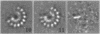Abstract
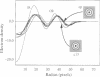
Large cyclical oligomers may be formed by (curvi-) linear polymerization of monomers until the n(th) monomer locks in with the first member of the chain. The subunits in incomplete structures exhibit a natural curvature with respect to each other which can be perturbed when the oligomer closes cyclically. Using cryo-electron microscopy and multivariate statistical image processing we report herein a direct structural observation of this effect. A sub-population (approximately 15%) of incomplete oligomers was found within a sample of SPP1 bacteriophage portal proteins embedded in vitreous ice. Whereas the curvature between adjacent subunits of the closed circular 13-fold symmetric oligomer is 27.7 degrees, in these incomplete oligomers the angle is only 25.8 degrees, a value which almost allows for a 14-subunit cyclical arrangement. A simple model for the association of large cyclical oligomers is suggested by our data.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazinet C., Benbasat J., King J., Carazo J. M., Carrascosa J. L. Purification and organization of the gene 1 portal protein required for phage P22 DNA packaging. Biochemistry. 1988 Mar 22;27(6):1849–1856. doi: 10.1021/bi00406a009. [DOI] [PubMed] [Google Scholar]
- Bazinet C., King J. The DNA translocating vertex of dsDNA bacteriophage. Annu Rev Microbiol. 1985;39:109–129. doi: 10.1146/annurev.mi.39.100185.000545. [DOI] [PubMed] [Google Scholar]
- Berriman J., Unwin N. Analysis of transient structures by cryo-microscopy combined with rapid mixing of spray droplets. Ultramicroscopy. 1994 Dec;56(4):241–252. doi: 10.1016/0304-3991(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Butler P. J., Bloomer A. C., Finch J. T. Direct visualization of the structure of the "20 S" aggregate of coat protein of tobacco mosaic virus. The "disk" is the major structure at pH 7.0 and the Proto-helix at lower pH. J Mol Biol. 1992 Mar 20;224(2):381–394. doi: 10.1016/0022-2836(92)91002-7. [DOI] [PubMed] [Google Scholar]
- Butler P. J. The current picture of the structure and assembly of tobacco mosaic virus. J Gen Virol. 1984 Feb;65(Pt 2):253–279. doi: 10.1099/0022-1317-65-2-253. [DOI] [PubMed] [Google Scholar]
- Carazo J. M., Donate L. E., Herranz L., Secilla J. P., Carrascosa J. L. Three-dimensional reconstruction of the connector of bacteriophage phi 29 at 1.8 nm resolution. J Mol Biol. 1986 Dec 20;192(4):853–867. doi: 10.1016/0022-2836(86)90033-1. [DOI] [PubMed] [Google Scholar]
- Carazo J. M., Santisteban A., Carrascosa J. L. Three-dimensional reconstruction of bacteriophage phi 29 neck particles at 2 X 2 nm resolution. J Mol Biol. 1985 May 5;183(1):79–88. doi: 10.1016/0022-2836(85)90282-7. [DOI] [PubMed] [Google Scholar]
- Carrascosa J. L., Viñuela E., García N., Santisteban A. Structure of the head-tail connector of bacteriophage phi 29. J Mol Biol. 1982 Jan 15;154(2):311–324. doi: 10.1016/0022-2836(82)90066-3. [DOI] [PubMed] [Google Scholar]
- Donate L. E., Herranz L., Secilla J. P., Carazo J. M., Fujisawa H., Carrascosa J. L. Bacteriophage T3 connector: three-dimensional structure and comparison with other viral head-tail connecting regions. J Mol Biol. 1988 May 5;201(1):91–100. doi: 10.1016/0022-2836(88)90441-x. [DOI] [PubMed] [Google Scholar]
- Driedonks R. A., Engel A., tenHeggeler B., van Driel Gene 20 product of bacteriophage T4 its purification and structure. J Mol Biol. 1981 Nov 15;152(4):641–662. doi: 10.1016/0022-2836(81)90121-2. [DOI] [PubMed] [Google Scholar]
- Dube P., Tavares P., Lurz R., van Heel M. The portal protein of bacteriophage SPP1: a DNA pump with 13-fold symmetry. EMBO J. 1993 Apr;12(4):1303–1309. doi: 10.1002/j.1460-2075.1993.tb05775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubochet J., Adrian M., Chang J. J., Homo J. C., Lepault J., McDowall A. W., Schultz P. Cryo-electron microscopy of vitrified specimens. Q Rev Biophys. 1988 May;21(2):129–228. doi: 10.1017/s0033583500004297. [DOI] [PubMed] [Google Scholar]
- Durham A. C., Klug A. Structures and roles of the polymorphic forms of tobacco mosaic virus protein. 3. A model for the association of A-protein into disks. J Mol Biol. 1972 Jun 20;67(2):315–332. doi: 10.1016/0022-2836(72)90244-6. [DOI] [PubMed] [Google Scholar]
- Fuller S. D., Berriman J. A., Butcher S. J., Gowen B. E. Low pH induces swiveling of the glycoprotein heterodimers in the Semliki Forest virus spike complex. Cell. 1995 Jun 2;81(5):715–725. doi: 10.1016/0092-8674(95)90533-2. [DOI] [PubMed] [Google Scholar]
- Hirth L., Richards K. E. Tobacco mosaic virus: model for structure and function of a simple virus. Adv Virus Res. 1981;26:145–199. doi: 10.1016/s0065-3527(08)60423-6. [DOI] [PubMed] [Google Scholar]
- Kochan J., Carrascosa J. L., Murialdo H. Bacteriophage lambda preconnectors. Purification and structure. J Mol Biol. 1984 Apr 15;174(3):433–447. doi: 10.1016/0022-2836(84)90330-9. [DOI] [PubMed] [Google Scholar]
- Kocsis E., Cerritelli M. E., Trus B. L., Cheng N., Steven A. C. Improved methods for determination of rotational symmetries in macromolecules. Ultramicroscopy. 1995 Sep;60(2):219–228. doi: 10.1016/0304-3991(95)00070-2. [DOI] [PubMed] [Google Scholar]
- Tsuprun V., Anderson D., Egelman E. H. The bacteriophage phi 29 head-tail connector shows 13-fold symmetry in both hexagonally packed arrays and as single particles. Biophys J. 1994 Jun;66(6):2139–2150. doi: 10.1016/S0006-3495(94)81009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N. Acetylcholine receptor channel imaged in the open state. Nature. 1995 Jan 5;373(6509):37–43. doi: 10.1038/373037a0. [DOI] [PubMed] [Google Scholar]
- Valpuesta J. M., Carrascosa J. L. Structure of viral connectors and their function in bacteriophage assembly and DNA packaging. Q Rev Biophys. 1994 May;27(2):107–155. doi: 10.1017/s0033583500004510. [DOI] [PubMed] [Google Scholar]
- Valpuesta J. M., Fujisawa H., Marco S., Carazo J. M., Carrascosa J. L. Three-dimensional structure of T3 connector purified from overexpressing bacteria. J Mol Biol. 1992 Mar 5;224(1):103–112. doi: 10.1016/0022-2836(92)90579-9. [DOI] [PubMed] [Google Scholar]
- van Heel M., Harauz G., Orlova E. V., Schmidt R., Schatz M. A new generation of the IMAGIC image processing system. J Struct Biol. 1996 Jan-Feb;116(1):17–24. doi: 10.1006/jsbi.1996.0004. [DOI] [PubMed] [Google Scholar]
- van Heel M., Stöffler-Meilicke M. Characteristic views of E. coli and B. stearothermophilus 30S ribosomal subunits in the electron microscope. EMBO J. 1985 Sep;4(9):2389–2395. doi: 10.1002/j.1460-2075.1985.tb03944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]