Cheon Y. Chronic clozapine reduces rat brain arachidonic acid metabolism by reducing plasma arachidonic acid availability. J Neurochem. 2013;124:376–387. doi: 10.1111/jnc.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery P, Richardson AJ. Omega-3 fatty acids for bipolar disorder. Cochrane Database Syst Rev. 2008:CD005169. doi: 10.1002/14651858.CD005169.pub2. [DOI] [PubMed] [Google Scholar]
- Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am J Psychiatry. 2003;160:2222–2227. doi: 10.1176/appi.ajp.160.12.2222. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863-844. [DOI] [PubMed] [Google Scholar]
- Plourde M, Cunnane SC. Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements. Appl Physiol Nutr Metab. 2007;32:619–634. doi: 10.1139/H07-034. [DOI] [PubMed] [Google Scholar]
- Poudel-Tandukar K, Nanri A, Iwasaki M, Mizoue T, Matsushita Y, Takahashi Y, Noda M, Inoue M, Tsugane S Japan Public Health Center-based Prospective Study G. Long chain n-3 fatty acids intake, fish consumption and suicide in a cohort of Japanese men and women--the Japan Public Health Center-based (JPHC) prospective study. J Affect Disord. 2011;129:282–288. doi: 10.1016/j.jad.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Quirk SE, Williams LJ, O’Neil A, Pasco JA, Jacka FN, Housden S, Berk M, Brennan SL. The association between diet quality, dietary patterns and depression in adults: a systematic review. BMC Psychiatry. 2013;13:175. doi: 10.1186/1471-244X-13-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden CE, Faurot KR, Zamora D, Suchindran CM, Macintosh BA, Gaylord S, Ringel A, Hibbeln JR, Feldstein AE, Mori TA, Barden A, Lynch C, Coble R, Mas E, Palsson O, Barrow DA, Mann JD. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: a randomized trial. Pain. 2013a;154:2441–2451. doi: 10.1016/j.pain.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden CE, Ringel A, Feldstein AE, Taha AY, MacIntosh BA, Hibbeln JR, Majchrzak-Hong SF, Faurot KR, Rapoport SI, Cheon Y, Chung YM, Berk M, Mann JD. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot Essent Fatty Acids. 2012;87:135–141. doi: 10.1016/j.plefa.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, Suchindran CM, Ringel A, Davis JM, Hibbeln JR. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ. 2013b;346:e8707. doi: 10.1136/bmj.e8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport SI, Basselin M, Kim HW, Rao JS. Bipolar disorder and mechanisms of action of mood stabilizers. Brain Res Rev. 2009;61:185–209. doi: 10.1016/j.brainresrev.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Cruz M, Sanchez Gonzalez R, Sanchez Garcia AM, Lopez-Alarcon M. Coexisting role of fasting or feeding and dietary lipids in the control of gene expression of enzymes involved in the synthesis of saturated, monounsaturated and polyunsaturated fatty acids. Gene. 2012;496:28–36. doi: 10.1016/j.gene.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Rondanelli M, Giacosa A, Opizzi A, Pelucchi C, La Vecchia C, Montorfano G, Negroni M, Berra B, Politi P, Rizzo AM. Effect of omega-3 fatty acids supplementation on depressive symptoms and on health-related quality of life in the treatment of elderly women with depression: a double-blind, placebo-controlled, randomized clinical trial. J Am Coll Nutr. 2010;29:55–64. doi: 10.1080/07315724.2010.10719817. [DOI] [PubMed] [Google Scholar]
- Sanhueza C, Ryan L, Foxcroft DR. Diet and the risk of unipolar depression in adults: systematic review of cohort studies. J Hum Nutr Diet. 2013;26:56–70. doi: 10.1111/j.1365-277X.2012.01283.x. [DOI] [PubMed] [Google Scholar]
- Saunders EF, Nazir R, Kamali M, Ryan KA, Evans S, Langenecker S, Gelenberg AJ, McInnis MG. Gender differences, clinical correlates, and longitudinal outcome of bipolar disorder with comorbid migraine. J Clin Psychiatry. 2014;75:512–519. doi: 10.4088/JCP.13m08623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn N, Milte CM, Street SJ, Buckley JD, Coates AM, Petkov J, Howe PR. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: a 6-month randomised controlled trial. Br J Nutr. 2012;107:1682–1693. doi: 10.1017/S0007114511004788. [DOI] [PubMed] [Google Scholar]
- Taha AY, Cheon Y, Faurot KF, Macintosh B, Majchrzak-Hong SF, Mann JD, Hibbeln JR, Ringel A, Ramsden CE. Dietary omega-6 fatty acid lowering increases bioavailability of omega-3 polyunsaturated fatty acids in human plasma lipid pools. Prostaglandins Leukot Essent Fatty Acids. 2014;90:151–157. doi: 10.1016/j.plefa.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Shen J, Abecasis GR, Kisialiou A, Ordovas JM, Guralnik JM, Singleton A, Bandinelli S, Cherubini A, Arnett D, Tsai MY, Ferrucci L. Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI Study. PLoS Genet. 2009;5:e1000338. doi: 10.1371/journal.pgen.1000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanskanen A, Hibbeln JR, Hintikka J, Haatainen K, Honkalampi K, Viinamaki H. Fish consumption, depression, and suicidality in a general population. Arch Gen Psychiatry. 2001;58:512–513. doi: 10.1001/archpsyc.58.5.512. [DOI] [PubMed] [Google Scholar]
- Tsai AC, Lucas M, Okereke OI, O’Reilly EJ, Mirzaei F, Kawachi I, Ascherio A, Willett WC. Suicide mortality in relation to dietary intake of n-3 and n-6 polyunsaturated fatty acids and fish: equivocal findings from 3 large US cohort studies. Am J Epidemiol. 2014;179:1458–1466. doi: 10.1093/aje/kwu086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Willett WC. The role of dietary n-6 fatty acids in the prevention of cardiovascular disease. J Cardiovasc Med (Hagerstown) 2007;8 (Suppl 1):S42–45. doi: 10.2459/01.JCM.0000289275.72556.13. [DOI] [PubMed] [Google Scholar]