Abstract
Background
Vascular pathologies constitute a major cause of graft rejection following organ transplantation. Recent studies have documented an improvement in transplant outcome when organs are preserved through pulsatile perfusion, however, the underlying mechanisms of these observations are poorly characterized. We hypothesized that the temporary absence of flow occurring in the context of organ cold storage conditions disrupts endothelial vasoprotective programs, and that this consequence of stasis may be a target for pharmacological modulation.
Methods
The expression of the transcription factor KLF2 and its vasoprotective target genes were assessed during cold storage conditions in cultured human endothelial cells and murine aortic segments. In addition, we evaluated the effect of simvastatin use as a supplement in a cold preservation solution on the expression of vasoprotective genes, and on endothelial activation and apoptosis.
Results
The expression of endothelial KLF2 and its vasoprotective transcriptional targets were rapidly lost during cold preservation in vitro and ex vivo. Importantly, simvastatin treatment blocked the decay of KLF2, sustaining a vasoprotective phenotype, and preventing endothelial activation and apoptosis.
Conclusions
Flow stasis leads to acute endothelial dysfunction and apoptosis in the context of cold storage conditions. Supplementation of organ preservation solutions with pharmacologic agents that restore endothelial vasoprotective programs via KLF2 upregulation may represent a significant advancement of current organ preservation techniques.
Keywords: cold storage, endothelium, statins, KLF2, shear stress
Introduction
Organ transplantation is the treatment of choice in several end-stage-organ diseases. Despite the critical advancement in surgical techniques, immunosuppressive therapies, and postoperative care, which have all improved short- and long-term graft survival, early organ damage continues to be an important problem, and remains a major focus of therapeutic attention (1). In general, organ damage is a consequence of multiple and diverse factors including alloantigen dependent and independent events (2-4), and of several types of vasculopathies, which are initiated in part by endothelial dysfunction and other vascular pathological consequences of ischemia-reperfusion injury (5).
Under normal physiological conditions, the vascular endothelium is constantly exposed to the flow of blood, which stimulates the production of several factors that regulate vascular tone (e.g., nitric oxide), and the anti-inflammatory and anti-thrombotic properties characteristic of this vascular interface (6, 7). However, during organ procurement and storage, in the context of transplantation, the endothelial interface experiences an abrupt change in hemodynamic conditions. Notably, the pathological consequences of this cessation of flow on endothelial cell biology and organ viability are just beginning to be elucidated. In order to optimize organ viability, different strategies focusing on improving organ preservation solutions and techniques have been pursued (8-11). For example, recent studies have demonstrated that organ procurement using hypothermic continuous perfusion significantly improves post-transplant organ function in comparison to the static cold storage standard technique (12, 13). Nevertheless, the cellular and molecular mechanisms behind these observations remain poorly characterized.
Kruppel-like factors (KLFs) are a subclass of the zinc finger family of transcription factors which regulate cellular function and tissue development (14). Kruppel-like factor 2 (KLF2) is expressed in the endothelium of the vasculature and is required for normal vessel development (15, 16). Our laboratory and others have demonstrated that KLF2 acts as a transcriptional integrator of the vasoprotective phenotype. KLF2 expression attenuates cytokine-mediated induction of pro-inflammatory molecules like E-selectin and VCAM-1, induces gene expression of anti-thrombotic molecules (e.g. thrombomodulin, TM), and potently activates endothelial nitric oxide synthase (eNOS) expression and activity (17-19).
Two of the most potent known inducers of KLF2 expression in the endothelium are blood flow-derived shear stress and statins (19-21). Previous studies have focused on the flow-dependent KLF2-mediated vasoprotective phenotype in vascular endothelial cells (19, 21). However, the effect of flow cessation on the KLF2-mediated endothelial vasoprotection has not been explored. Therefore, the present study aimed to characterize how flow cessation affects the endothelial vasoprotective phenotype and to evaluate if the consequence of stasis on the vascular endothelium could be pharmacologically modulated.
Materials and Methods
Endothelial cells culture
Primary human umbilical vein endothelial cells (EC) were isolated, cultured and subjected to vasoprotective flow as previously described (19, 22). Briefly, EC cultured in Medium-199 (BioWhittaker) supplemented with 50 mg/mL endothelial cell growth supplement (BT-203, Biomedical Technologies Inc.), 100 mg/mL heparin (Sigma), 100 units/mL penicillin plus 100 μg/mL streptomycin (BioWhittaker), 2mM L-Glutamine (GIBCO) and 20% Fetal Bovine Serum (BioWhittaker) were plated on 0.1% gelatin (Difco)–coated plastics disks and maintained at 37°C 5% CO2 for 24h of static culture. After 24h, the static EC growth medium was exchanged for shear medium (Medium-199, 20% FBS, 2 mM L-Glutamine, 100 units/mL penicillin plus 100 ug/mL streptomycin and 2% dextran (Sigma). In order to achieve physiological levels of KLF2 expression in cultured EC, which we have previously shown conferred an endothelial vasoprotective phenotype, we exposed EC to vasoprotective flow using a dynamic flow system composed by a cone and plate device equipped with a computer-controlled user interface that generates shear stress, and maintains cultured cells at 37°C in a 5% CO2 environment previously developed and used in our laboratory (19, 22).
Endothelial cells culture upon flow cessation
After 24h of vasoprotective flow stimulation, EC were washed twice with phosphate buffered saline (PBS) and incubated under static conditions for different periods of time at 37°C in complete EC culture media or at 4°C in UWS supplemented with Simvastatin (Calbiochem) or its vehicle DMSO (0.1% v/v).
To evaluate endothelial activation, following cold static preservation cells were washed with PBS and incubated for 4h in complete EC culture media at 37°C with IL-1β (0.1U/mL) or vehicle (PBS).
siRNA experiments
KLF2 expression was silenced using siRNA transfection as previously described (19). Briefly, 24h before flow stimulus initiation, EC were transfected with a siRNA targeting human KLF2 (100nM, L-006928-00-00005, Thermo Scientific), or with a control siRNA (100nM, 4390844, Ambion Inc.) using oligofectamine reagent (Invitrogen Corp.) according to the manufacturer's instructions.
Cold preservation of aortic segments
Female C57BL/6J mice (5 weeks old) were obtained from Jackson Laboratories (Bar Harbor, Maine). All animal procedures and care were performed with approval from the Institutional Animal Care and Use Committee and complied with the “Guide for the Care and Use of Laboratory Animals” (National Institutes of Health, NIH publication 86-23, revised 1985). Murine thoracic aortas (n=5) were harvested and divided in three segments, one was snap frozen immediately, and the other two were incubated at 4°C for 6h in UWS supplemented with Simvastatin (10μM) or with vehicle (DMSO 0.1% v/v) and snap frozen afterwards. In additional experiments, murine thoracic aortas (n=5) were divided in three pieces, one was snap frozen and the other two were incubated for 24h as described above and frozen afterwards.
RNA processing and real-time Taqman PCR analysis
Total RNA from EC was isolated and purified using the Prism Nucleic Acid Prep-Station (Applied Biosystems) according to manufacturer's protocol. Total RNA from mouse tissues was isolated and purified using RNeasy Mini Kit (Qiagen) according to manufacturer's instructions. RNA quality was verified using Agilent's 2100 Bioanalyzer. RNA was reverse-transcribed to cDNA using a MultiScribe-based reaction (Applied Biosystems). cDNA templates were amplified by real-time Taqman PCR on an ABI Prism 7900HT Fast Detection System (Applied Biosystems). Expression of KLF2, eNOS, TM, c-type natriuretic peptide (CNP), E-Selectin and Vascular Cell Adhesion Molecule-1 (VCAM-1) was analyzed using pre-designed Gene-Expression-Assays obtained from Applied Biosystems according to the manufacturer's protocol and reported relative to endogenous controls GAPDH or VE-Cadherin. All PCR reactions were performed in duplicate and using nuclease free water as no template control.
Protein analysis
EC were lysed using RIPA buffer (Sigma) supplemented with Complete Mini Protease inhibitor (Roche) and Halt Phosphatase inhibitor (Pierce) and protein expression was assessed by western blot. Briefly, aliquots from each sample containing equal amounts of protein were run on a 7.5% SDS-polyacrylamide gel, and transferred to a nitrocellulose membrane. After the transfer, the blots were subsequently blocked for 1 hour with Tris-buffered saline containing 0.05% (vol/vol) Tween 20 and 5% (wt/vol) nonfat dry milk and subsequently incubated with KLF2 (1:1000) or eNOS (1:500) primary antibodies overnight at 4°C. Then, the membrane was incubated with anti-mouse HRP-conjugated secondary antibody (1:10000, Pierce) for 1 hour at room temperature. After stripping, blots were assayed for α-tubulin (Santa Cruz Biotechnology) expression as standardization of sample loading.
Flow cytometric analysis
TM, VCAM-1 and E-Selectin protein expression was analyzed using flow cytometry as previously described (19, 22). Briefly, EC were trypsinized, washed with PBS and resuspended in PBS containing 5% fetal calf serum (Sigma) and an anti-TM antibody (1:100, PE mouse anti-human TM, BD Pharmingen), an anti-VCAM-1 antibody (1:100, APC mouse anti-human VCAM-1, BD Pharmingen) or anti-E-selectin antibody (1:400, H18/7 mouse monoclonal IgG anti-human-E-selectin, a gift from Dr. Michael A. Gimbrone, Jr.). The cell suspension was incubated at 4°C for 30 min, centrifuged at 1000 rpm and washed twice with PBS. Secondary antibody incubation was performed for E-selectin analysis samples (1:500, Molecular Probes) followed by two washes with PBS. FACS analysis was performed in a Becton Dickinson FACScan flow cytometer.
Leukocyte adhesion assay
The adhesion of leukocytes to EC was determined using the HL-60 cells as previously described (19). Briefly, EC were washed twice with PBS and were incubated with one million of HL-60 cells labeled using CellTrace CFSE (Molecular Probes). After 10 minutes of horizontal rotation, EC monolayers were gently washed with PBS and HL-60 adhesion was visualized using a microscope with a 488 filter. For quantification, all cells were lysed with 200uL of lysis buffer (0.1% NaOH/0.01% SDS) and fluorescence was quantified.
Analysis of apoptosis
Analysis of cell apoptosis was performed by FACS analysis using PE-annexin V (BD Pharmingen) and 7-amino-actinomycin (7AAD, BD Pharmingen) stainings following manufacturer's protocol. Apoptotic cells were defined as annexin V-positive and 7AAD-negative cells (23). In brief, cells were trypsinized, washed, resuspended in annexin V binding buffer (BD Pharmingen) and incubated with PE annexin V and 7AAD for 15 min at room temperature. Reaction was stopped by adding 400 uL of annexin V binding buffer and analyzed using FACS as described above.
In situ hybridization
Aortas were harvested from C57BL/6 mice (10 weeks old), fixed in 4% paraformaldehyde, and then embedded in OCT. 14 μm sections were then taken and in situ hybridization was carried out using digoxigenin (DIG)-labeled mouse KLF2 riboprobe as previously described (24). A PCR amplified fragment of mouse KLF2 cDNA (Primer F: 5′- TGAATTCATGCGCGACTGTGGC-3′ and Primer R: 5′- TTGCTCTAGAGCAGAGATGCCA-3′) was used as templates for synthesis of the riboprobe.
Statistical analysis
Statistical significance was determined using Student's t test or one-way ANOVA, with differences considered significant at P<0.05.
Results
The expression of KLF2 in endothelial cells rapidly decays after flow cessation
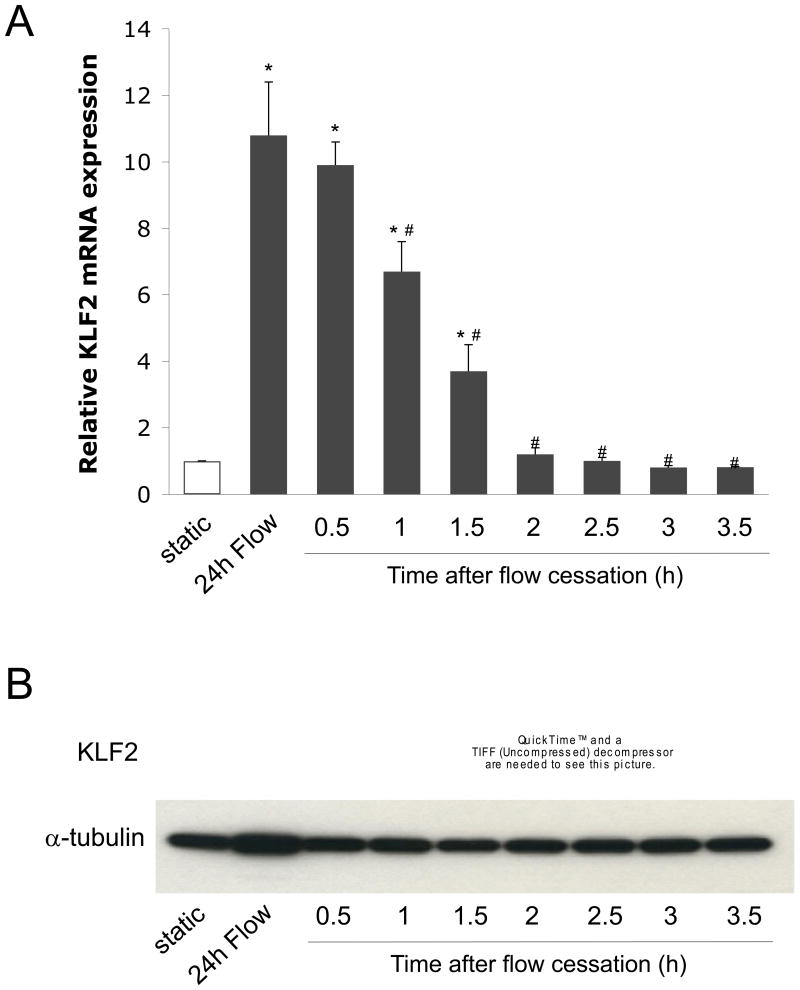
We have previously demonstrated that EC exposed to vasoprotective flow for 24h exhibit a marked increase in the expression of the transcription factor KLF2 (19). In order to document the expression of KLF2 in EC upon flow cessation, KLF2 mRNA and protein expression were determined in EC exposed for 24h to vasoprotective flow and then maintained under static (no flow) culture conditions for different periods of time. As seen in Figure 1, EC cultured for 24h under vasoprotective flow showed a significant increase in KLF2 mRNA (Fig. 1A) compared to static controls. Notably, this expression was rapidly lost after flow cessation. Indeed, after 2h of flow cessation KLF2 mRNA expression level was comparable to the one documented under static conditions. Similarly, 24h of vasoprotective flow stimulus induced a remarkable increase in KLF2 protein expression that was rapidly lost once flow was stopped (Fig. 1B).
Figure 1. KLF2 expression in the endothelium is rapidly lost upon flow cessation.
A) KLF2 mRNA expression was determined in EC exposed for 24h to vasoprotective flow, followed by static (no flow) culture conditions at 37°C for the indicated periods of time (*p<0.05 vs. static; #p<0.05 vs. 24h flow. n=3). B) Representative western blot of KLF2 and α-tubulin protein expression determined in EC cultured as described in A.
Simvastatin maintains the expression of KLF2 and its vasoprotective genes during cold storage
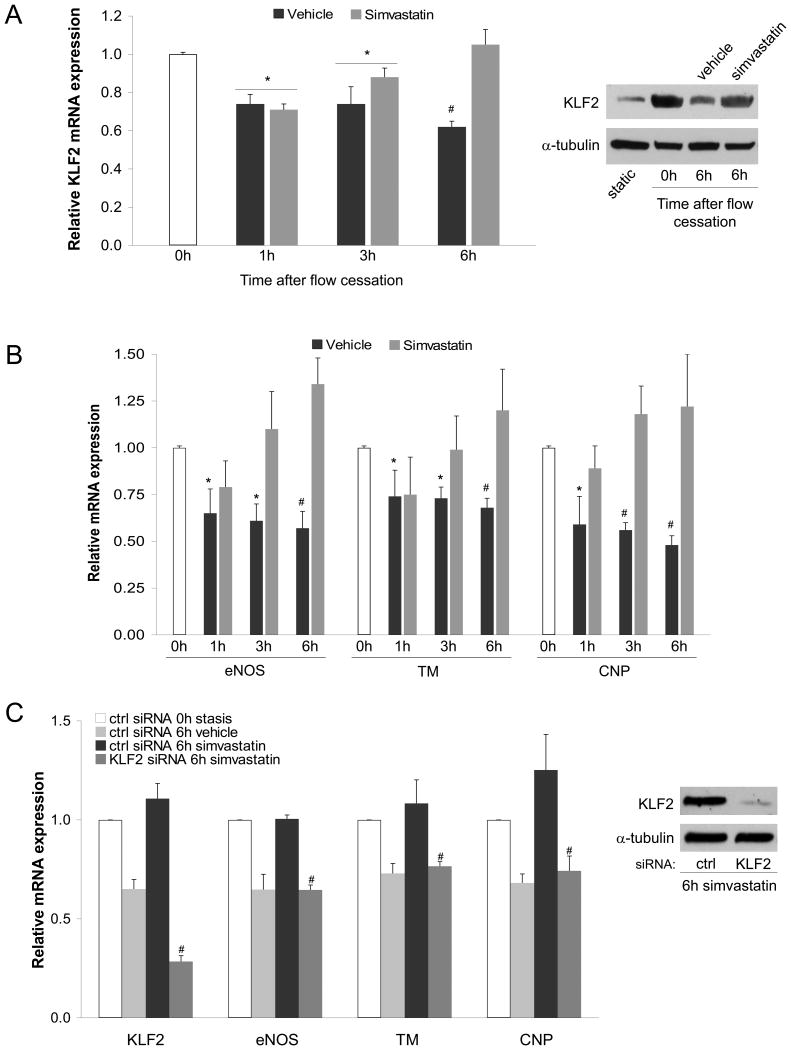
Since KLF2 rapidly decreases upon flow cessation in EC cultured at 37°C, we aimed to characterize KLF2 expression upon flow cessation and EC preservation in cold storage conditions. As seen in Figure 2A, EC cultured for 24h under vasoprotective flow and then placed under cold static conditions (4°C) in University of Wisconsin Solution (UWS) exhibited a significant time-dependent reduction in KLF2 expression. Considering that we and others have shown that flow-derived KLF2 induces the expression of a variety of vasoprotective genes (19, 21), we next aimed to test the effects of flow cessation on the expression of KLF2-dependent vasoprotective genes. As shown in Figure 2B, the reduction in KLF2 observed after 6h of cold preservation was accompanied by a significant and time dependent decrease in the expression of the KLF2 vasoprotective target genes eNOS, TM and CNP.
Figure 2. Simvastatin maintains endothelial KLF2 and its vasoprotective target genes expression during static cold storage.
A) Left, KLF2 mRNA expression determined in EC exposed for 24h to vasoprotective flow followed by static preservation at 4°C for 0h, 1h, 3h and 6h in University of Wisconsin solution supplemented with simvastatin (1μM) or its vehicle. Right, representative western blot of KLF2 and α-tubulin protein expression determined in EC under indicated conditions. B) Expression of KLF2-dependent vasoprotective target genes determined in EC described above (*p<0.05 vs 0h; #p<0.01 vs. 0h. n=3). C) Expression of KLF2 and its vasoprotective target genes in EC transfected with specific siRNA against KLF2 or control, cultured for 24h under flow conditions and preserved at 4°C for 0h or 6h in University of Wisconsin solution supplemented with simvastatin (1μM) or its vehicle (#p<0.05 vs. ctrl siRNA 6h simvastatin, n=3). Insert shows western blot demonstrating KLF2 silencing efficiency.
Next, to examine if the loss of KLF2 expression during cold storage could be pharmacologically rescued, simvastatin was added to the cold storage solution. The results of these experiments demonstrated that simvastatin addition to UWS blocked the decay of KLF2 and its vasoprotective target genes expression in EC maintained in cold preservation (Fig. 2A and 2B). Similar results were obtained using a higher simvastatin concentration (see Fig. 2, Supplemental Digital Content).
Importantly, the ability of simvastatin to prevent the decay in the expression of the studied vasoprotective genes during static cold storage was completely abrogated when KLF2 expression was muted via siRNA silencing (Fig. 2C), thus demonstrating that the expression of KLF2 mediates the simvastatin-induced maintenance of the vasoprotective phenotype lost upon flow cessation.
Simvastatin-mediated KLF2 expression during cold storage prevents endothelial activation
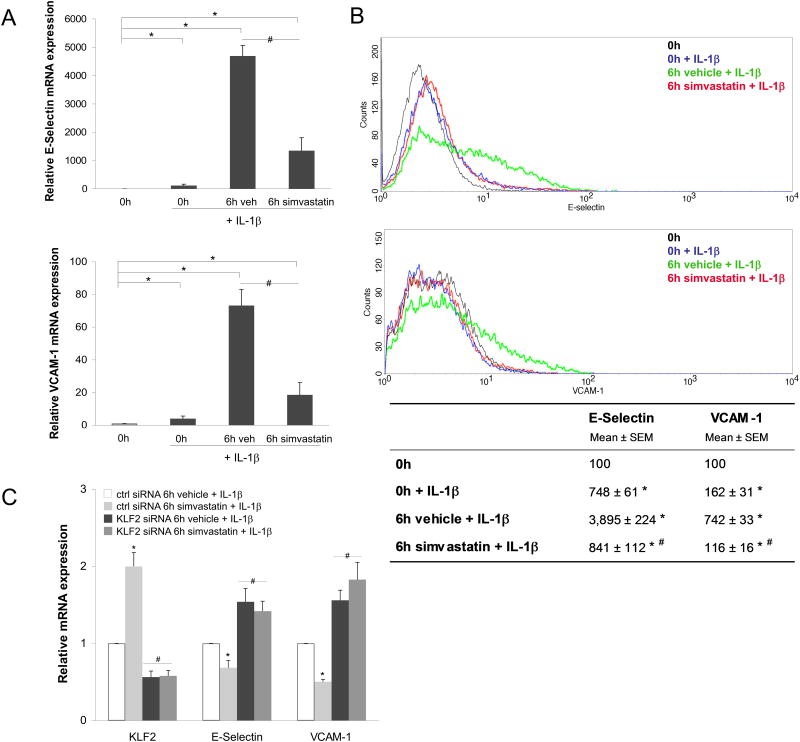
To investigate the functional consequences of flow cessation and cold storage conditions on endothelial cell activation we studied the effects of a pro-inflammatory stimulus after cold preservation. To this end, endothelial cells were cultured for 24h under vasoprotective flow, followed by cold preservation for 6h in UWS, and then stimulated with IL-1β for 4h at 37°C. As shown in Figure 3, IL-1β induced a significant increase in the expression of the adhesion molecules E-Selectin and VCAM-1 in endothelial cells maintained in cold preservation conditions. Importantly, this up-regulation of adhesion molecules was accompanied by a significant increase in the degree of leukocyte adhesion (Fig. 4).
Figure 3. Simvastatin preserves the endothelial anti-inflammatory phenotype upon cold storage through a KLF2-dependent mechanism.
A) E-Selectin (top) and VCAM-1 (bottom) mRNA expression determined in EC expose to vasoprotective flow for 24h and statically cold preserved for 0h or 6h with simvastatin or its vehicle, and afterwards stimulated for 4h with IL-1β (0.1U/mL) in warm culture conditions (n=3). B) Top, representative flow cytometry histogram of E-Selectin and VCAM-1 cell surface expression determined in EC described above. Bottom, flow cytometry quantitative analysis of four independent experiments (*p<0.05 vs. 0h; #p<0.05 vs. 6h vehicle+IL1β). C) Expression of KLF2 and inflammatory genes VCAM-1 and E-Selectin in EC transfected with specific siRNA against KLF2 or control, cultured for 24h under vasoprotective flow, preserved at 4°C for 6h in University of Wisconsin Solution supplemented with simvastatin (1μM) or its vehicle, and stimulated for 4h with IL1β (0.1U/mL) in warm culture conditions (*p<0.05 vs. ctrl siRNA 6h vehicle. n=3).
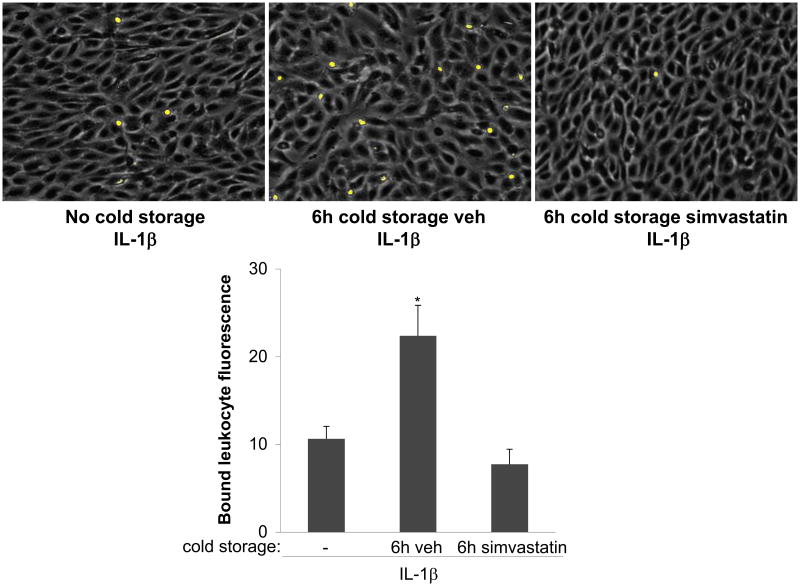
Figure 4. Simvastatin inhibits endothelial activation after cold storage.
Top, EC monolayers were cultured for 24h under vasoprotective flow, followed by the immediate addition of IL-1β 0.1 U/mL, 4h, 37°C, left), or by cold storage for 6h with simvastatin (1 μM, right) or its vehicle (center), and then treated for 4h with IL-1β (as above). All groups were then incubated with fluorescently labeled HL-60 cells. Shown are representative fields of EC monolayers (gray) and attached HL60 cells (yellow). Bottom, quantitative fluorescence analysis of bound HL-60 cells (*p<0.05 vs. no cold storage and 6h simvastatin, n=4).
We next evaluated if simvastatin, used as a cold storage solution supplement, could prevent endothelial cell activation after cold preservation. As shown in Figures 3 and 4, the inflammatory response and the increase in leukocyte adhesion observed in EC cold preserved in UWS and stimulated with IL-1β were prevented when cells were cold stored in presence of simvastatin. Notably, the effects of simvastatin preventing the up-regulation of E-selectin and VCAM-1 were completely abrogated when KLF2 expression was specifically silenced (Fig. 3C), demonstrating the key role of this transcription factor maintaining the vasoprotective endothelial phenotype.
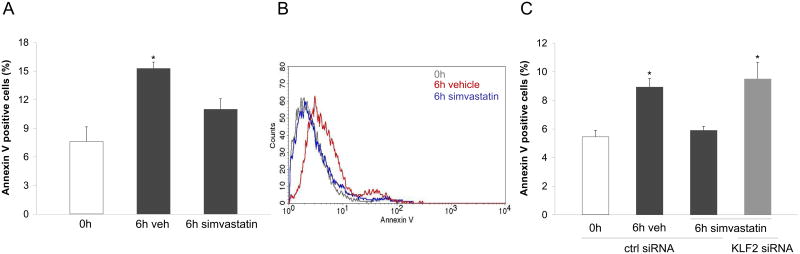
Simvastatin prevents endothelial apoptosis triggered by cold storage conditions via a KLF2-dependent mechanism
Cell death has been documented as one of the most detrimental effects of ischemia/reperfusion injury. In particular, apoptosis has been described as a major mechanism of cell death in the vasculature of transplanted organs (25-27). Therefore, we next aimed to define the effect of flow cessation and cold storage on endothelial cell apoptosis. Using annexin V staining, apoptosis was determined in endothelial cells cultured during 24h under vasoprotective flow and then cold stored for 6h in UWS. As shown in Figure 5A, cold storage induced a significant increase in the number of cells undergoing apoptosis. Remarkably, the addition of simvastatin to the organ preservation solution blocked the induction of apoptosis during cold storage by a KLF2-dependent mechanism (Fig. 5C). Indeed, simvastatin protection against endothelial apoptosis was not observed when KLF2 expression was specifically silenced, revealing that KLF2 mediates the anti-apoptotic effects of simvastatin during cold storage conditions.
Figure 5. Simvastatin prevents endothelial apoptosis during cold storage through a KLF2-dependent mechanism.
A) Expression of the apoptosis marker Annexin V in EC exposed for 24h to vasoprotective flow and statically cold stored for 0h or 6h in University of Wisconsin Solution supplemented with simvastatin or its vehicle (* p<0.05 vs. 0h and 6h simvastatin. n=4). B) Representative flow cytometry histogram of Annexin V expression determined in EC described above. C) Expression of Annexin V in EC transfected with specific KLF2 siRNA or control, cultured for 24h under vasoprotective flow and statically cold preserved for 0h or 6h with simvastatin or its vehicle (*p<0.05 vs. ctrl siRNA 0h and ctrl siRNA 6h vehicle. n=3).
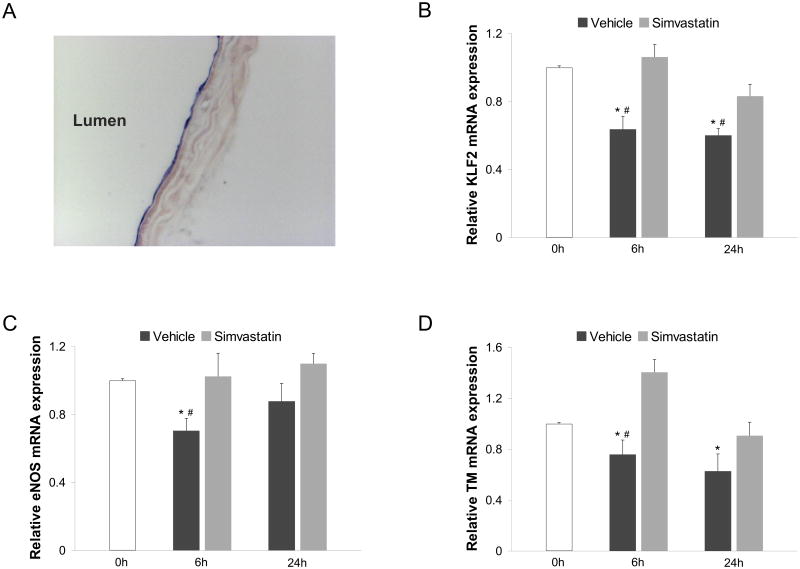
KLF2 and its transcriptional targets decrease in murine aortas during cold storage
We next validated our in vitro observations using an ex vivo model of vascular stasis. To this end, we first documented the endothelial specific expression of KLF2 within the vascular wall by in situ hybridization. As shown in Figure 6A, KLF2 expression in murine aortas was specifically detected in the vascular endothelium. Once we confirmed that KLF2 was uniquely expressed in the aortic endothelial layer, we used quantitative gene expression analysis to assess how two different periods of cold storage affect the expression of KLF2 and its downstream transcriptional targets. As shown in Figure 6, murine aortas cold stored for 6h in UWS exhibited a significant reduction in the expression of KLF2 (Fig 6B) and in the expression of eNOS and TM (Fig. 6C and 6D, respectively) compared to aortic segments obtained from the same animals but frozen immediately after their isolation. Similar results were obtained in murine aortic segments cold preserved for 24h (Fig. 6). Importantly, simvastatin addition to UWS maintained the vascular expression of KLF2 and its vasoprotective target genes during 6h or 24h of cold storage in all studied samples, validating the protective effect of this compound in an ex vivo model of cold preservation.
Figure 6. Simvastatin maintains the expression of endothelial KLF2 and its vasoprotective target genes during cold storage ex vivo.
A) KLF2 mRNA localization determined by in situ hybridization in murine aorta. B) KLF2 and its target genes eNOS (C) and thrombomodulin (D) mRNA expression in murine aortas cold preserved for 0h, 6h or 24h in University of Wisconsin solution supplemented with simvastatin (10μM) or its vehicle (*p<0.05 vs. 0h; #p<0.05 vs. simvastatin. n=5 per condition).
Discussion
Successful recovery of organ function following transplantation is dependent on the degree of tissue protection achieved during organ procurement and preservation (28). Currently, the most common method for organ preservation is cold storage, consisting in a rapid vascular perfusion followed by an immersion and preservation of the organ in a cold storage solution. Several studies have shown that ischemia, occurring during hypothermic organ storage triggers several cellular and molecular processes, leading to the deterioration of endothelial function and organ viability following reperfusion (29, 30). It remains unknown, however, if the temporary absence of biomechanical stimulation resulting from cold storage contributes to organ endothelial dysfunction and overall viability.
The present study demonstrates that shear-stress-dependent expression of the transcription factor KLF2 in vascular endothelial cells is rapidly lost upon flow cessation. Our observations, together with previous reports (31, 32), indicate that both the KLF2 mRNA and protein have a short half-life. Indeed, protein and mRNA levels decline in parallel in a time-dependent manner, and KLF2 mRNA and protein expression are completely lost after 2 and 2.5 hours of stasis, respectively.
Our laboratory and others have demonstrated that KLF2 acts as a critical mediator for the establishment of the vasoprotective phenotype induced by flow (19, 21), however, the effect of KLF2 decay upon flow cessation on the protein expression of KLF2 downstream targets was unknown. Here, we documented that shortly after flow cessation, EC exhibit a marked decrease in the cell surface expression levels of the anti-thrombotic gene TM, whereas no differences in eNOS protein were observed (see Fig. 1, Supplemental Digital Content). These data demonstrate that critical KLF2-dependent vasoprotective targets are down-regulated at the protein level after flow cessation.
Since the most established organ preservation technique to date is cold storage, we aimed to investigate how KLF2 expression and its vasoprotective phenotype are influenced by these conditions. Our experiments demonstrated that cold storage preservation of endothelial cells leads to a significant decrease in KLF2 expression in cultured EC and murine aortic segments. Moreover, the reduction in KLF2 expression due to cold storage is accompanied by a decrease in the expression of KLF2-dependent vasoprotective genes in cultured EC and in murine aortas.
It is has been previously demonstrated that statins induce the expression of KLF2 in EC leading to the establishment of a vasoprotective phenotype (20, 33). Although the precise mechanisms linking statins to KLF2 induction in endothelial cells remain to be elucidated, some signaling molecules including MEF2 and Akt have been described to be necessary for statin-mediated KLF2 expression (33, 34). Recently, this class of drugs has been shown to improve ischemia/reperfusion injury if used as a donor prophylactic agent (35, 36). Here we report that simvastatin, when used as a supplement in an organ preservation solution, is capable of sustaining the expression of key vasoprotective genes in human endothelial cells maintained under cold storage conditions through a KLF2-dependent mechanism.
To understand the functional consequences of KLF2 decay during cold storage we performed a series of experiments using IL-1β stimulation to partially mimic some of the conditions experienced by the vascular endothelium following organ cold preservation. This experimental approach revealed that endothelial cells with a reduced KLF2 expression, due to flow cessation and cold storage, are primed to response to pro- inflammatory challenges, and exhibit an increased level of adhesion molecules expression and leukocyte adhesion. These results are in agreement with previous reports describing an increased expression of adhesion molecules following reperfusion (37, 38). Thus, it can be speculated that endothelial protection may provide an important link between innate and adaptive immune responses. Importantly, our experiments also evaluated the effect of simvastatin on endothelial cell activation and demonstrated that simvastatin, when used as supplement in cold storage solutions, prevents endothelial activation by restoring KLF2 expression.
Another detrimental consequence of reperfusion injury is vascular cell death (27). Our study documents that flow cessation and cold storage conditions per se also induce a significant increase in endothelial cell apoptosis. Importantly, these effects are blocked when KLF2 expression is sustained using simvastatin.
Collectively, our data support the concept that flow cessation results in the loss of the endothelial vasoprotective phenotype leading to acute endothelial dysfunction and apoptosis. In the context of organ transplantation, this vascular dysfunctional state could be present for several hours during reperfusion, which may be a critical determinant for the pathological vascular responses documented during this period, and for subsequent organ function.
It has been suggested that cold storage may not be the best option to satisfactorily preserve organs (10). According to this idea, an organ preservation technique based on hypothermic continuously perfusion has recently re-emerged as a promising alternative (12, 13). By using this procedure a significant improvement in graft function is achieved, which we recently suggested may be due, at least in part, from the preservation of flow-dependent endothelial vasoprotective programs (11). Since flow, as well as statins, induce a KLF2-dependent vasoprotective endothelial phenotype, it is possible that both of these stimuli may sustain in a similar manner endothelial function during organ preservation. Therefore, our data provide a rationale to investigate whether the use of statins as an organ preservation solution supplement could partially mimic the positive effects of machine perfusion, thereby representing an easier and more cost-effective alternative.
Acknowledgments
We thank Kay Case and Vannessa Davis for isolation and culture of the human endothelial cells.
Funding: This work was supported by grants from the NIH (HL-076686 and HL-090856 to G.G.-C.); the Department of Innovation, Universities and Enterprise, Government of Catalonia, Spain (BPA-2007-00137 to J.G.-S.), the Spanish Association for the Study of the Liver (to J.G.-S.); the Howard Hughes Medical Institute Research Training Fellowship for Medical Students and the American Federation for Aging Research (NIA T35-AG026781 to G.V.).
Abbreviations
- CNP
C-type natriuretic peptide
- eNOS
Endothelial nitric oxide synthase
- IL-1β
Interleukin 1 beta
- KLF2
Kruppel-like factor 2
- TM
Thrombomodulin
- UWS
University of Wisconsin Solution
- VCAM-1
Vascular cell adhesion molecule-1
Footnotes
Author's contributions: J.G.-S. designed the research, conceived ideas, wrote the manuscript, performed experiments and analyzed data. G.V., Y.Z. J.X.Y. and Y.L. performed experiments and analyzed data. S.G.T. conceived ideas and contributed to research design. G.G.-C. designed the research, conceived ideas, wrote the manuscript, analyzed data and directed the project. All authors edited and reviewed the final manuscript.
Conflict of interests: J.G.-S., G.V., Y.Z., J.X.Y. Y.L., S.G.T. and G.G.-C. have nothing to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tambyraja AL, Mitchell R, Driscoll PJ, et al. Glutathione supplementation to University of Wisconsin solution causes endothelial dysfunction. Transpl Immunol. 2007;18:146. doi: 10.1016/j.trim.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Najafian B, Kasiske BL. Chronic allograft nephropathy. Curr Opin Nephrol Hypertens. 2008;17:149. doi: 10.1097/MNH.0b013e3282f4e514. [DOI] [PubMed] [Google Scholar]
- 3.Desai M, Neuberger J. Chronic liver allograft dysfunction. Transplant Proc. 2009;41:773. doi: 10.1016/j.transproceed.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 4.Khan UA, Williams SG, Fildes JE, Shaw SM. The pathophysiology of chronic graft failure in the cardiac transplant patient. Am J Transplant. 2009;9:2211. doi: 10.1111/j.1600-6143.2009.02807.x. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto K, Pearson PJ, Schaff HV, Cartier R. Endothelial cell dysfunction after ischemic arrest and reperfusion: a possible mechanism of myocardial injury during reflow. J Thorac Cardiovasc Surg. 1991;102:688. [PubMed] [Google Scholar]
- 6.Fleming I, Busse R. NO: the primary EDRF. J Mol Cell Cardiol. 1999;31:5. doi: 10.1006/jmcc.1998.0839. [DOI] [PubMed] [Google Scholar]
- 7.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 8.Belzer FO, Ashby BS, Gulyassy PF, Powell M. Successful seventeen-hour preservation and transplantation of human-cadaver kidney. N Engl J Med. 1968;278:608. doi: 10.1056/NEJM196803142781108. [DOI] [PubMed] [Google Scholar]
- 9.Peralta C, Closa D, Hotter G, Gelpi E, Prats N, Rosello-Catafau J. Liver ischemic preconditioning is mediated by the inhibitory action of nitric oxide on endothelin. Biochem Biophys Res Commun. 1996;229:264. doi: 10.1006/bbrc.1996.1790. [DOI] [PubMed] [Google Scholar]
- 10.Maathuis MH, Leuvenink HG, Ploeg RJ. Perspectives in organ preservation. Transplantation. 2007;83:1289. doi: 10.1097/01.tp.0000265586.66475.cc. [DOI] [PubMed] [Google Scholar]
- 11.Tullius SG, Garcia-Cardena G. Organ procurement and perfusion before transplantation. N Engl J Med. 2009;360:78. doi: 10.1056/NEJMe0809215. [DOI] [PubMed] [Google Scholar]
- 12.Guarrera JV, Estevez J, Boykin J, et al. Hypothermic machine perfusion of liver grafts for transplantation: technical development in human discard and miniature swine models. Transplant Proc. 2005;37:323. doi: 10.1016/j.transproceed.2004.12.094. [DOI] [PubMed] [Google Scholar]
- 13.Moers C, Smits JM, Maathuis MH, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360:7. doi: 10.1056/NEJMoa0802289. [DOI] [PubMed] [Google Scholar]
- 14.Bieker JJ. Kruppel-like factors: three fingers in many pies. J Biol Chem. 2001;276:34355. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- 15.Kuo CT, Veselits ML, Barton KP, Lu MM, Clendenin C, Leiden JM. The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 1997;11:2996. doi: 10.1101/gad.11.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson KP, Kern CB, Crable SC, Lingrel JB. Isolation of a gene encoding a functional zinc finger protein homologous to erythroid Kruppel-like factor: identification of a new multigene family. Mol Cell Biol. 1995;15:5957. doi: 10.1128/mcb.15.11.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SenBanerjee S, Lin Z, Atkins GB, et al. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Z, Kumar A, SenBanerjee S, et al. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ Res. 2005;96:e48. doi: 10.1161/01.RES.0000159707.05637.a1. [DOI] [PubMed] [Google Scholar]
- 19.Parmar KM, Larman HB, Dai G, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parmar KM, Nambudiri V, Dai G, Larman HB, Gimbrone MA, Jr, Garcia-Cardena G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J Biol Chem. 2005;280:26714. doi: 10.1074/jbc.C500144200. [DOI] [PubMed] [Google Scholar]
- 21.Dekker RJ, van Thienen JV, Rohlena J, et al. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol. 2005;167:609. doi: 10.1016/S0002-9440(10)63002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai G, Kaazempur-Mofrad MR, Natarajan S, et al. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A. 2004;101:14871. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haendeler J, Tischler V, Hoffmann J, Zeiher AM, Dimmeler S. Low doses of reactive oxygen species protect endothelial cells from apoptosis by increasing thioredoxin-1 expression. FEBS Lett. 2004;577:427. doi: 10.1016/j.febslet.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 24.Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 25.Schumer M, Colombel MC, Sawczuk IS, et al. Morphologic, biochemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. Am J Pathol. 1992;140:831. [PMC free article] [PubMed] [Google Scholar]
- 26.Miller LW, Granville DJ, Narula J, McManus BM. Apoptosis in cardiac transplant rejection. Cardiol Clin. 2001;19:141. doi: 10.1016/s0733-8651(05)70200-9. [DOI] [PubMed] [Google Scholar]
- 27.Hall AV, Jevnikar AM. Significance of endothelial cell survival programs for renal transplantation. Am J Kidney Dis. 2003;41:1140. doi: 10.1016/s0272-6386(03)00345-7. [DOI] [PubMed] [Google Scholar]
- 28.Vos IH, Briscoe DM. Endothelial injury: cause and effect of alloimmune inflammation. Transpl Infect Dis. 2002;4:152. doi: 10.1034/j.1399-3062.2002.t01-1-02002.x. [DOI] [PubMed] [Google Scholar]
- 29.Thurman RG, Bunzendahl H, Lemasters JJ. Role of sinusoidal lining cells in hepatic reperfusion injury following cold storage and transplantation. Semin Liver Dis. 1993;13:93. doi: 10.1055/s-2007-1007341. [DOI] [PubMed] [Google Scholar]
- 30.de Groot H, Rauen U. Ischemia-reperfusion injury: processes in pathogenetic networks: a review. Transplant Proc. 2007;39:481. doi: 10.1016/j.transproceed.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 31.van Thienen JV, Fledderus JO, Dekker RJ, et al. Shear stress sustains atheroprotective endothelial KLF2 expression more potently than statins through mRNA stabilization. Cardiovasc Res. 2006;72:231. doi: 10.1016/j.cardiores.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Liu M, Kluger MS, D'Alessio A, Garcia-Cardena G, Pober JS. Regulation of arterial-venous differences in tumor necrosis factor responsiveness of endothelial cells by anatomic context. Am J Pathol. 2008;172:1088. doi: 10.2353/ajpath.2008.070603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sen-Banerjee S, Mir S, Lin Z, et al. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112:720. doi: 10.1161/CIRCULATIONAHA.104.525774. [DOI] [PubMed] [Google Scholar]
- 34.Ali F, Zakkar M, Karu K, et al. Induction of the cytoprotective enzyme heme oxygenase-1 by statins is enhanced in vascular endothelium exposed to laminar shear stress and impaired by disturbed flow. J Biol Chem. 2009;284:18882. doi: 10.1074/jbc.M109.009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joyce M, Kelly C, Winter D, Chen G, Leahy A, Bouchier-Hayes D. Pravastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, attenuates renal injury in an experimental model of ischemia-reperfusion. J Surg Res. 2001;101:79. doi: 10.1006/jsre.2001.6256. [DOI] [PubMed] [Google Scholar]
- 36.Lai IR, Chang KJ, Tsai HW, Chen CF. Pharmacological preconditioning with simvastatin protects liver from ischemia-reperfusion injury by heme oxygenase-1 induction. Transplantation. 2008;85:732. doi: 10.1097/TP.0b013e3181664e70. [DOI] [PubMed] [Google Scholar]
- 37.Nagano H, Tilney NL. Chronic allograft failure: the clinical problem. Am J Med Sci. 1997;313:305. doi: 10.1097/00000441-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Dragun D, Hoff U, Park JK, et al. Prolonged cold preservation augments vascular injury independent of renal transplant immunogenicity and function. Kidney Int. 2001;60:1173. doi: 10.1046/j.1523-1755.2001.0600031173.x. [DOI] [PubMed] [Google Scholar]