Abstract
T cells directed to endogenous tumor antigens are powerful mediators of tumor regression. Recent immunotherapy advances have identified effective interventions to unleash tumor-specific T cell activity in patients who naturally develop them. Eliciting T cell responses to a patient's individual tumor remains a major challenge. Radiation therapy can induce immune responses to model antigens expressed by tumors, but it remains unclear if it can effectively prime T cells specific for endogenous antigens expressed by poorly immunogenic tumors. We hypothesized that TGFβ activity is a major obstacle hindering the ability of radiation to generate an in situ tumor vaccine. Here we show that antibody-mediated TGFβ neutralization during radiation therapy effectively generates CD8+ T cell responses to multiple endogenous tumor antigens in poorly immunogenic mouse carcinomas. Generated T cells were effective at causing regression of irradiated tumors and non-irradiated lung metastases or synchronous tumors (abscopal effect). Gene signatures associated with IFNγ and immune-mediated rejection were detected in tumors treated with radiation therapy and TGFβ blockade in combination but not as single agents. Upregulation of programmed death (PD) ligand-1 and -2 in neoplastic and myeloid cells and PD-1 on intratumoral T cells limited tumor rejection resulting in rapid recurrence. Addition of anti-PD-1 antibodies extended survival achieved with radiation and TGFβ blockade. Thus, TGFβ is a fundamental regulator of radiation therapy ability to generate an in situ tumor vaccine. The combination of local radiation therapy with TGFβ neutralization offers a novel individualized strategy for vaccinating patients against their tumors.
Keywords: Abscopal effect, Breast cancer, PD-1, T cells, TGFβ
INTRODUCTION
Recent progress in cancer immunotherapy has demonstrated that unleashing the power of tumor-reactive T cells with immune checkpoint inhibitors is an effective treatment in a substantial percentage of patients with metastatic melanoma, renal cell and lung cancer (1-3). However, the majority of cancer patients do not respond to these treatments due to the presence of multiple immunosuppressive mechanisms or the absence of tumor-reactive T cells. Therapeutic cancer vaccines have shown some success in inducing T cells specific for tumor antigens, but their efficacy is limited by the intrinsic genomic instability of tumors generating highly mutable targets (4). Thus, a strategy to induce effective anti-tumor immunity should combine an individualized vaccination approach with targeted interventions to overcome immunosuppression.
Experimental data and clinical observations indicate that radiation therapy (RT) has the potential to convert the irradiated tumor into an in situ vaccine (5). Data in mouse tumors expressing OVA or other model antigens have shown that RT induces cross-priming of T cells to these relatively strong antigens and that T cells contribute to the therapeutic effect of RT (6,7). Rejection of irradiated tumors is facilitated by RT-induced modulation of chemokines and cell surface molecules that enhance T cell recruitment (8,9) and the interaction of CTLs with tumor cells (10-12). RT-elicited cross-priming of tumor-specific T cells depends on generation of the molecular signals that define an immunogenic cell death (13) and requires type I IFN production by infiltrating immune cells (14). However, rejection of non-irradiated metastases and synchronous tumors is usually not achieved by RT alone (15-17) and, despite the widespread use of RT in cancer treatment, the clinical response of metastases outside of the radiation field (abscopal effect) is an extremely rare occurrence (18).
These observations suggest that generation of a tumor vaccine by RT may be impeded by other radiation effects. Of particular concern is the activation of TGFβ (19). The latter is mediated by RT-induced reactive oxygen species (ROS) that cause a conformational change of the latency-associated peptide (LAP)-TGFβ complex releasing active TGFβ (20,21). We have previously shown that activated TGFβ reduces radiosensitivity of tumor cells by promoting the DNA damage response (22). Importantly, TGFβ is a powerful immunosuppressive cytokine that hinders cross-priming of T cells by impairing the antigen-presenting function of dendritic cells and the functional differentiation of T cells into effectors (23).
We hypothesized that TGFβ may be a major obstacle to the optimal activation of anti-tumor T cell responses by RT. Here we show that TGFβ neutralizing antibodies administered during RT uncover the ability of RT to induce T cell responses to endogenous tumor antigens in pre-clinical models of metastatic breast cancer. Importantly, only the combination of RT with anti-TGFβ, but not each treatment alone, induced T cell-mediated rejection of the irradiated tumor and non-irradiated metastases in mice, indicating that blocking TGFβ unleashes the potential of RT to generate an in situ tumor vaccine. Although adaptive immune resistance that developed in responding tumors limited the efficacy of this approach, it could be overcome by additional blockade of the immune checkpoint receptor PD-1.
MATERIALS AND METHODS
Mice
Six weeks old BALB/c female mice from Taconic Animal Laboratory (Germantown, NY) were maintained under pathogen-free conditions in the animal facility at NYU Langone Medical Center. All experiments were approved by the Institutional Animal Care and Use Committee.
Cells and reagents
4T1 and TSA cells were obtained from F. Miller (24) and P.L. Lollini (25), respectively. Cells were authenticated by morphology, phenotype, growth and pattern of metastasis in vivo, and routinely screened for Mycoplasma. Cells were cultured in DMEM (Invitrogen Corporation) supplemented with 2 mmol/L L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 2.5 × 10−5 mol/L 2-mercapthoethanol, and 10% FBS (Life technologies). 1D11, a pan-isoform, TGFβ-neutralizing mouse monoclonal antibody (mAb) that binds only to active TGFβ (26) or 13C4 isotype mAb were provided by Genzyme Inc. Anti-PD-1 RMP1-14 mAb was purchased from BioXCell.
Tumor challenge and treatment
4T1 model: mice were injected subcutaneously (s.c.) in the right flank with 5×104 4T1 cells on day 0. TSA model: mice were injected s.c with 1×105 TSA cells in the right flank (primary tumor) on day 0, and in the left flank (secondary tumor) on day 2. Perpendicular tumor diameters were measured with a Vernier caliper, and tumor volumes calculated as length × width2 × 0.52. On day 12, when tumors reached 60-80 mm3, mice were randomly assigned to treatment groups. RT was delivered to the tumor volume as previously described with some modifications (16). Briefly, all mice (including mice receiving sham radiation) were anesthetized by intra-peritoneal (i.p.) injection of avertin (240 mg/kg) and the primary tumors irradiated with 6 Gy on days 13, 14, 15, 16 and 17 using the Small Animal Radiation Research Platform (SARRP Xstrahl Ltd, Surrey, UK). 13C4 and 1D11 mAbs were administered i.p. (200μg/mouse) every other day from day 12 to day 28. In some experiments, anti-PD-1 mAb RMP1-14 was injected i.p. (200μg/mouse) on days 18, 22, 26 and 30.
Depletion of CD4+ and CD8+ T-cells was achieved by injecting GK1.5 or 2.43 mAb (BioXCell) given i.p. at 100 μg/mouse on 3 consecutive days, starting at day 10, and was maintained by weekly injections. Depletion was confirmed by staining spleen and tumor draining lymph node (TDLN) cells with non–cross-reactive FITC-RMA4-4 and PE-anti-CD8β mAb (BD PharMingen).
Flow cytometric analysis
Single cell suspensions of collagenase-digested tumors were stained with the following antibodies purchased from eBioscience: fixable viability dye efluor 450, CD69-FITC, PD-1-PE, CD4-PeCy7, CD45-Alexa Fluor 700, CD8a-PE efluor 610, CD40-FITC, CD70-PE, MHC-II IAd-APC, CD11c-PE efluor 610, CD45-APC, CD3-PerCP-Cy5.5, CD11b-PerCP-Cy5.5, EpCAM-PeCy7, PD-L1-PE and PD-L2-FITC. Phospho-Smad2/3 levels were assessed as previously described (27). Briefly, TDLN cells were stained with anti-mouse CD4-PE and anti-mouse CD8-FITC (eBioscience), fixed, permeabilized (Foxp3 Fixation/Permeabilization Concentrate and Diluent kit, eBioscience) and stained with goat anti-phospho-Smad2/3 (Ser 423/425) followed by APC-labeled donkey anti-goat IgG (Santa Cruz Biotechnology). All samples were acquired with LSRII flow cytometer and analyzed with FlowJo software (version 7.3.6).
Analysis of IFN-γ production by CD8 T cells
5×105 TDLN cells were stimulated ex vivo with 1μM of the following peptides (GenScript): AH1A5 (SPSYVYHQF), Survivin-1 (GWEPDDNPI), Survivin-2 (AFLTVKKQM), Twist (LYQVLQSDEL), pMCMV (YPHFMPTNL) (17,25,28,29). After 72h culture in 1mL T cell medium (RPMI 1640 medium supplemented with 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μM 2-mercapthoethanol, 10% FBS) supplemented with 10U/mL of human rIL-2, cell-free supernatants were assessed for IFN-γ concentration using the FlowCytomix kit (eBioscience).
Genome-wide microarray analysis
Total RNA was purified from 4T1 tumors with Qiagen RNeasy Mini kit. The 260/280 nm ratio was calculated using Nanodrop ND-1000. RNA integrity of each sample was confirmed by capillary electrophoresis resolving the 18S and 28S ribosomal RNA profile on the Agilent Technologies 2100 Bioanalyzer. Genome-wide microarray analyses were performed with 100ng of total RNA from three independent biological replicates per group using Affymetrix mouse genome 430 2.0 arrays (3’ IVT labeling). The data obtained have been deposited in the Gene Expression Omnibus (GEO) database (GSE61208). Affymetrix CEL files were normalized using Robust Multichip Average algorithm (30) in GeneSpring GX software (Agilent Technologies) and each probe was normalized to the median value of the control specimens (Sham+Isotype). Differentially regulated genes were identified by feature selection algorithm Pavlidis template matching (31) using a p-value <0.05, to identify gene sets for subsequent pathway analysis. The most altered canonical pathways and gene networks were identified using the Ingenuity Pathway Analysis (IPA) software.
Immunostaining of tumor sections
Tumors were fixed for 1h at 4°C in a 4% paraformaldehyde, incubated overnight in 30% sucrose, and frozen in optimum cutting temperature (OCT) medium. Sections were incubated with 0.1% Tween 20 and 0.01% Triton X-100 for 20 min, followed by blocking with 4% rat serum in 4% bovine serum albumin/PBS and staining with PE-Texas Red–rat anti-mouse CD4 or PE-rat anti-mouse CD8α (Caltag, Carlsbad, CA), and 5 μg/mL 4′,6-diamidino-2-phenylindole (Sigma). Images were obtained using a Leica SNC400F fluorescence slide scanner. CD4 and CD8 T cells were counted in five randomly selected (×200) fields in each tumor.
Statistical analysis
The unpaired Student T test was used for analysis of IFNγ levels, cell number and phenotype. Treatment effect on tumor growth was assessed using random coefficient regression. The dependent variable was the natural log of tumor volume at all available time points. Exact Mann-Whitney tests were used to compare treatment groups in terms of lung metastasis count on day 28. ANOVA based on ranks was used to test the interactions of RT and 1D11 exposure in terms of their impact on lung metastasis count. Survival differences were assessed using log-rank tests. The Kaplan-Meier method was used to estimate median survival times. All reported p values are two-sided and are declared as significant at the level of 5%. The statistical computations were carried out using SAS for windows, version 9.3 (SAS Institute).
RESULTS
Priming of CD8+ T cells reactive to multiple endogenous tumor antigens by RT requires TGFβ blockade
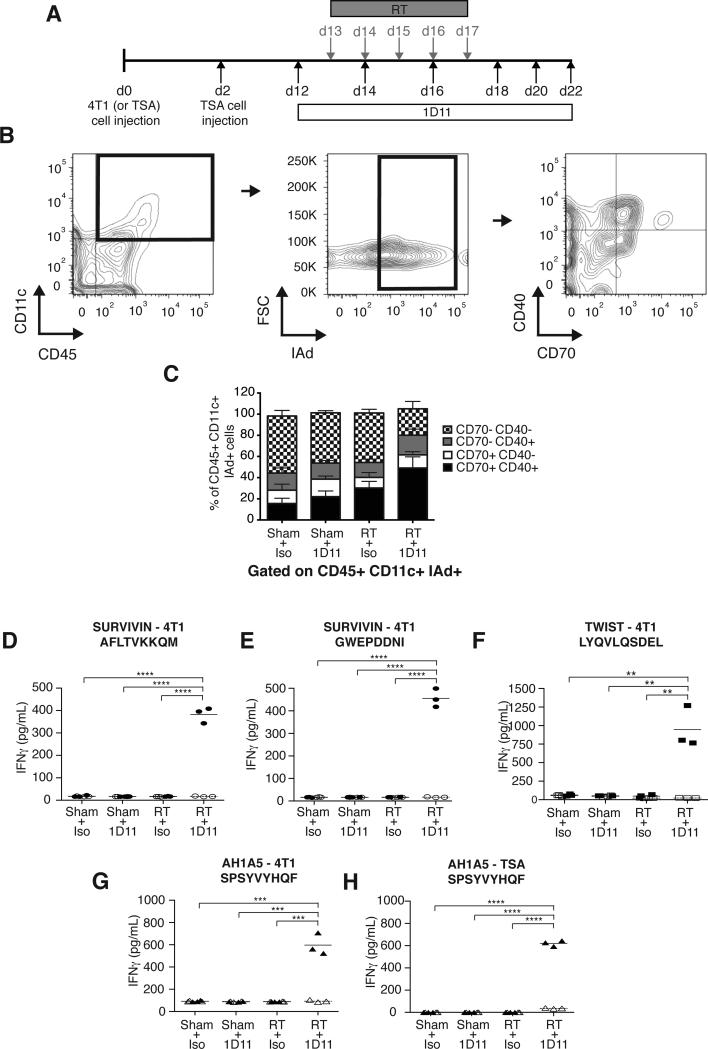
Radiation has been shown to promote DC activation while TGFβ hinders it (23,32). To determine whether blocking TGFβ improves RT-induced tumor-infiltrating dendritic cells (TIDC) activation, mice bearing established flank tumors from the 4T1 mouse breast carcinoma received i.p. injection of 1D11, a pan-isoform neutralizing TGFβ mAb or its isotype control starting at day 12 post-tumor cells injection. In half of the mice in each group, tumors were treated with RT given in 5 daily doses of 6 Gy starting on day 13 (Figure 1A). TIDC, defined as CD45+CD11c+MHC-II+ cells, were analyzed at day 22 for expression of activation markers CD40 and CD70, which plays a critical role in controlling priming versus tolerance induction of CD8+ T cells (33). The percentage of CD40+CD70+ TIDC was increased by RT and TGFβ blockade used alone (22.3% ± 4.9 in 1D11 and 30.2% ± 6.2 in RT versus 15.5% ± 4.9 in control, p<0.05 and p<0.005, respectively) but the increase was far larger when they were combined (49.1% ± 10.5 in RT+1D11; p<0.0005 compared to control; p<0.005 compared to 1D11 or RT). Overall, about 80% of TIDC expressed at least one activation marker in mice treated with RT + TGFβ blockade (Figure 1B-C).
Figure 1. TGFβ blockade with RT enhances TIDC activation and induces CD8+ T cells responses to endogenous tumor antigens.
(A) Treatment schema. (B-C) Analysis of 4T1 TIDC at day 22 (n=9/group). To obtain sufficient material, 3 tumors were pooled to obtain 3 independent samples for each group. Viable cells were gated on CD45+CD11c+IAd+ TIDC and analyzed for expression of activation markers CD40 and CD70. (B) Representative dot plots. (C) Bar graphs showing significant increase in mean percentage of TIDC expressing CD40 and CD70 in tumors of mice treated with RT+1D11 (P<0.005 compared to all other groups). (D-H) IFNγ production by CD8+ T cells from LN draining 4T1 (D-G) or TSA (H) tumors in response to peptides derived from survivin (D and E, closed circles), Twist (F, closed squares,), and gp70 (AH1A5) (G and H, closed triangles) or irrelevant peptide (open symbols). Each symbol represents one animal. Horizontal lines indicate the mean of antigen-specific (solid lines) or control (dashed lines) IFNγ concentration. Data are representative of three independent experiments. **p<0.005; ***p<0.0005; ****p<0.00005.
Next, TDLN were analyzed to assess the effect of RT and TGFβ blockade on priming of 4T1 tumor antigen-specific CD8+ T cells. TGFβ signaling leads to downstream phosphorylation of Smad2/3, which was similarly detected in T cells of untreated mice and mice receiving RT (supplementary Figure 1), indicating that baseline production of active TGFβ is sufficient to affect T cell responses. Administration of 1D11 effectively abrogated TGFβ signaling in T cells of both untreated and RT-treated mice. However, CD8+ T cell responses to four MHC class I-restricted peptides derived from three endogenous tumor antigens, survivin, Twist and gp70 (25,28,29), were detected only in mice treated with the combination of RT and 1D11 (Figure 1 D-G). The requirement for TGFβ blockade to achieve priming of CD8+ T cells to gp70 was confirmed in the TSA mouse breast carcinoma (Figure 1H). These data indicate that priming of T cell responses to endogenous tumor antigens is promoted by tumor irradiation but is blocked by TGFβ.
Inhibition of tumor growth and metastases by the combination of RT and TGFβ blockade
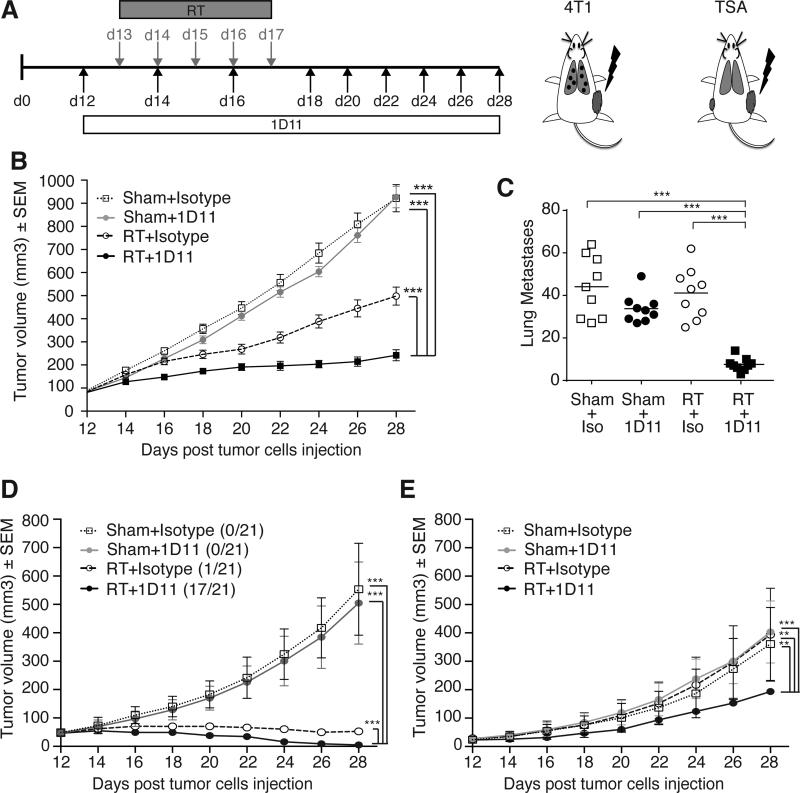
To determine whether priming of tumor-specific T cells by RT and TGFβ blockade was associated with therapeutic activity we used two experimental settings. First, mice bearing day 12 subcutaneous 4T1 tumors, a time at which this aggressive carcinoma has already metastasized to the lungs (24), were treated as above, but 1D11 administration was continued until day 28 (Figure 2A). Mice were followed for tumor growth and lung metastases were evaluated at day 28. TGFβ blockade by itself did not have any effect on either growth of the subcutaneous tumor or number of lung metastases (Figure 2B-C). RT caused a significant growth delay of the irradiated tumor (p<0.0005 RT versus control) without any effects on lung metastases. However, when RT was given with TGFβ blockade, both the irradiated tumor and non-irradiated lung metastases were markedly inhibited (p<0.0005 RT+1D11 versus all other groups), demonstrating a synergistic interaction of the two treatments.
Figure 2. TGFβ blockade with RT inhibits irradiated tumor and non-irradiated metastases.
(A) Tumor models and treatment schema. (B, C) Tumor volume overtime (B) and lung metastases quantified at day 28 (C) in 4T1 tumor-bearing mice treated with Sham+Isotype (n=9, open squares), Sham+1D11 (n=8, grey circles), RT+Isotype (n=9, open circles) and RT+1D11 (n=9, black squares). Data are representative of three independent experiments. (D, E) Tumor volume overtime of primary (right flank) (D) and secondary (left flank) (E) TSA tumors in mice treated with Sham+Isotype (n=21, open squares), Sham+1D11 (n=21, grey circles), RT (primary only) +Isotype (n=20, open circles) and RT (primary only) +1D11 (n=21, black squares). Data are representative of two experiments. *p<0.05; **p<0.005; ***p<0.0005.
In the second experimental setting, 1×105 TSA cells were injected subcutaneously on day 0 to generate a “primary” tumor and on day 2 in the contralateral flank to generate a “secondary” tumor. Treatment was started when both tumors were palpable, but only the primary larger tumor was irradiated (Figure 2A). Similarly to what was observed with 4T1 tumor, TGFβ blockade did not have any effect on either of the two tumors (Figure 2D-E). When combined with RT, however, TGFβ blockade significantly improved response of the irradiated tumor with 81% showing complete regression compared to only 5% with RT alone (p<0.001). Importantly, growth of the contralateral, non-irradiated tumors was significantly inhibited only when RT was given with TGFβ blockade (p<0.001). Taken together, these data indicate that TGFβ blockade during radiotherapy allows priming of tumor-specific CD8+ T cells to occur, and both improves the local response to RT and elicits abscopal responses.
Gene signatures associated with immune-mediated tissue rejection are upregulated in irradiated tumors only when TGFβ is blocked
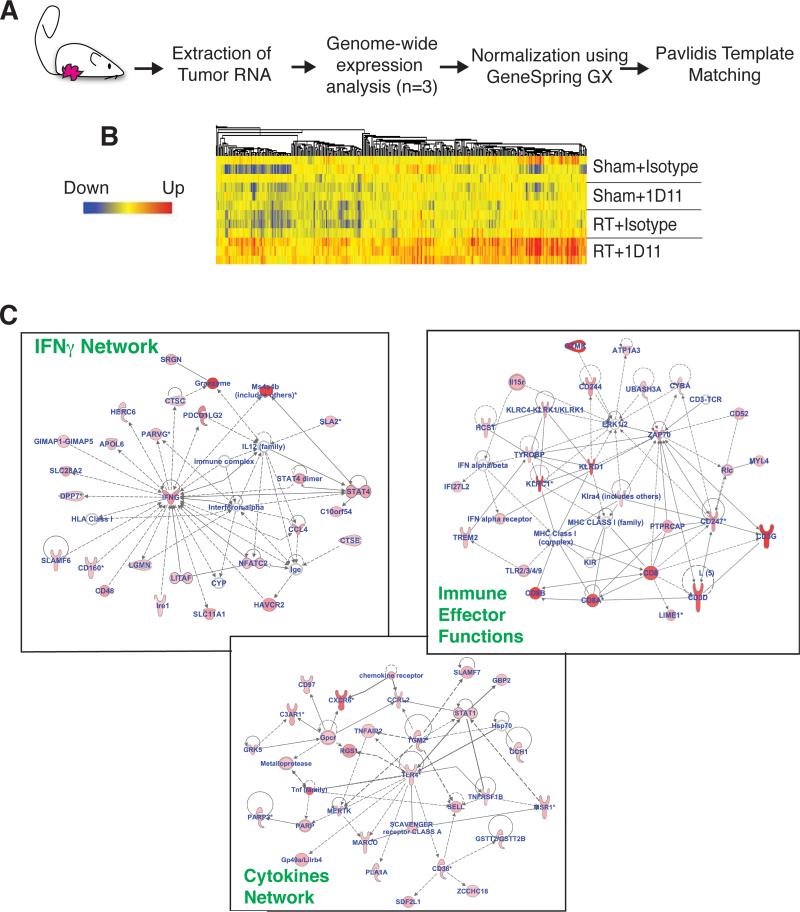
TGFβ is a pleiotropic cytokine that promotes tumor growth and metastases by acting on multiple cell compartments including the neoplastic cells themselves, the tumor stroma and the immune system (34). Once tumors were established, blocking TGFβ did not impair growth of 4T1 subcutaneous tumors or lung metastases (Figure 2), suggesting that at more advanced stages these processes are less dependent on TGFβ. However, TGFβ inhibition also impairs the DNA damage response thereby increasing radiosensitivity of breast and other cancer cells (22,35). To gain a comprehensive portrait of the effects of RT and TGFβ blockade on gene expression in tumors, we performed microarray analysis of 4T1 tumors four days after completion of RT. Using Pavlidis template matching (31) we identified 500 genes selectively upregulated in tumors treated with RT+1D11 (Figure 3A and B). The enriched data set was analyzed using IPA software to obtain the molecular pathways differentially expressed in the combination treatment group. The top 20 significantly upregulated canonical pathways were all immune-related (supplementary Figure 2). Importantly, many of these pathways were found to be upregulated in regressing melanoma metastases from patients responding to immunotherapy (36) and to be associated with a favorable prognosis in human breast tumors (37). Furthermore, the top three self-organizing gene interaction networks generated by IPA indicated activation of a process encompassing the coordinated production of chemokines and cytokines that promote the recruitment of CTLs, and the activation of immune effector function genes and of IFNγ (Figure 3C). Overall, these data indicate that the dominant change in tumors treated with RT and concomitant TGFβ blockade is activation of immune-mediated tissue rejection pathways (38).
Figure 3. TGFβ blockade with RT activates immune-mediated tissue rejection pathways.
(A) Experimental schema. Genome-wide analysis of RNA from 4T1 tumor of mice treated as indicated in Figure 1 and harvested at day 22 (n=3/group). (B) Heat map showing the top 500 genes selectively upregulated in RT+1D11 tumors. (C) Top 3 self-organizing networks according to IPA representing schematic relationship among genes upregulated in RT+1D11 tumors. Upregulated genes and gene complexes are in red, while no color fill designates genes that are part of the network but not of the gene list. Bold lines indicate direct interaction. Dotted lines indicate indirect interaction.
The therapeutic effect of RT in combination with TGFβ blockade is T cell-dependent
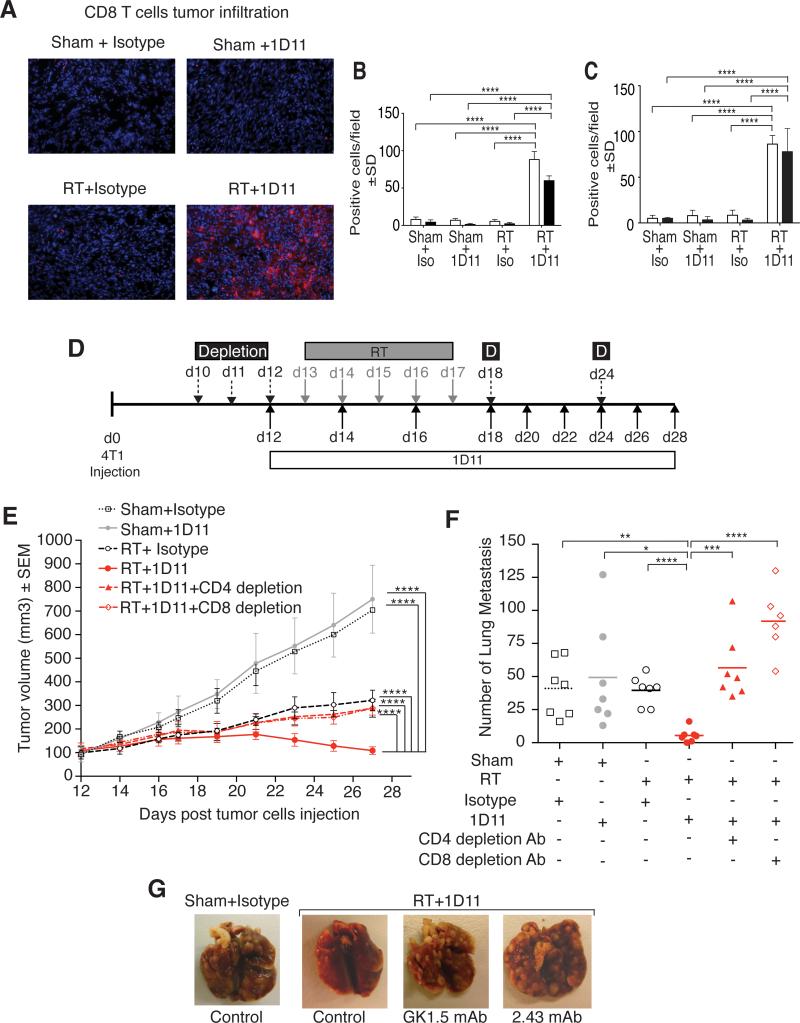
To confirm that TGFβ blockade elicited immune-mediated tumor rejection, tumor sections were stained for T cell markers. Rare T cells were present in untreated 4T1 tumors and their density was not altered significantly by either RT or TGFβ blockade alone. In contrast, when the two treatments were combined CD4+ and CD8+ T cells infiltrating the irradiated 4T1 tumors markedly increased (Figure 4A and B). Importantly, a marked increase in tumor-infiltrating T cells was also seen with TGFβ blockade in non-irradiated tumors of mice bearing two TSA tumors only one of which was irradiated (Figure 4C), suggesting that immune-mediated tumor rejection occurs systemically. Consistent with this, depletion of CD4+ or CD8+ T cells abrogated regression of the irradiated 4T1 tumors and restored the number of lung metastases in mice treated with TGFβ inhibition + RT (Figure 4 E-G). Overall, these data demonstrate that tumor-specific T cells activated in mice treated with RT and TGFβ blockade are key mediators of regression of irradiated tumors and of the abscopal responses.
Figure 4. The therapeutic effect of local RT and TGFβ blockade is T cell-dependent.
4T1 primary (A, B) and TSA secondary (C) tumors of mice treated as indicated in Figure 1 were harvested at day 22 (n=3/group) and stained for T cell markers. (A) Representative fields (x200) showing CD8+ T cells (red). Nuclei were stained with DAPI (blue). Mean number ± SD of CD4+ (white bars) and CD8+ (black bars) cells/field in 3 mice/group in 4T1 (B) and TSA (C) tumors. (D) Treatment schema for depletion of CD4+ or CD8+ T cells in 4T1 tumor-bearing mice treated with RT and TGFβ blockade. (E) Tumor volume and (F) lung metastases at day 28 in mice treated with Sham+Isotype (open squares, n=7), Sham+1D11 (grey circles, n=7), RT+Isotype (open circles, dashed black line), RT+1D11 (red circle, red line, n=7), RT+1D11+CD4 depletion (red triangles, dashed red line, n=7) and RT+1D11+CD8 depletion (open red diamond, dashed and dotted red line, n=7). Data are representative of two independent experiments. (G) Representative photographs of lungs. *p<0.05; **p<0.005; ***p<0.0005; ****p<0.00005.
Adaptive immune resistance mediated by PD-1 pathway limits the therapeutic efficacy of RT with TGFβ blockade
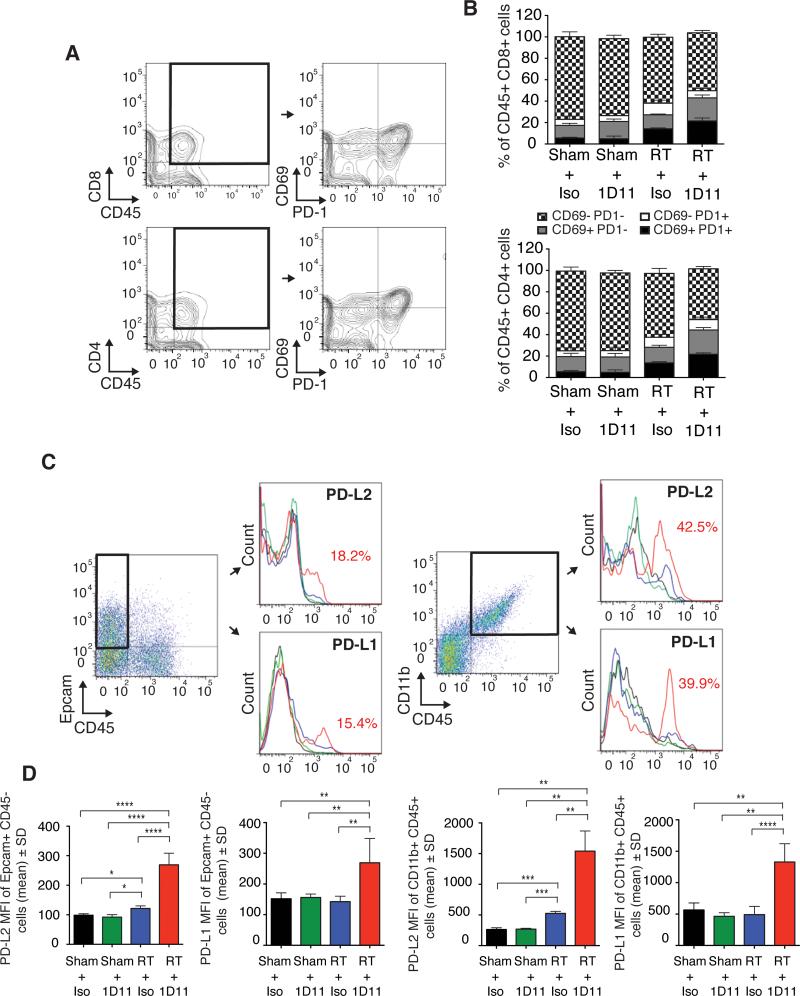
Despite the prominent T cell infiltrate the majority of 4T1 tumors in mice treated with RT and TGFβ blockade did not undergo complete regression, suggesting that additional immunosuppressive mechanisms hinder effector T cell function in the tumor. Since interactions between the immune checkpoint receptor PD-1 and its ligands PD-L1 and PD-L2 are an important mechanism inhibiting tumor rejection by T cells (39), we next investigated the expression of PD-1 and its ligands in the tumor.
Intratumoral CD4+ and CD8+ T cells showed increased expression of the activation marker CD69 in tumors treated with RT, which was further enhanced by TGFβ blockade (Figure 5A-B). Interestingly, about half of CD69+ T cells co-expressed PD-1, while only a minor subset was PD1+CD69−, suggesting that most PD1+ T cells are activated tumor-specific T cells (40). Expression of both PD-L1 and PD-L2 was induced by treatment with RT and TGFβ blockade in a small subset of 4T1 cells, identified as EpCAM+CD45− cells, and in a larger subset of intratumoral CD45+CD11b+ myeloid cells (Figure 5C-D). Irradiation in the absence of TGFβ blockade had only a minor effect on the expression of PD-L1 and PD-L2, while TGFβ blockade had no effect.
Figure 5. Enhanced expression of PD-1 and its ligands in tumors treated with TGFβ blockade and RT.
4T1 tumors of mice treated as indicated in Figure 1 were harvested at day 22 (n=9/group). To obtain sufficient material, 3 tumors were pooled to obtain 3 independent samples for each group. (A-B) Expression of CD69 and PD-1 was analyzed in CD4+ and CD8+ T cells. Representative dot plots. (B) Bar graphs show mean percentage of T cells expressing one or both markers, as indicated. (C) Representative dot plots showing the gating strategy for 4T1 and myeloid cells, and expression of PD-1 ligands. (D) Bar graphs show PD-L2 and PD-L1 mean fluorescence intensity (MFI) ± SD on gated Epcam+CD45− 4T1 cells and CD45+CD11b+ myeloid cells. *p<0.05; **p<0.005; ***p<0.0005.
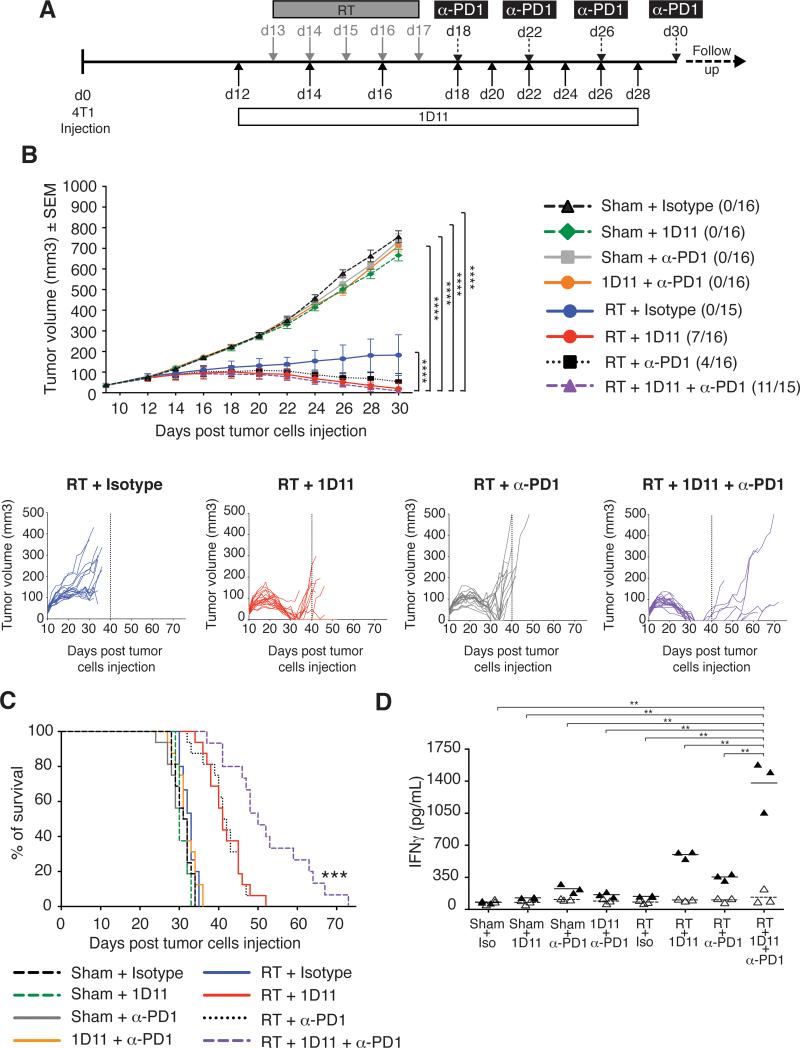
We then tested the hypothesis that treatment with PD-1 blocking antibody could further improve the therapeutic effect achieved with RT and TGFβ blockade. Blockade of PD-1 alone or in combination with TGFβ blockade did not have any effect on tumor growth or survival (Figure 6B-C). RT alone, as expected, caused growth delay of the irradiated tumor without an effect on survival. RT given with either TGFβ or PD-1 blockade improved control of the irradiated tumor with 44% and 25% complete regressions, respectively, and a significant extension of survival (p<0.001 compared to control and RT), but in both groups all tumors recurred before day 40. In contrast, 75% of the mice treated with RT and blockade of both PD-1 and TGFβ achieved complete regression of the irradiated tumor, and tumor recurrence was delayed, resulting in a significant increase in survival compared to all other groups (P<0.001). ANOVA based on ranks demonstrated a significant interaction among the three interventions (p=0.006), suggesting complementary effects. Interestingly, PD-1 blockade in the presence of RT promoted priming of tumor-specific CD8+ T cells in TDLN, and further enhanced the response achieved with TGFβ blockade (Figure 6D). Thus, when used with RT anti-PD-1 may have effects at both effector and priming phase that contribute to tumor rejection. Thus, these data support the rational targeting of multiple inhibitory pathways in combination with RT.
Figure 6. Blocking PD-1 in mice treated with RT and TGFβ blockade improves tumor rejection and survival.
(A) Treatment schema. (B) Mean tumor volume in each group up to day 30, and individual mice tumor growth curves for groups receiving RT. (C) Survival of mice treated with Sham+Isotype (dashed black line, n=16), Sham+1D11 (dashed green line, n=16), Sham+anti-PD-1 (grey line, n=16), Sham+1D11+anti-PD-1 (orange line, n=16), RT+Isotype (blue line, n=15), RT+1D11 (red line, n=16), RT+ anti-PD-1 (dotted black line, n=16) and RT+1D11+anti-PD-1 (dashed purple line, n=15). Data are representative of two independent experiments. (D) IFNγ production by CD8+ T cells from LN draining 4T1 tumors in response to AH1A5 peptide (closed triangles, n=3) or irrelevant peptide (n=3, open triangles). Each symbol represents one animal. Horizontal lines indicate the mean of antigen-specific (solid lines) or control (dashed lines) IFNγ concentration. Data are representative of two experiments. *p<0.05; **p<0.005; ***p<0.005.
DISCUSSION
Recent successes of immunotherapy have shown that unleashing the power of the immune system to fight cancer can change the outcome for metastatic disease. Although responses are seen in a relatively small percentage of patients, the durability of the response is far superior to other types of treatment (41,42). Therefore, combination treatments that can extend the benefits of immunotherapy to more patients are under active investigation. We previously proposed that RT has effects that may complement those of at least some immunotherapies (43), a concept that has recently gained broader support (44,45). Here we show that irradiation of tumors unresponsive to targeting of two key immunosuppressive pathways alone, or even in combination, enables effective anti-tumor immune responses.
Accumulating evidence suggests that the presence of substantial pre-existing tumor-specific T cells predicts which patients will respond to immunotherapy (46), emphasizing the inability of most currently tested treatments to effectively induce de novo T cell priming to endogenous tumor antigens. Although RT has been previously shown to prime T cells to model antigens expressed by tumors (6,7), evidence that it can prime T cell responses to less immunogenic endogenous tumor antigens has been limited (47). We have uncovered that RT-induced T cell priming is impeded by radiation-induced TGFβ. TGFβ blockade has effects on multiple immune cells that contribute to tumor control (34). Radiation elicits TGFβ activation (20,21); notably, our data show that blocking TGFβ in established tumors is insufficient to slow growth of the primary tumor or its lung metastases. While we cannot exclude the contribution of other immune cells to the therapeutic effect achieved with RT and TGFβ blockade, depletion of T cells completely abrogated the inhibition of the primary irradiated tumor and non-irradiated lung metastases, demonstrating the critical role of T cells. When TGFβ was neutralized in irradiated tumors, we detected T cells reactive against multiple tumor antigens, including the anti-apoptotic protein survivin and the transcription factor Twist, which promotes epithelial to mesenchymal transition and metastasis (48). Thus, TGFβ blockade is likely to be required to greatly enhance radiation-induced vaccination to breast and other cancers.
The prominent IFNγ signature and T cell infiltration in tumors of mice treated with RT and TGFβ blockade are associated with development of adaptive immune resistance mediated by the PD-1 pathway (49). In fact, PD-L1 and PD-L2 were upregulated on both cancer cells and myeloid cells in tumors of mice treated with this combination. Increased expression of PD-1 was also seen in T cells infiltrating irradiated tumors, and was larger in the presence of TGFβ blockade. Administration of anti-PD-1 antibody enhanced tumor regression and delayed recurrence, demonstrating that PD-1 limits the function of T cells primed by RT and TGFβ blockade. Interestingly, tumor antigen-specific IFNγ production by CD8+ T cells was enhanced by PD-1 blockade in the presence of RT, suggesting that improved priming of tumor-specific T cells may contribute to the therapeutic effect when PD-1 blockade is combined with RT-induced vaccination. Overall, data suggest a model whereby TGFβ controls priming of tumor-specific T cells by RT-released antigens, and PD-1 negatively modulates T cell activation by acting at both the induction and effector phases of the anti-tumor response.
Despite the improved responses achieved with addition of anti-PD-1 to RT and TGFβ blockade tumors still recurred within a relatively short time after discontinuation of anti-PD-1 treatment. It is possible that prolonged administration of anti-PD-1 would have further delayed or prevented recurrence. Alternatively, although T cell responses to multiple tumor antigens were induced by RT and TGFβ blockade, it is possible that the critical antigenic target for tumor rejection is a tumor-specific mutant antigen (50) that might be lost allowing for tumor escape from immune control.
Overall, our findings support targeting of the PD-1 pathway in future clinical trials testing the combination of RT and TGFβ blockade, currently being tested in metastatic breast cancer patients (NCT01401062).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Genzyme Inc. for providing antibodies, and the Immunohistochemistry, Immunomonitoring, Genome Technology Center, and Flow Cytometry Core Facilities of the Laura and Isaac Perlmutter Cancer Center for assistance.
Grant Support: Supported by grants from the USA Department of Defense Breast (DOD) Cancer Research Program (BCRP) (W81XWH-11-1-0532), Breast Cancer Research Foundation, Breast Cancer Alliance, and The Chemotherapy Foundation. CV-B is supported by a Post-doctoral fellowship from the DOD BCRP (W81XWH-13-1-0012). Laura and Isaac Perlmutter Cancer Center at NYU Langone Medical Center is supported by NIH/NCI P30CA016087.
Footnotes
Potential conflicts of interest: S. Demaria has served as consultant for Sanofi US Inc. and Regeneron Pharmaceuticals, Inc. MH Barcellos-Hoff has received a grant from Varian Medical Systems, and honoraria from Genzyme and Isarna Speaker Bureau.
REFERENCES
- 1.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunol Rev. 2011;239(1):27–44. doi: 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Formenti SC, Demaria S. Radiotherapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys. 2012;84(4):879–80. doi: 10.1016/j.ijrobp.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 7.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114(3):589–95. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099–107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180(5):3132–9. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]
- 10.Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–47. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 11.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruocco MG, Pilones KA, Kawashima N, Cammer M, Huang J, Babb JS, et al. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves anti-tumor effects. J Clin Invest. 2012;122(10):3718–30. doi: 10.1172/JCI61931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Y, Kepp O, Ghiringhelli F, Apetoh L, Aymeric L, Locher C, et al. Chemotherapy and radiotherapy: cryptic anticancer vaccines. Semin Immunol. 2010;22(3):113–24. doi: 10.1016/j.smim.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71(7):2488–96. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demaria S, Ng B, Devitt M-L, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–70. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Demaria S, Kawashima N, Yang AM, Devitt M-L, Babb JS, Allison JP, et al. Immune-mediated inhibition of metastases following treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–34. [PubMed] [Google Scholar]
- 17.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–88. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10(7):718–26. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barcellos-Hoff MH, Cucinotta FA. New tricks for an old fox: impact of TGFbeta on the DNA damage response and genomic stability. Science signaling. 2014;7(341):re5. doi: 10.1126/scisignal.2005474. [DOI] [PubMed] [Google Scholar]
- 20.Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA. Transforming growth factor-beta activation in irradiated murine mammary gland. J Clin Invest. 1994;93(2):892–9. doi: 10.1172/JCI117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jobling MF, Mott JD, Finnegan MT, Jurukovski V, Erickson AC, Walian PJ, et al. Isoform-specific activation of latent transforming growth factor beta (LTGF-beta) by reactive oxygen species. Radiat Res. 2006;166(6):839–48. doi: 10.1667/RR0695.1. [DOI] [PubMed] [Google Scholar]
- 22.Bouquet F, Pal A, Pilones KA, Demaria S, Hann B, Akhurst RJ, et al. TGFβ1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin Cancer Res. 2011;17(21):6754–65. doi: 10.1158/1078-0432.CCR-11-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007;13:5262–70. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 24.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–405. [PubMed] [Google Scholar]
- 25.Rosato A, Santa SD, Zoso A, Giacomelli S, Milan G, Macino B, et al. The cytotoxic T-lymphocyte response against a poorly immunogenic mammary adenocarcinoma is focused on a single immunodominant class I epitope derived from the gp70 Env product of an endogenous retrovirus. Cancer Res. 2003;63:2158–63. [PubMed] [Google Scholar]
- 26.Dasch JR, Pace DR, Waegell W, Inenaga D, Ellingsworth L. Monoclonal antibodies recognizing transforming growth factor-beta. Bioactivity neutralization and transforming growth factor beta 2 affinity purification. J Immunol. 1989;142(5):1536–41. [PubMed] [Google Scholar]
- 27.Donkor MK, Sarkar A, Savage PA, Franklin RA, Johnson LK, Jungbluth AA, et al. T cell surveillance of oncogene-induced prostate cancer is impeded by T cell-derived TGF-beta1 cytokine. Immunity. 2011;35(1):123–34. doi: 10.1016/j.immuni.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel S, Wagner A, Schmitz N, Zeis M. Induction of antitumour immunity using survivin peptide-pulsed dendritic cells in a murine lymphoma model. Br J Haematol. 2003;122:911–4. doi: 10.1046/j.1365-2141.2003.04535.x. [DOI] [PubMed] [Google Scholar]
- 29.Demaria S, Wang B, Yang AM, Santori F, Kawashima N, Matsumura S. Immunotherapy against metastatic breast cancer with a twist Breast cancer research & treatment. 2007;106(1):S31–S31. [Google Scholar]
- 30.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19(2):185–93. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 31.Pavlidis P, Noble WS. Analysis of strain and regional variation in gene expression in mouse brain. Genome Biol. 2001;2(10):RESEARCH0042. doi: 10.1186/gb-2001-2-10-research0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189(2):558–66. doi: 10.4049/jimmunol.1200563. [DOI] [PubMed] [Google Scholar]
- 33.Keller AM, Schildknecht A, Xiao Y, van den Broek M, Borst J. Expression of costimulatory ligand CD70 on steady-state dendritic cells breaks CD8+ T cell tolerance and permits effective immunity. Immunity. 2008;29(6):934–46. doi: 10.1016/j.immuni.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Nam JS, Terabe M, Mamura M, Kang MJ, Chae H, Stuelten C, et al. An anti-transforming growth factor beta antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Res. 2008;68:3835–43. doi: 10.1158/0008-5472.CAN-08-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardee ME, Marciscano AE, Medina-Ramirez CM, Zagzag D, Narayana A, Lonning SM, et al. Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-β. Cancer Res. 2012;72(16):4119–29. doi: 10.1158/0008-5472.CAN-12-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carretero R, Wang E, Rodriguez AI, Reinboth J, Ascierto ML, Engle AM, et al. Regression of melanoma metastases after immunotherapy is associated with activation of antigen presentation and interferon-mediated rejection genes. Int J Cancer. 2012;131(2):387–95. doi: 10.1002/ijc.26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ascierto ML, De Giorgi V, Liu Q, Bedognetti D, Spivey TL, Murtas D, et al. An immunologic portrait of cancer. J Transl Med. 2011;9:146. doi: 10.1186/1479-5876-9-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang E, Worschech A, Marincola FM. The immunologic constant of rejection. Trends Immunol. 2008;29(6):256–62. doi: 10.1016/j.it.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207–12. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014;124(5):2246–59. doi: 10.1172/JCI73639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Postow MA, Callahan MK, Wolchok JD. The antitumor immunity of ipilimumab: (T-cell) memories to last a lifetime? Clin Cancer Res. 2012;18(7):1821–3. doi: 10.1158/1078-0432.CCR-12-0409. [DOI] [PubMed] [Google Scholar]
- 42.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–30. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys. 2005;63(3):655–66. doi: 10.1016/j.ijrobp.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. J Clin Invest. 2013;123(7):2756–63. doi: 10.1172/JCI69219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105(4):256–65. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–22. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaue D, Comin-Anduix B, Ribas A, Zhang L, Goodglick L, Sayre JW, et al. T-cell responses to survivin in cancer patients undergoing radiation therapy. Clin Cancer Res. 2008;14(15):4883–90. doi: 10.1158/1078-0432.CCR-07-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–81. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.