Abstract
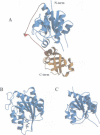
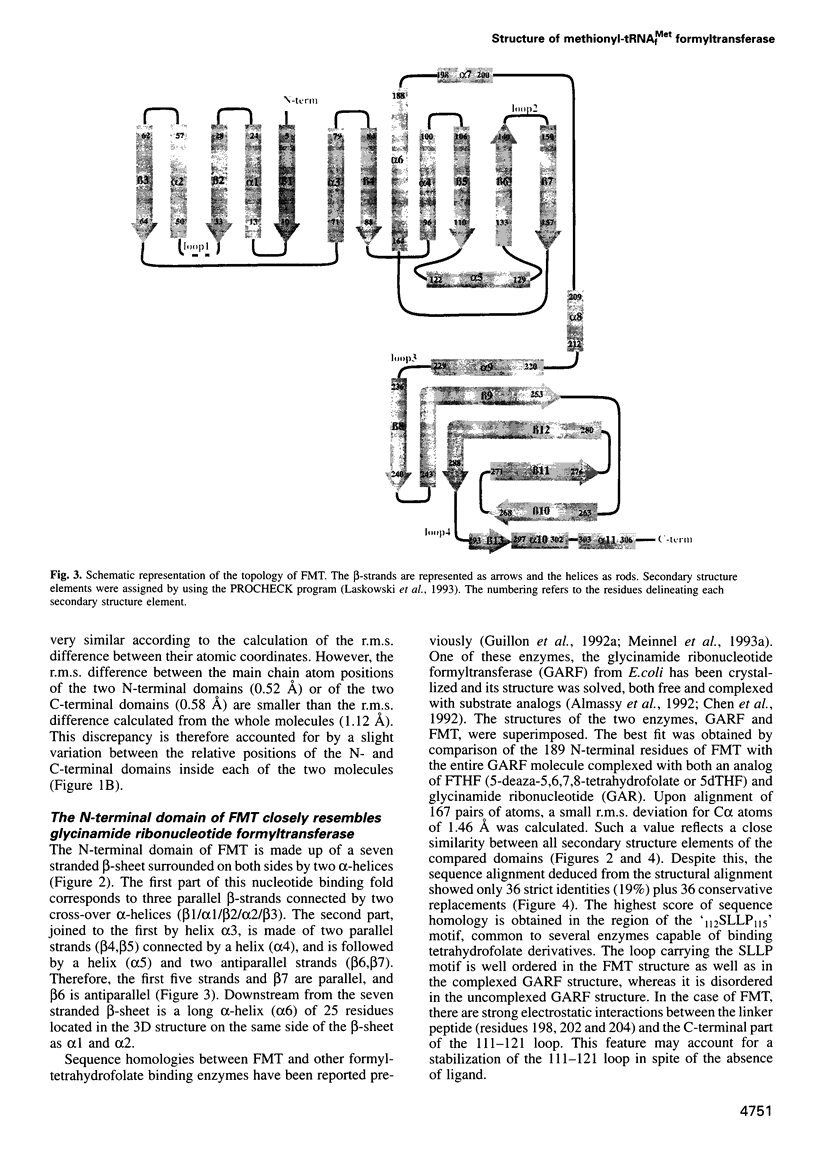
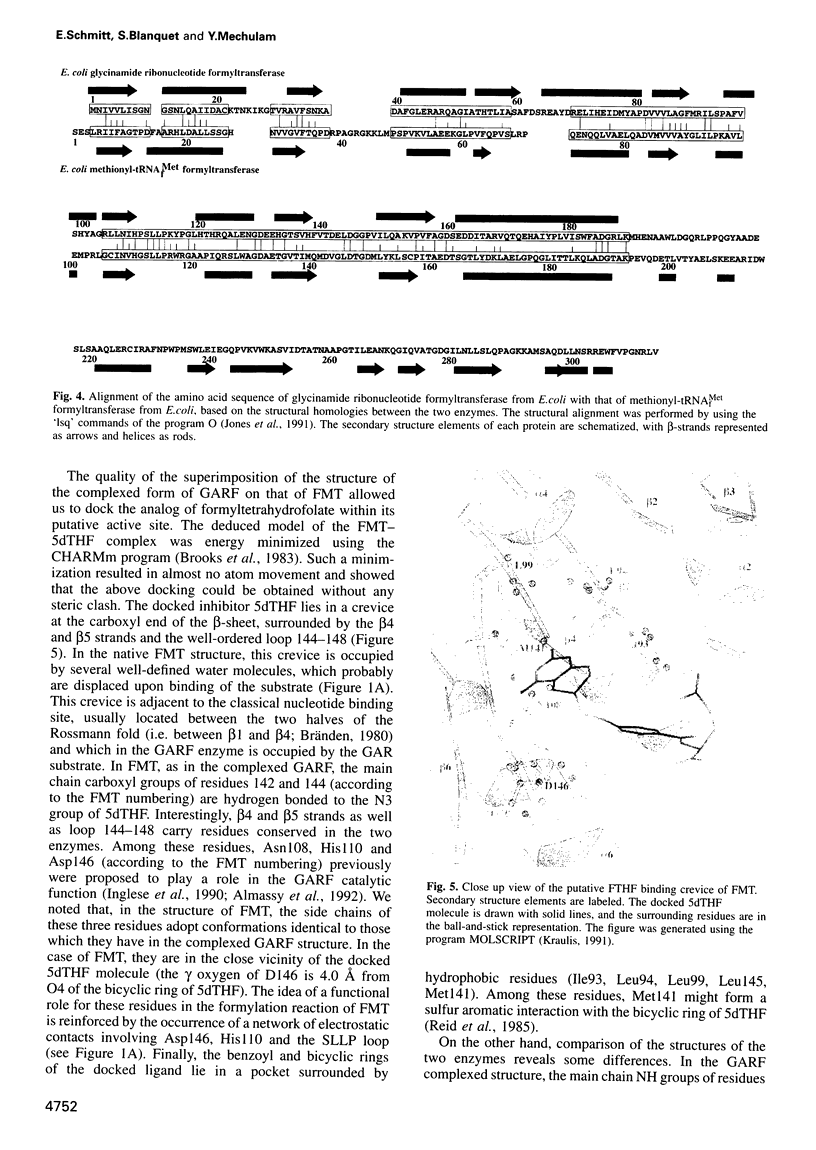
Formylation of the methionyl moiety esterified to the 3' end of tRNA(f)Met is a key step in the targeting of initiator tRNA towards the translation start machinery in prokaryotes. Accordingly, the presence of methionyl-tRNA(f)Met formyltransferase (FMT), the enzyme responsible for this formylation, is necessary for the normal growth of Escherichia coli. The present work describes the structure of crystalline E.coli FMT at 2.0 A, resolution. The protein has an N-terminal domain containing a Rossmann fold. This domain closely resembles that of the glycinamide ribonucleotide formyltransferase (GARF), an enzyme which, like FMT, uses N-10 formyltetrahydrofolate as formyl donor. However, FMT can be distinguished from GARF by a flexible loop inserted within its Rossmann fold. In addition, FMT possesses a C-terminal domain with a beta-barrel reminiscent of an OB fold. This latter domain provides a positively charged side oriented towards the active site. Biochemical evidence is presented for the involvement of these two idiosyncratic regions (the flexible loop in the N-terminal domain, and the C-terminal domain) in the binding of the tRNA substrate.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almassy R. J., Janson C. A., Kan C. C., Hostomska Z. Structures of apo and complexed Escherichia coli glycinamide ribonucleotide transformylase. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6114–6118. doi: 10.1073/pnas.89.13.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanquet S., Dessen P., Kahn D. Properties and specificity of methionyl-tRNAfMet formyltransferase from Escherichia coli. Methods Enzymol. 1984;106:141–152. doi: 10.1016/0076-6879(84)06013-4. [DOI] [PubMed] [Google Scholar]
- Blanquet S., Iwatsubo M., Waller J. P. The mechanism of action of methionyl-tRNA synthetase from Escherichia coli. 1. Fluorescence studies on tRNAMet binding as a function of ligands, ions and pH. Eur J Biochem. 1973 Jul 2;36(1):213–226. doi: 10.1111/j.1432-1033.1973.tb02903.x. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Chen P., Schulze-Gahmen U., Stura E. A., Inglese J., Johnson D. L., Marolewski A., Benkovic S. J., Wilson I. A. Crystal structure of glycinamide ribonucleotide transformylase from Escherichia coli at 3.0 A resolution. A target enzyme for chemotherapy. J Mol Biol. 1992 Sep 5;227(1):283–292. doi: 10.1016/0022-2836(92)90698-j. [DOI] [PubMed] [Google Scholar]
- Commans S., Plateau P., Blanquet S., Dardel F. Solution structure of the anticodon-binding domain of Escherichia coli lysyl-tRNA synthetase and studies of its interaction with tRNA(Lys). J Mol Biol. 1995 Oct 13;253(1):100–113. doi: 10.1006/jmbi.1995.0539. [DOI] [PubMed] [Google Scholar]
- Fleischmann R. D., Adams M. D., White O., Clayton R. A., Kirkness E. F., Kerlavage A. R., Bult C. J., Tomb J. F., Dougherty B. A., Merrick J. M. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995 Jul 28;269(5223):496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- Guillon J. M., Mechulam Y., Blanquet S., Fayat G. Importance of formylability and anticodon stem sequence to give a tRNA(Met) an initiator identity in Escherichia coli. J Bacteriol. 1993 Jul;175(14):4507–4514. doi: 10.1128/jb.175.14.4507-4514.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon J. M., Mechulam Y., Schmitter J. M., Blanquet S., Fayat G. Disruption of the gene for Met-tRNA(fMet) formyltransferase severely impairs growth of Escherichia coli. J Bacteriol. 1992 Jul;174(13):4294–4301. doi: 10.1128/jb.174.13.4294-4301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon J. M., Meinnel T., Mechulam Y., Lazennec C., Blanquet S., Fayat G. Nucleotides of tRNA governing the specificity of Escherichia coli methionyl-tRNA(fMet) formyltransferase. J Mol Biol. 1992 Mar 20;224(2):359–367. doi: 10.1016/0022-2836(92)91000-f. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989 Apr;5(2):151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Hirel P. H., Schmitter M. J., Dessen P., Fayat G., Blanquet S. Extent of N-terminal methionine excision from Escherichia coli proteins is governed by the side-chain length of the penultimate amino acid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8247–8251. doi: 10.1073/pnas.86.21.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglese J., Smith J. M., Benkovic S. J. Active-site mapping and site-specific mutagenesis of glycinamide ribonucleotide transformylase from Escherichia coli. Biochemistry. 1990 Jul 17;29(28):6678–6687. doi: 10.1021/bi00480a018. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kahn D., Fromant M., Fayat G., Dessen P., Blanquet S. Methionyl-transfer-RNA transformylase from Escherichia coli. Purification and characterisation. Eur J Biochem. 1980 Apr;105(3):489–497. doi: 10.1111/j.1432-1033.1980.tb04524.x. [DOI] [PubMed] [Google Scholar]
- Lee C. P., Seong B. L., RajBhandary U. L. Structural and sequence elements important for recognition of Escherichia coli formylmethionine tRNA by methionyl-tRNA transformylase are clustered in the acceptor stem. J Biol Chem. 1991 Sep 25;266(27):18012–18017. [PubMed] [Google Scholar]
- Mangroo D., RajBhandary U. L. Mutants of Escherichia coli initiator tRNA defective in initiation. Effects of overproduction of methionyl-tRNA transformylase and the initiation factors IF2 and IF3. J Biol Chem. 1995 May 19;270(20):12203–12209. doi: 10.1074/jbc.270.20.12203. [DOI] [PubMed] [Google Scholar]
- Meinnel T., Blanquet S. Characterization of the Thermus thermophilus locus encoding peptide deformylase and methionyl-tRNA(fMet) formyltransferase. J Bacteriol. 1994 Dec;176(23):7387–7390. doi: 10.1128/jb.176.23.7387-7390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinnel T., Blanquet S. Maturation of pre-tRNA(fMet) by Escherichia coli RNase P is specified by a guanosine of the 5'-flanking sequence. J Biol Chem. 1995 Jun 30;270(26):15908–15914. doi: 10.1074/jbc.270.26.15908. [DOI] [PubMed] [Google Scholar]
- Meinnel T., Mechulam Y., Blanquet S. Methionine as translation start signal: a review of the enzymes of the pathway in Escherichia coli. Biochimie. 1993;75(12):1061–1075. doi: 10.1016/0300-9084(93)90005-d. [DOI] [PubMed] [Google Scholar]
- Meinnel T., Mechulam Y., Lazennec C., Blanquet S., Fayat G. Critical role of the acceptor stem of tRNAs(Met) in their aminoacylation by Escherichia coli methionyl-tRNA synthetase. J Mol Biol. 1993 Jan 5;229(1):26–36. doi: 10.1006/jmbi.1993.1005. [DOI] [PubMed] [Google Scholar]
- Murzin A. G. OB(oligonucleotide/oligosaccharide binding)-fold: common structural and functional solution for non-homologous sequences. EMBO J. 1993 Mar;12(3):861–867. doi: 10.1002/j.1460-2075.1993.tb05726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls A., Sharp K. A., Honig B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins. 1991;11(4):281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- Onesti S., Miller A. D., Brick P. The crystal structure of the lysyl-tRNA synthetase (LysU) from Escherichia coli. Structure. 1995 Feb 15;3(2):163–176. doi: 10.1016/s0969-2126(01)00147-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Moras D., Olsen K. W. Chemical and biological evolution of nucleotide-binding protein. Nature. 1974 Jul 19;250(463):194–199. doi: 10.1038/250194a0. [DOI] [PubMed] [Google Scholar]
- Ruff M., Krishnaswamy S., Boeglin M., Poterszman A., Mitschler A., Podjarny A., Rees B., Thierry J. C., Moras D. Class II aminoacyl transfer RNA synthetases: crystal structure of yeast aspartyl-tRNA synthetase complexed with tRNA(Asp). Science. 1991 Jun 21;252(5013):1682–1689. doi: 10.1126/science.2047877. [DOI] [PubMed] [Google Scholar]
- Skala J., Van Dyck L., Purnelle B., Goffeau A. The sequence of an 8 kb segment on the left arm of chromosome II from Saccharomyces cerevisiae identifies five new open reading frames of unknown functions, two tRNA genes and two transposable elements. Yeast. 1992 Sep;8(9):777–785. doi: 10.1002/yea.320080911. [DOI] [PubMed] [Google Scholar]
- Stein P. E., Boodhoo A., Tyrrell G. J., Brunton J. L., Read R. J. Crystal structure of the cell-binding B oligomer of verotoxin-1 from E. coli. Nature. 1992 Feb 20;355(6362):748–750. doi: 10.1038/355748a0. [DOI] [PubMed] [Google Scholar]
- Wallis N. G., Dardel F., Blanquet S. Heteronuclear NMR studies of the interactions of 15N-labeled methionine-specific transfer RNAs with methionyl-tRNA transformylase. Biochemistry. 1995 Jun 13;34(23):7668–7677. doi: 10.1021/bi00023a013. [DOI] [PubMed] [Google Scholar]