Abstract
The aim of this work was to determine the parameters that have decisive roles in microwave-assisted reactions and to develop a model, using computational chemistry, to predict a priori the type of reactions that can be improved under microwaves. For this purpose, a computational study was carried out on a variety of reactions, which have been reported to be improved under microwave irradiation. This comprises six types of reactions. The outcomes obtained in this study indicate that the most influential parameters are activation energy, enthalpy, and the polarity of all the species that participate. In addition to this, in most cases, slower reacting systems observe a much greater improvement under microwave irradiation. Furthermore, for these reactions, the presence of a polar component in the reaction (solvent, reagent, susceptor, etc.) is necessary for strong coupling with the electromagnetic radiation. We also quantified that an activation energy of 20–30 kcal mol−1 and a polarity (μ) between 7–20 D of the species involved in the process is required to obtain significant improvements under microwave irradiation.
Keywords: activation energy, computational chemistry, microwave irradiation, organic synthesis, polarity
Introduction
Since the first publication on microwave-assisted organic synthesis (MAOS),1 this unconventional heating technology has been extended to medicinal chemistry,2 biochemistry,3 materials science,4 and analytical, environmental, and green chemistry.5 Microwave irradiation has proven to be an interesting alternative for heating chemical reactions6 and offers considerable advantages over conventional heating methods, including shorter reaction times, simple purification procedures, and higher product purity as decomposition is minimized.
The best known characteristic of microwave-assisted reactions is the spectacular acceleration of most processes to give reaction times that cannot be achieved with conventional heating. However, more important effects include the possibility of accessing products and reactions that are not achievable by conventional methods and the possibility of modifying or even inverting the selectivity of reactions (induced and inverted selectivity).7
Despite the great developments and applications of microwave-assisted chemistry, there is an ongoing debate in the scientific community about the origin of the effects caused by microwave irradiation. It has been widely postulated that some of the improvements observed in MAOS can be attributed to the so-called ‘nonthermal’ effect.[8] Nowadays, it is accepted that in most cases, the observed enhancements in microwave-heated reactions are, in fact, the result of purely thermal/kinetic effects.9
The existence of so-called ‘specific thermal microwave effects’, which cannot be duplicated by conventional heating and result from the uniqueness of the microwave dielectric heating phenomenon, is widely documented.7,8b Nowadays, it is generally accepted that the action of microwave irradiation affects chemical reactions by thermal effects: overheating,10 hot-spot formation,11 selective heating of catalysts, solvents, and reagents (molecular radiators),12 and the elimination of wall effects by inverted temperature gradients.13
‘Nonthermal microwave effects’ are highly controversial and have caused great debates among experts in MAOS.8,9b,14 Such effects are rare and very difficult to prove because the conversion of electromagnetic energy into kinetic energy is slower than the conversion of kinetic energy into thermal energy. On the other hand, it is very difficult to separate experimentally thermal heating from other possible effects of electromagnetic radiation. In any case, MAOS has proven to be an incredibly effective methodology in chemistry that provides results and conditions that are hardly reproducible under conventional heating.
The physical principles behind this method and the factors that influence the successful use of microwave technology in chemical applications are not particularly familiar to chemists, possibly because electric field theory is generally taught in engineering or physics rather than in chemistry. Further study is therefore required on the physical factors that can influence MAOS. Several aspects, like temperature, stirring, or reaction vessel characteristics, have been studied to some extent.15 Other aspects, like the influence of the polarity of the species, activation energy, and enthalpy, have barely been studied at all. We present here a broad study on the influence of these factors in MAOS by using computational chemistry. It is noteworthy that, considering the different characteristics of the two methods of heating, it is extremely difficult to design procedures that strictly have the same reaction conditions under microwave irradiation and conventional heating. In this regard, computational studies allow us to determine the thermodynamic, kinetic, and physical parameters and also the reaction mechanisms separately.
Our research group has significant experience in MAOS. In this respect, we have an interest in the computational study of microwave-assisted reactions in order to understand and predict the effects of microwave irradiation in organic synthesis.16
The goal of our research is to determine the influence of the activation energy, enthalpy and polarity of some selected reactions in an attempt to gain a deeper understanding of MAOS and to develop a general model that will allow the use of simple calculations to predict whether a reaction could be improved under microwave irradiation.
Some specific microwave effects have been explained by using the Arrhenius law [Eq. (1)]17 and by considering modifications in each of term in the equation. Several reports suggest that the electric component of microwaves leads to orientation effects on dipolar molecules or intermediates and hence changes the pre-exponential factor (A) or the activation energy (Ea) term in the Arrhenius equation. This effect is particularly important under solvent-free conditions or in a nonpolar medium.8a
| 1 |
Polarity is another important factor to be considered in MAOS. The polarities of all components present in the reaction medium play an important role in the heating rate with microwaves. The ability of highly polar substrates to absorb microwave energy completely is generally accepted. This property has been exploited to heat reactions under microwave irradiation when carried out in poorly absorbing solvents by using ionic additives such as ionic liquids18 or tetrabutylammonium bromide, or by adding highly microwave-absorbing silicon carbide plugs or graphite.19
Additionally, if a polar reaction mechanism occurs, where the relative polarity increases from the reactant to the transition structure, it is claimed that acceleration due to an increase in microwave absorbance of the intermediate could occur. This effect is particularly important in product-like transition states according to Hammonds postulate.8a
Although the importance of these parameters in MAOS is known, to the best of our knowledge, they have not yet been quantified. With this aim in mind, we have used computational calculations as predictive tools to determine quantitatively when a reaction can be improved under microwave conditions, determining the predicted activation energies, reaction rates, and species polarity and using these as indicators of efficiency under microwave conditions.
Our preliminary conclusions, which are reported in previous publications,16f are summarized in Table 1. The results obtained show that the activation energy of the reaction is a good indicator to determine when a reaction can be improved under microwave conditions. The following conclusions can be drawn:
Table 1.
Preliminary conclusions on the influence of activation energy (Ea) in MAOS.
| Reaction | Ea [kcal mol−1] | Conclusions |
|---|---|---|
| Type A | <20 | Not improved |
| Type B | 20–30 | Improved |
| Type C | >30 | Improved with susceptors |
Reactions with activation energies below 20 kcal mol−1 occur easily by conventional heating and improvements are not expected under microwave conditions.
Reactions with activation energies from 20 to 30 kcal mol−1 can be improved under microwave irradiation without the use of harsh reaction conditions (e.g., high pressure, pyrolysis).
Reactions with activation energies above 30 kcal mol−1 cannot be performed either under conventional heating or microwave irradiation. However, the use microwave susceptors such as ionic liquids or highly polar solvents (microwave flash heating) can improve these processes.
In this paper, we report computational calculations on a variety of previously described reactions in order to refine our model and to study the influence of enthalpy and polarity in the reaction improvement observed when using MAOS. For this purpose, we have divided each type of reaction (A, B and C) in two different ones taking into account the enthalpy.
Results and Discussion
The selected reactions represent different kinds of processes, including cycloadditions, organometallic reactions, and substitution reactions, with different characteristics, such as polarity, activation free energy, enthalpy, and the presence of polar components like susceptors, polar solvents, etc.; these reactions can be classified into Types I–VI. Comparisons between the results obtained under conventional heating and microwave irradiation are listed along with the principal parameters studied, namely the polarity of the initial state, variations in the transition state, activation energy and enthalpy.
The objective was to confirm and refine our preliminary model and to provide a method to determine with simple calculations when a reaction can be improved under microwave irradiation. Note that, in order to clarify the obtained results, we have summarized previous computational studies of our group (Types I, III and VI) with new studies (Types II, IV, V and some examples of Type III).
Type I reactions
Type I reactions are exothermic reactions (ΔH<0 kcal mol−1) with low activation energies (Ea<20 kcal mol−1). Loupy and co-workers20 performed the cycloaddition reaction of azidomethylphosphonate and functionalized enamines under solvent-free conditions and under microwave irradiation (Scheme 1). Experimentally, triazole 5 was obtained in good yields (70–86 %) under conventional heating in solvent-free conditions at 100 °C but very long reaction times were required (6 h). Under these conditions, the reaction is 100 % regioselective. For shorter times (20 min), there was no reaction under conventional heating, whereas the reaction proceeded with a 55 % yield under microwave irradiation under comparable conditions but with lower regioselectivity (5/6 ratio, 85:15).
Scheme 1.
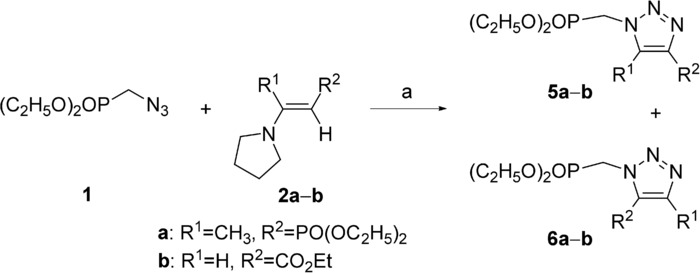
Cycloaddition reaction of azidomethylphosphonate and functionalized enamines. Reaction conditions: a) conventional conditions: 90 °C, 6 h, 86 % (5/6 ratio =100:0); microwave irradiation: 90 °C (120 W), 5–20 min, 55 % (5/6 ratio =85:15). Result: modification of the selectivity.
In order to understand the influence of microwave irradiation on the selectivity, we performed computational calculations at the B3LYP/6-31G* level (Table 2). The results of these calculations showed that cycloadduct 5 is the kinetic regioisomer; the activation energy for this isomer lies between 13.6 and 18.2 kcal mol−1. In contrast, the activation energy for cycloadduct 6 is higher (21.6–30.6 kcal mol−1), although this regioisomer is thermodynamically more stable (Scheme 2).
Table 2.
Computational calculated parameters for a Type I 1,3-dipolar reaction (Scheme 1).
| Solvent | μ [D] (most polar species) | Δμ[a] [D] | Ea [kcal mol−1] | ΔH[b] [kcal mol−1] |
|---|---|---|---|---|
| Solvent-free | 4.7 (Reactant 2 a) 7.0 (Reactant 2 b) | −1.1 to 3.5 | 13.6 to 30.6 | −16.5 to −22 |
[a] Δμ=μ TS−μ reactants; [b] ΔH=ΔHproducts−ΔHreactants.
Scheme 2.
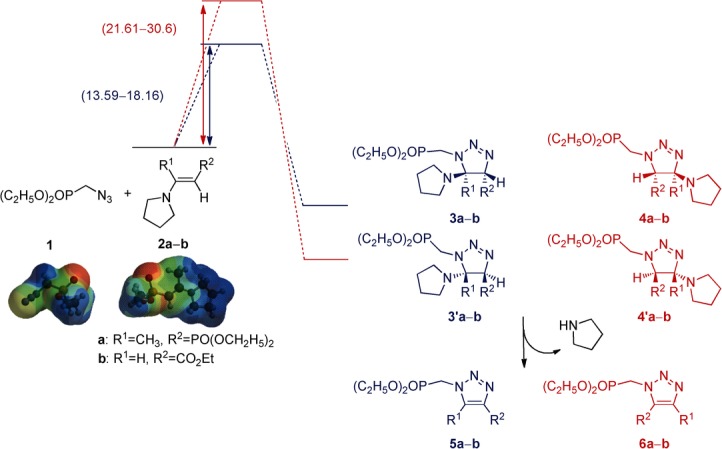
Calculated reaction path of cycloaddition reaction of azidomethylphosphonate and functionalized enamines.
The polarities of the reagents and their variation along the reaction coordinate were determined in an attempt to determine the influence of this parameter. The outcomes indicate that the reactants have moderate polarity (μ=4.7–7 D) and a negligible variation in the transition structures (μ=1.1–6.2 D). Taking in to account that the reaction was performed in solvent-free conditions, the interaction of microwaves with the polar reactants should be optimal. This situation leads to rapid heating and, consequently, a reduction in the selectivity through a clear thermal effect. In this regard, these species could be acting as ‘molecular radiators’. These species are reported to be formed when a polar reactant is irradiated in a nonpolar medium. It was suggested that ‘molecular radiators’ can directly couple with microwaves, thus creating microscopic hot spots that improve the absorption of microwave radiation and might improve the reaction through a thermal effect.21
In summary, modification of the regioselectivity under microwave irradiation can be explained through a thermal effect. The high activation energy required for cycloadduct 6 (21.6–30.6 kcal mol−1) can be easily achieved by efficient heating under microwaves but not under conventional heating. In conclusion, the formation of isomer 5 is not improved but the formation of isomer 6 is favored under microwaves.
Type II reactions
This group includes endothermic reactions (ΔH>0 kcal mol−1) with low activation energies (Ea<20 kcal mol−1). The chemical transformation used to test our model was the 1,3-dipolar reaction indicated in Scheme 3.
Scheme 3.
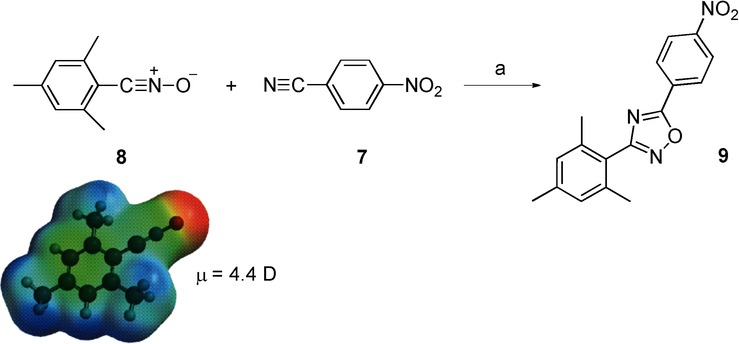
Reaction between 4-nitrobenzonitrile (7) and 2,4,6-trimethylbenzonitrile oxide (8). Reagents and conditions: a) alumina magnetite bath; conventional conditions: 135 °C, 8 min, 48 %; microwave irradiation: 135 °C (450 W), 8 min, 68 %. Result: acceleration rate=1.7.
When this reaction was performed in a domestic microwave oven,22 under solvent-free conditions, a moderate increase in the yield was observed compared with conventional heating (68 % vs 48 %).22 Computational calculations predicted a concerted mechanism that takes place through a near synchronous transition structure. In this case, the calculated polarity (μ) of the reactants is 4.4 D, and a slight increase in the polarity (Δμ=+2.1 D) takes place from the reactants to the transition structure (Scheme 2).23 It is noteworthy that, although the activation energy is low (14.8 kcal mol−1), this is an endothermic process (ΔH=14.5 kcal mol−1) (Table 3).
Table 3.
Computational calculated parameters for a Type II 1,3-dipolar reaction (Scheme 3).
| Solvent | μ [D] (most polar species) | Δμ[a] [D] | Ea [kcal mol−1] | ΔH[b] [kcal mol−1] |
|---|---|---|---|---|
| Solvent-free Al2O3/Fe3O4 bath | 4.4 (Reactant 8) | 2.1 | 14.8 | 14.5 |
[a] Δμ=μ TS−μ reactants; [b] ΔH=ΔHproducts−ΔHreactants.
In the original study,22 this reaction was carried out in a household microwave oven and was performed using an alumina–magnetite bath (Al2O3/Fe3O4, 5:1). These conditions present two main problems: firstly, microwave radiation is absorbed by the polar alumina–magnetite bath and the reaction is therefore heated rapidly but by conventional mechanisms (convection and conduction from the surface of the sample), and efficient stirring/agitation of the reaction mixture is not assured. This fact might lead to temperature gradients as a consequence of poor stirring.
In an effort to overcome this problem, we performed the reaction in a single-mode microwave oven without the mineral bath and using a nonpolar solvent (toluene) in an attempt to increase the homogeneity of the medium and to ensure absorption of microwave irradiation by the reagents. In this case, the rate was 1.7 times faster under microwave irradiation than with conventional heating. Based on these results, it was established that Type II reactions with lower Ea and positive ΔH values can be slightly improved under microwave irradiation. The presence of a polar bath or moderate polar reactants allows the efficient absorption of microwave energy and produces an acceleration effect through a clear thermal effect.
Type III reactions
This group contains a series of reactions that were previously studied theoretically in our research group.16f,16h Type III reactions are exothermic (ΔH<0 kcal mol−1) and have high activation energies (Ea=20–30 kcal mol−1). The first reaction studied was the intramolecular hetero-Diels–Alder cycloaddition of alkenyl-tethered 2(1H)-pyrazinones 10 a–b (Scheme 4). The reaction mixture was irradiated in a single-mode microwave cavity using a preselected maximum temperature of 190 °C (300 W maximum power) in a sealed vessel, with dichloroethane as the solvent and 0.035 mmol of 1-butyl-3-methylimidazolium hexafluorophosphate ([BMIM][PF6]). The reaction took place within 10 min and gave good yields (67–77 %). Under these conditions, the complete conversion of 10 took place in about 8–10 min, which represents a considerable decrease in the reaction time as compared with the 1–2 days required under conventional reflux conditions in chlorobenzene (131 °C).24
Scheme 4.
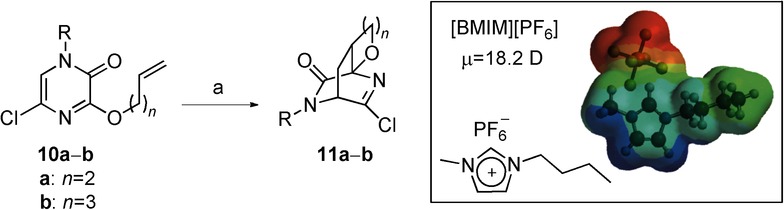
Intramolecular hetero Diels–Alder cycloaddition of alkenyl-tethered 2(1H)-pyrazinones (10). Reagents and conditions: a) 1,2-dichloroethane, [BMIM][PF6]; conventional conditions: 132 °C, 1–2 d, 54%; microwave irradiation: 170 °C (300 W), 10 min, 67–77 %. Box: structure of [BMIM][PF6]. Result: acceleration.
The potential surface of this reaction was explored at the B3LYP(PCM)/6-31G*+ΔZPVE level using dichloroethane as the solvent (Table 4).16f The outcomes indicated that the process has high activation energies (25.7–28.3 kcal mol−1 when n=2 and 37.2–39.7 kcal mol−1 when n=3), low Gibbs energy and is moderately exothermic. Moreover, the polarity of the reactants is moderate (μ=5.1–5.2 D) and only increases slightly from the reactants to the transition structure. With these characteristics, this reaction should not occur under microwave irradiation.
Table 4.
Computational calculated parameters for a Type III reaction: intramolecular hetero-Diels–Alder cycloaddition (Scheme 4).
| Solvent | μ [D] (most polar species) | Δμ[a] [D] | Ea [kcal mol−1] | ΔH[b] [kcal mol−1] |
|---|---|---|---|---|
| Dichloroethane ionic liquid ([BMIM][PF6]) | 18.2 ([BMIM][PF6]) | 0.3 to 1.4 | 25.7 to 39.7 | −7.5 to −8.9 |
[a] Δμ=μ TS−μ reactants; [b] ΔH=ΔHproducts−ΔHreactants.
The question then arises, why does this reaction proceed in such short reaction times? The addition of small amounts of a strongly microwave-absorbing ionic liquid ([BMIM][PF6]) (μ=18.2 D) as a doping agent changes the dielectric properties of the reaction mixture (Scheme 3). Ionic liquids interact very efficiently with microwaves through the ionic conduction mechanism. So, rapid heating (<1 min) to 190 °C is possible; a heating rate that cannot be achieved under classical heating.
The second example involves a nucleophilic aromatic substitution of a fluoro substituent by a piperidine ring in a deactivated benzene scaffold (Scheme 5).25 We carried out this transformation in the polar solvent dimethyl sulfoxide (DMSO) with both classical and microwave heating. According to the experimental kinetic data obtained under microwave irradiation (k*) and by classical heating (k), the k*/k ratio for the reaction between and piperidine at 462 K is 3.2.25c
Scheme 5.
Nucleophilic aromatic substitution of p-halonitrobenzenes (12). Reagents and conditions: a) DMSO; conventional conditions: 190 °C, 2 min; microwave irradiation: 190 °C (300 W), 2 min. Result: acceleration rate=3.2.
The computational study was performed at the B3LYP(PCM)/6-31G*+ΔZPVE level using DMSO as solvent (Table 5). The reaction paths are collected in Scheme 6. In all processes, very polar intermediates are involved (Table 5). The activation energy in all three cases (X=F, Cl, Br) is not very high and, as expected, is lower when the substituent (X) is a fluoro group. It is interesting to note that in this reaction a large increase in the polarity occurs due to the high polarity of both the transition state and the intermediate. On the basis of these outcomes, it can be concluded that in this process, the theory proposed by Loupy is valid: “Specific microwave effects can be expected for the polar mechanism, when the polarity is increased during the reaction on going from the reactants to the transition structures”.8a The stabilization of the transition state is more effective than that of ground state, which results in an enhancement in the reactivity through a decrease in the activation energy. The conclusion in this case is that in this process the acceleration effect is a consequence of the strong absorption of microwave irradiation by the intermediates present in the reaction mixture.
Table 5.
Computational calculated parameters for Type III reaction: nucleophilic aromatic substitution (Scheme 5).
| Solvent | X | μ [D] (most polar species) | Δμ[a] [D] | Ea [kcal mol−1] | ΔH[b] [kcal mol−1] |
|---|---|---|---|---|---|
| DMSO | F | 17.5 (Intermediate) | 13.1 | 16.7 | −10.7 |
| Cl | 14.0 (TS) | 10.3 | 20.8 | −23.5 | |
| Br | 15.6 (TS) | 11.6 | 18.2 | −24.1 |
[a] Δμ=μ TS−μ reactants; [b] ΔH=ΔHproducts−ΔHreactants.
Scheme 6.
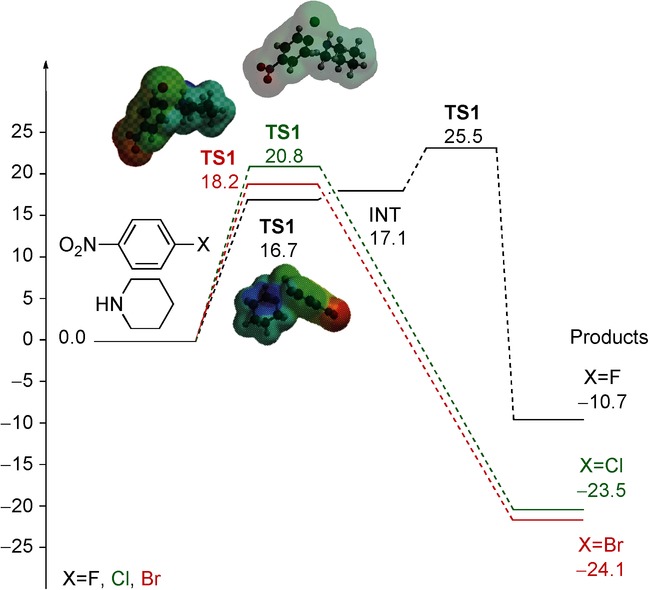
Energy profile for the reaction between 4-halonitrobenzene (12) and piperidine (13).
The third example concerns a ring-closing metathesis (RCM) reaction. Kiddle and co-workers26 reported the RCM of diallyl derivatives using ruthenium-based catalysts (Scheme 7). The reaction can be rapidly conducted in either an ionic liquid, such as 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]), or in a microwave-transparent solvent (MTS) such as dichloromethane. In both cases, the reaction was successfully improved under microwave irradiation. The best results were obtained using ionic liquids, although a dramatic decrease in reaction time was not observed when using dichloromethane as the solvent. It is important to note that the reaction temperature did not exceed 33 °C.
Scheme 7.
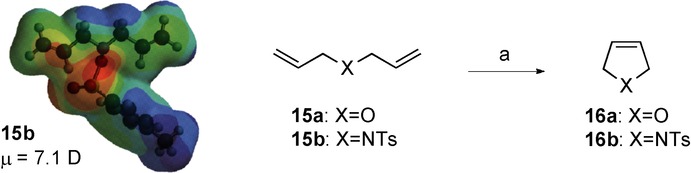
General scheme for ring-closing metathesis of diallyl derivatives. Reagents and conditions: a) Ru catalyst, BMIM; conventional conditions: 33 °C, 2 min, 4 % (16 a), 45 % (16 b); microwave irradiation: 33 °C (110 W), 2 min, 85 % (16 a), 91 % (16 b). Result: acceleration.
The authors suggested that microwave energy produced nonthermal effects that might involve direct coupling to one or both reactants in these transformations. However, they could not ascertain which component was coupled with the microwave energy or if other factors related to the medium were influencing the microwave heating. Careful comparisons by Kappe and co-workers27 indicated that the observed rate enhancements were not the result of a nonthermal microwave effect. They confirmed experimentally that the diene showed significant microwave absorption and that the absorption of the Grubbs catalyst was negligible. The heating profile of the reaction mixture was very close to that of the diene. However, it was argued that the comparatively rapid metathesis transformations could be rationalized solely by taking into account thermal effects (Arrhenius equation).
A density functional theory (DFT) computational mechanistic study of this reaction with diallylether (15 a) or N,N-diallyl-para-toluenesulfonamide (15 b), catalyzed by second-generation Grubbs-type ruthenium carbene complexes, was carried out by our group.16h This study was performed at the B3LYP/6-311+G(2d,p)//B3LYP/SDD theory level (Table 6). Once again, as in the cases outlined above, high activation energy in the precatalytic step was found. In another sense, polarity values of all the species were determinant. These outcomes are completely in agreement with Kappes postulates. N,N-Diallyl-para-toluensulfonamide (15 b) is much more polar (μ=7.1 D) than diallylether (15 a) (μ=1.7 D). However, this trend is not observed for diene 15 b alone, but is consistent for all species present in the reaction path in which the tosyl group is involved.
Table 6.
Computational calculated parameters for a Type III reaction: ring-closing metathesis (Scheme 7).
| Solvent | μ [D] (most polar species) | Δμ[a] [D] | Ea [kcal mol−1] | ΔH[b] [kcal mol−1] |
|---|---|---|---|---|
| CH2Cl2 | 12 to 13 (Intermediates) | 4 to 5 | 19 to 25 | – |
[a] Δμ=μ TS−μ reactants; [b] ΔH=ΔHproducts−ΔHreactants.
Once again, the improvement produced in this reaction is a consequence of the enhanced polarity of the reaction mixture produced by one reagent, which acts as molecular radiator. However, dielectric properties are group properties and cannot be modelled by an interaction between a single dipole and an electric field.28 Considering that the reagents are in a homogeneous reaction mixture, the presence of a polar reagent might increase the polarity of the medium and hence the absorption of microwave radiation. As a consequence, rather than a selective heating by one component (molecular radiator), the enhancement produced in this reaction is a consequence of the enhancement of the dielectric properties of the reaction mixture; again, a clear thermal effect.
In summary, Type III reactions include exothermic reactions with relatively high activation energies. The higher reaction rate and the improvements achieved under microwave irradiation are caused by an effective interaction between the material and the electromagnetic field due to the presence of a polar component that increases the polarity of the medium, i.e., the presence of molecular radiators, doping agents or intermediates.
Type IV reactions
Type IV reactions include endothermic reactions (ΔH>0 kcal mol−1) with high activation energies (Ea=20–30 kcal mol−1). We have included in this section a [2π+2π] cycloaddition and a 1,3-dipolar cycloaddition reaction.
The first reaction was the intramolecular [2π+2π] cycloaddition of alkynyl allenes (17) to afford bicyclomethylenecyclobutenes (18). The reaction occurs in 15 min at 250 °C under microwave heating and gives high yields (74 %) of compounds 18.29 The high temperature necessary for the reaction to occur was achieved by doping the toluene with an ionic liquid (1-ethyl-3-methylimidazolium hexafluorophosphate, [EMIM][PF6]) (Scheme 8). The computed activation energy (Ea=29.7 kcal mol−1) and enthalpy (ΔH=28.8 kcal mol−1) show that this reaction requires very harsh conditions (Table 7). Once again, the presence of an ionic liquid that couples efficiently with microwaves makes it possible to exceed the harsh conditions under which the reaction is carried out.87
Scheme 8.
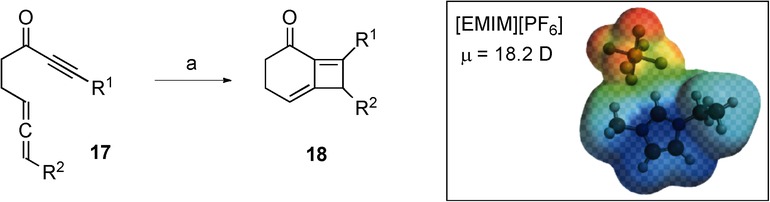
Microwave irradiation of alkynyl allenes leads to an intramolecular [2π+2π] cycloaddition reaction to provide bicyclomethylenecyclobutenes. Reagents and conditions: a) [EMIM][PF6] (3 m), toluene; conventional conditions: no reaction; microwave irradiation: 250 °C, 15 min, 74 %. Result: reaction takes place only under microwave irradiation.
Table 7.
Computational calculated parameters for a Type IV reaction: intramolecular [2π+2π] cycloaddition (Scheme 8).
| Solvent | μ [D] (most polar species) | Δμ[a] [D] | Ea [kcal mol−1] | ΔH[b] [kcal mol−1] |
|---|---|---|---|---|
| Toluene ionic liquid ([EMIM][PF6]) | 18.2 ([EMIM][PF6]) | 0.6 | 29.7 | 28.8 |
[a] Δμ=μ TS−μ reactants; [b] ΔH=ΔHproducts−ΔHreactants.
The second reaction is a 1,3-dipolar cycloaddition between pyridinium dicyanomethylidene and phenylacetylene under microwave irradiation (Scheme 9). De la Hoz and co-workers30 studied several parameters (molar ratio, incident power, temperature and irradiation time) on the yield and selectivity. The extent of the cycloaddition depends on the temperature necessary for the reaction to proceed while preventing decomposition of pyridinium N-dicyanomethylide. In this way, the best results were obtained on using an ylide/dipolarophile molar ratio of 1:1.5 under solvent-free conditions or using bentonite as a mineral support and catalyst. Only traces of cycloadducts were detected on using conventional heating and yields could not be improved even on extending the reaction time to several days.
Scheme 9.
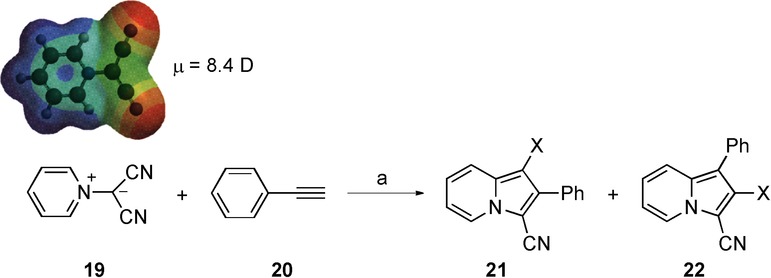
1,3-Dipolar cycloaddition of pyridinium dicyanomethylidene and phenylacetylene. Reagents and conditions: a) conventional conditions: 150 °C, 180 min, trace amounts of product; microwave irradiation: 180 °C (120 W), 180 min, 50 %. Result: reaction takes place only under microwave irradiation.
The computational study performed at the B3LYP/6-31+G* level indicated that this process is highly endothermic (ΔH=20.3–22.4 kcal mol−1), although the activation energy is only moderate (Ea=20.5–22.6 kcal mol−1) (Table 8). The polarity values obtained indicate that pyridinium dicyanomethylidene 19 is a polar species (μ=8.4 D) (Scheme 9). It should be noted that this reagent is used in excess. This fact, together with the presence of bentonite and the use of solvent-free conditions, allows an efficient absorption of microwave energy by the substrates.
Table 8.
Computational calculated parameters for a Type IV reaction: 1,3-dipolar cycloaddition (Scheme 9).
| Solvent | μ [D] (most polar species) | Δμ[a] [D] | Ea [kcal mol−1] | ΔH[b] [kcal mol−1] |
|---|---|---|---|---|
| Solvent-free bentonite bath | 8.4 (Reactant 19) | −3 | 20.5 to 22.6 | 20.3 to 22.4 |
[a] Δμ=μ TS−μ reactants; [b] ΔH=ΔHproducts−ΔHreactants.
Once again, in Type IV reactions, the presence of a polar reaction medium or polar reagents allows the reaction to be improved under a polarizing field such as microwaves.
Type V reactions
The Type V reaction group includes exothermic reactions (ΔH<0 kcal mol−1) with activation energies (Ea) above 30 kcal mol−1. The selected reaction is a Diels–Alder cycloaddition between azoniaanthracene (24) and 1,1-bis-2-thienylethene (23), which leads to 6,11-ethanobenzo-[b]quinolizinium bromide (25). This process was performed in a domestic microwave oven in trifluoroethanol (TFE) or 10 % acetic acid in TFE as the solvent and was highly accelerated under microwave irradiation (3 min) to give the product in good yields (78 %) (Scheme 10). Under conventional heating, the reaction takes place in two hours.31
Scheme 10.
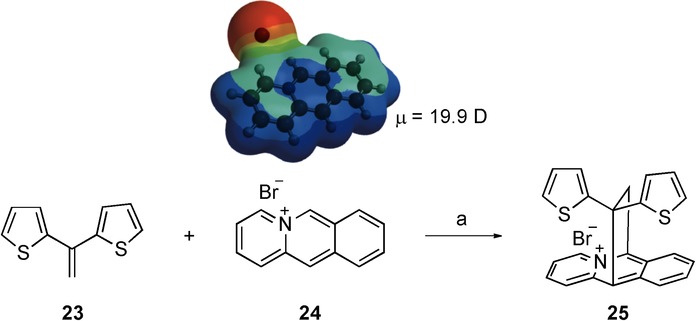
Diels–Alder cycloaddition between azoniaanthracene and bis-substituted olefin leading to 6,11-ethanobenzo-[b]quinolizinium. Reagents and conditions: a) H2O/HOAc (10 %), trifluoroethanol (TFE); conventional conditions: 100 °C, 120 min, 76 %; microwave irradiation: 100 °C (500 W), 3 min, 78 %. Result: acceleration.
The computational study performed at the B3LYP/6-31+G* level indicated that this process is exothermic, but its activation energy is as high as 41.8 kcal mol−1 (Table 9). These harsh conditions can be overcome due to the high polarity of azoniaanthracene (μ=19.9 D), which is similar to that of an ionic liquid.
Table 9.
Computational calculated parameters for a Type V reaction: Diels–Alder cycloaddition (Scheme 10).
| Solvent | μ [D] (most polar species) | Δμ[a] [D] | Ea [kcal mol−1] | ΔH[b] [kcal mol−1] |
|---|---|---|---|---|
| H2O or trifluoroethanol | 19.9 (Reactant 24) | −7.1 | 41.8 | −4.5 |
[a] Δμ=μ TS−μ reactants; [b] ΔH=ΔHproducts−ΔHreactants.
Once again, the presence of a very polar reagent leads to an increase in the absorption of microwaves; a clear thermal effect.
Type VI reactions
Finally, Type VI reactions are endothermic processes (ΔH>0 kcal mol−1) with high activation energy (Ea>30 kcal mol−1). Reaction between 4-nitroimidazole (27) and cyclohexadiene (26) does not occur under microwave irradiation,32 although nitroimidazole (27) is polar (μ=8.6 D) (Scheme 1). In contrast, the reaction between 4-nitropyrazole and cyclohexadiene gives a high yield (80 %), although the polarity and nucleophilicity of nitropyrazole are lower (μ=5.5 D), and this represents a significant improvement in relation to conventional heating (5 %).
Computational calculations at the B3LYP(PCM)/6-31 G* level showed different aspects in the mechanism for both reactions. In both cases, the mechanisms involve protonation of cyclohexadiene and subsequent nucleophilic attack. The difference is that while the addition of pyrazole (Mechanism 1, Scheme 2) involves synchronous protonation and addition of pyrazole, the addition of imidazole is asynchronous (Mechanism 2, Scheme 2).
Scheme 11.
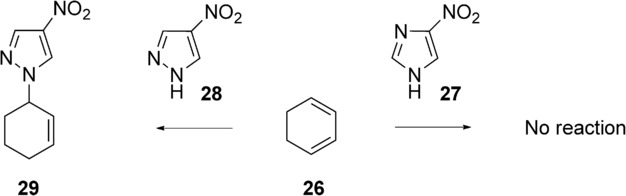
Reaction of 4-nitroimidazole (27) and 4-nitropyrazole (29) with cyclohexadiene (26).
Scheme 12.
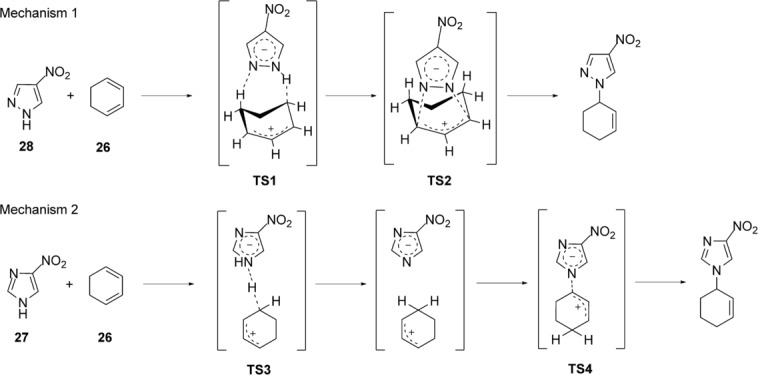
Computed mechanisms for the reactions of 4-nitropyrazole (28) and imidazole (27) with cyclohexadiene (26).
The calculated values for the activation barriers are 29.5 kcal mol−1 for mechanism 1 and 35.3 kcal mol−1 for mechanism 2 (Table 10). As expected for the proposed mechanism, there is an increase in the polarity from the starting material to the TS (TS1: μ=15.0 D; TS2: μ=10.4 D; TS3: μ=15.2 D; and TS4: μ=10.3 D). However, regardless of the polarity of the TS, only the reaction of 4-nitropyrazole 29 actually takes place.
Table 10.
| Solvent | μ [D] (most polar species) | Δμ[a] [D] | Ea [kcal mol−1] | ΔH[b] [kcal mol−1] |
|---|---|---|---|---|
| Benzene | 15.0 (TS1) 15.2 (TS3) | 5.5 | 29.5 (mechanism 1) 35.3 (mechanism 2) | 5.6 |
[a] Δμ=μ TS−μ reactants; [b] ΔH=ΔHproducts−ΔHreactants.
These results are very representative, and they are consistent with a thermal effect and with our calculations, showing that under microwave heating reactions with high activation energies (Ea=29.5 kcal mol−1; Type IV reactions) can be successfully performed. However, when the activation energies are above 30 kcal mol−1, that is, Type VI reactions, the processes do not occur.
Computational methods
All of the geometry optimizations and energy calculations included here were performed using the GAUSSIAN 0333 and 0934 program suites. In order to perform the calculations at a homogenous computational level that provides quantitative accuracy at a reasonable computational cost, all calculations described were performed by DFT35 using a Beckes hybrid three-parameter functional (B3LYP)36 and the 6-31+G* basis set.37 This level of theory has proven to yield accurate activation parameters and geometries.38 Only for the metathesis reaction, in order to take ruthenium into account, were studies performed at the B3LYP/6-311+G(2d,p)//B3LYP/SDD39 theory level. All stationary points were characterized by harmonic analysis. All relative energies reported here include the zero-point vibrational energy (ZPVE) corrections.
To take into account the possible solvent effect of polar media for some reactions, calculations were carried out with the polarized-continuum model (PCM)40 using the appropriate solvent.
Conclusion
Computational calculations have been carried out on selected reactions in order to determine energy and physical parameters required to understand and predict the origin of improvements and selectivities observed in organic reactions performed under microwave irradiation. For this purpose, the influence of enthalpy (ΔH) and polarity (μ) in the reactions was studied. In order to obtain relevant information, a complete study of all reaction paths was performed. The main conclusions of this study are summarized in Table 11.
Table 11.
Main conclusions from the study.
| Reaction | Ea [kcal mol−1] | ΔH[a] [kcal mol−1] | Conclusion |
|---|---|---|---|
| Type I | <20 | <0 | Not improved |
| Type II | >0 | Improved | |
| Type III | 20–30 | <0 | Improved |
| Type IV | >0 | Improved | |
| Type V | >30 | <0 | Improved with susceptors |
| Type VI | >0 | Do not occur |
[a] ΔH=ΔHproducts−ΔHreactants.
Exothermic reactions with low activation energies (Type I) proceed well under conventional heating, and improvements should not be expected under microwave irradiation. However, the corresponding endothermic reactions (Type II) can be improved slightly under microwave irradiation under the conditions described above.
Endothermic and exothermic reactions with moderate or high activation energies (Types III and IV) can be improved under microwave irradiation in polar media (reagents, solvent, catalyst).
Reactions with very high activation energies can be performed under microwave irradiation when two requirements are fulfilled: the reaction is exothermic (Type V) and a polar medium is present. Endothermic reactions (Type VI) do not take place under either conventional heating or microwave irradiation.
In agreement with our previous studies, the outcomes obtained here provide evidence that slower reacting systems tend to show better effects under microwave irradiation. So, radiation is selectively absorbed by polar systems, a characteristic that leads to selective heating profiles. The presence of a polar solvent, reagent or support in the reaction media leads to strong coupling with the radiation. This fact is particularly important in heterogeneous systems where it could also generate microscopic hot spots or selective heating.
The presence of small amounts of a strongly microwave- absorbing ‘doping agent’ or ‘susceptor’, such as an ionic liquid, leads to very efficient interactions with microwaves through the ionic conduction mechanism. It is noteworthy that we also quantified that an activation energy of 20–30 kcal mol−1 and a polarity of the species involved in the process between 7 and 20 D is required to obtain improvements under microwave irradiation.
The results discussed in this paper highlight some features that can be used to predict, with simple calculations of activation energy, enthalpy and polarity, when a reaction can be improved under microwave irradiation. These characteristics could prove to be very useful for synthetic chemists.
Acknowledgments
Financial support from the Dirección General de Ciencia y Tecnología (DGCT) del Ministerio de Educación, Ciencia y Tecnología (España) through projects CTQ2011-22410/BQU and CTQ2011-28124-C02-01, from the Consejería de Educación y Ciencia de la Junta de Comunidades de Castilla-la Mancha (JCCM), España through project PII2I09-0100, and technical support from the High-Performance Computing Service of the University of Castilla–La Mancha (Spain) is gratefully acknowledged. A.M.R. gratefully acknowledges the Spanish Ministerio de Educación y Ciencias (MEC) for an FPU fellowship.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
References
- 1a.Gedye R, Smith F, Westaway K, Ali H, Baldisera L, Laberge L, Rousell R. Tetrahedron Lett. 1986;27:279–282. [Google Scholar]
- 1b.Giguere RJ, Bray TL, Duncan SM, Majetich G. Tetrahedron Lett. 1986;27:4945–4948. [Google Scholar]
- 2. Organic and medicinal references:
- 2a.de La Hoz A, Loupy A, editors. Microwaves in Organic Synthesis. 3. Weinheim: Wiley-VCH; 2012. rd . [Google Scholar]
- 2b.Hayes BL. Microwave Synthesis: Chemistry at the Speed of Light. Matthews (NC): CEM Publishing; 2002. [Google Scholar]
- 2c.Lidstöm P, Tierney JP, editors. Microwave-Assisted Organic Synthesis. Oxford: Blackwell Scientific; 2005. [Google Scholar]
- 2d.Kappe CO, Dallinger D, Murphree SS. Practical Microwave Synthesis for Organic Chemists–Strategies, Instruments, and Protocols. Weinheim: Wiley-VCH; 2009. [Google Scholar]
- 2e.Kappe CO. Angew. Chem. Int. Ed. 2004;43:6250–6284. doi: 10.1002/anie.200400655. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2004;116 [Google Scholar]
- 2f.Kappe CO. Chem. Soc. Rev. 2008;37:1127–1139. doi: 10.1039/b803001b. [DOI] [PubMed] [Google Scholar]
- 2g.Alcazar J, Diels G, Schoentjes B. Mini-Rev. Med. Chem. 2007;7:345–369. doi: 10.2174/138955707780363846. [DOI] [PubMed] [Google Scholar]
- 2h.Kappe CO, Stadlerand A, Dallinger D. Microwaves in Organic and Medicinal Chemistry. 2. Weinheim: Wiley-VCH; 2012. [Google Scholar]
- 3. Biosciences references:
- 3a.Lill JR. Microwave Assisted Proteomics. Cambridge: RSC Publishing; 2009. [Google Scholar]
- 3b.Pedersen SL, Tofteng AP, Malik L, Jensen KJ. Chem. Soc. Rev. 2012;41:1826–1844. doi: 10.1039/c1cs15214a. [DOI] [PubMed] [Google Scholar]
- 3c.Collins JM, Leadbeater NE. Org. Biomol. Chem. 2007;5:1141–1150. doi: 10.1039/b617084f. [DOI] [PubMed] [Google Scholar]
- 4. Materials science references:
- 4a.Bogdal D, Prociak A. Microwave-Enhanced Polymer Chemistry and Technology. Oxford: Wiley-Blackwell; 2007. [Google Scholar]
- 4b.Rao KJ, Vaidhyanathan B, Ganguli M, Ranmakrishnan PA. Chem. Mater. 1999;11:882–895. [Google Scholar]
- 4c.Horikoshi S, Serpone N, editors. Microwaves in Nanoparticle Synthesis: Fundamentals and Applications. Weinheim: Wiley-VCH; 2013. [Google Scholar]
- 5. Analytical and environmental science references:
- 5a.Jin Q, Liang F, Zang H, Zhao L, Huan Y, Song D. TrAC Trends Anal. Chem. 1999;18:479–484. [Google Scholar]
- 5b.Leadbeater NE, editor. Microwave Heating as a Tool for Sustainable Chemistry. Boca Raton: CRC Press; 2010. [Google Scholar]
- 5c.Varma RS. Green Chem. Lett. Rev. 2007;1:37–45. [Google Scholar]
- 5d.Strauss CR, Rooney DW. Green Chem. 2010;12:1340–1344. [Google Scholar]
- 6.Mingos DMP, Baghurst DR. Chem. Soc. Rev. 1991;20:1–47. [Google Scholar]
- 7.Díaz-Ortiz A, de La Hoz A, Carrillo JR, Herrero MA. In: Selectivity Modifications Under Microwave Irradiation in Microwaves in Organic Synthesis, Vol. 1. 3. de La Hoz A, Loupy A, editors. Weinheim: Wiley-VCH; 2012. pp. 209–244. rd Chapter 5. [Google Scholar]
- 8a.Perreux L, Loupy A. Tetrahedron. 2001;57:9199–9223. [Google Scholar]
- 8b.de La Hoz A, Díaz-Ortiz A, Moreno A. Chem. Soc. Rev. 2005;34:164–178. doi: 10.1039/b411438h. [DOI] [PubMed] [Google Scholar]
- 9a.Herrero MA, Kremsner JM, Kappe CO. J. Org. Chem. 2008;73:36–47. doi: 10.1021/jo7022697. [DOI] [PubMed] [Google Scholar]
- 9b.Kappe CO, Pieber B, Dallinger D. Angew. Chem. Int. Ed. 2013;52:1088–1094. doi: 10.1002/anie.201204103. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 125 [Google Scholar]
- 10a.Baghurst DR, Mingos DMP. J. Chem. Soc. Chem. Commun. 1992:674–677. [Google Scholar]
- 10b.Chemat F, Esveld E. Chem. Eng. Technol. 2001;24:735–744. [Google Scholar]
- 11a.Tsai Y-C, Coles BA, Compton RG, Marken F. J. Am. Chem. Soc. 2002;124:9784–9788. doi: 10.1021/ja026037w. [DOI] [PubMed] [Google Scholar]
- 11b.Horikoshi S, Osawa A, Abe M, Serpone N. J. Phys. Chem. C. 2011;115:23030–23035. [Google Scholar]
- 12a.Bogdal D, Lukasiewicz M, Pielichowsli J, Miciak A, Bednarz S. Tetrahedron. 2003;59:649–653. [Google Scholar]
- 12b.Lukasiewicz M, Bogdal D, Pielichowsli J. Adv. Synth. Catal. 2003;345:1269–1272. [Google Scholar]
- 12c.Will H, Scolz P, Ondruschka B. Chem. Ing. Tech. 2002;74:1057–1067. [Google Scholar]
- 12d.Raner KD, Strauss CR, Trainor RW, Thorn JS. J. Org. Chem. 1995;60:2456–2460. [Google Scholar]
- 13.Schanche J-S. Mol. Diversity. 2003;7:291–298. doi: 10.1023/b:modi.0000006866.38392.f7. [DOI] [PubMed] [Google Scholar]
- 14a.Dudley GB, Stiegman AE, Rosana MR. Angew. Chem. Int. Ed. 2013;52:7918–7923. doi: 10.1002/anie.201301539. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013;125 [Google Scholar]
- 14b.Kappe CO. Angew. Chem. Int. Ed. 2013;52:7924–7928. doi: 10.1002/anie.201304368. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013;125 [Google Scholar]
- 15a.Obermayer D, Gutmann B, Kappe CO. Angew. Chem. Int. Ed. 2009;48:8321–8324. doi: 10.1002/anie.200904185. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009;121 [Google Scholar]
- 15b.Robinson J, Kingman S, Irvine D, Licence P, Smith A, Dimitrakis G, Obermayer D, Kappe CO. Phys. Chem. Chem. Phys. 2010;12:4750–4758. doi: 10.1039/b922797k. [DOI] [PubMed] [Google Scholar]
- 15c.Moseley JD, Kappe CO. Green Chem. 2011;13:794–806. [Google Scholar]
- 15d.Kappe CO. Chem. Soc. Rev. 2013;42:4977–4990. doi: 10.1039/c3cs00010a. [DOI] [PubMed] [Google Scholar]
- 16a.Fraile JM, García JI, Gómez MA, de La Hoz A, Mayoral JA, Moreno A, Prieto P, Salvatella L, Vázquez E. Eur. J. Org. Chem. 2001:2891–2899. [Google Scholar]
- 16b.Díaz-Ortiz A, Carrillo JR, Cossío FP, Gómez-Escalonilla MJ, de La Hoz A, Moreno A, Prieto P. Tetrahedron. 2000;56:1569–1577. [Google Scholar]
- 16c.Arrieta A, Otaegui D, Zubia A, Cossío FP, Díaz-Ortiz A, de La Hoz A, Herrero MA, Prieto P, Foces-Foces C, Pizarro JL, Arriortua MI. J. Org. Chem. 2007;72:4313–4322. doi: 10.1021/jo062672z. [DOI] [PubMed] [Google Scholar]
- 16d.de La Hoz A, Prieto P, Rajzmann M, de Cózar A, Díaz-Ortiz A, Moreno A, Cossío FP. Tetrahedron. 2008;64:8169–8176. [Google Scholar]
- 16e.de Cózar A, Millán MC, Cebrián C, Prieto P, Díaz-Ortiz A, de La Hoz A, Cossío FP. Org. Biomol. Chem. 2010;8:1000–1009. doi: 10.1039/b922730j. [DOI] [PubMed] [Google Scholar]
- 16f.Rodriguez AM, Prieto P, Díaz-Ortiz A, de La Hoz A. Org. Biomol. Chem. 2011;9:2371–2377. doi: 10.1039/c0ob01037e. [DOI] [PubMed] [Google Scholar]
- 16g.Rodriguez AM, Cebrián C, Prieto P, García JI, de La Hoz A, Díaz-Ortiz A. Chem. Eur. J. 2012;18:6217–6224. doi: 10.1002/chem.201103560. [DOI] [PubMed] [Google Scholar]
- 16h.Rodríguez AM, Prieto P, de La Hoz A, Díaz-Ortiz A, García JI. Org. Biomol. Chem. 2014;12:2436–2445. doi: 10.1039/c3ob42536c. [DOI] [PubMed] [Google Scholar]
- 17.Berlan J, Giboreau P, Lefeuvre S, Marchand C. Tetrahedron Lett. 1991;32:2363–2366. [Google Scholar]
- 18.Leadbeater NE, Torrenius HM. J. Org. Chem. 2002;67:3145–3148. doi: 10.1021/jo016297g. [DOI] [PubMed] [Google Scholar]
- 19.Besson T, Kappe CO. In: Microwaves in Organic Synthesis, Vol. 1. 3. de La Hoz A, Loupy A, editors. Weinheim: Wiley-VCH; 2012. pp. 301–350. rd Chapter 7, [Google Scholar]
- 20.Louërat F, Bougrin K, Loupy A, Ochoa de Retama AM, Pagalday J, Palacios F. Heterocycles. 1998;48:161–170. [Google Scholar]
- 21a.Kaiser N-FK, Bremberg U, Larhed M, Moberg C, Hallberg A. Angew. Chem. Int. Ed. 2000;39:3595–3598. doi: 10.1002/1521-3773(20001016)39:20<3595::aid-anie3595>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2000;112 [Google Scholar]
- 21b.Steinreiber A, Stadler A, Mayer SF, Faber K, Kappe CO. Tetrahedron Lett. 2001;42:6283–6286. [Google Scholar]
- 22.Díaz-Ortiz A, Diez-Barra E, de La Hoz A, Moreno A, Gómez-Escalonilla MJ, Loupy A. Heterocycles. 1996;43:1021–1030. [Google Scholar]
- 23a.Díaz-Ortiz A, de la Hoz A, Alcázar J, Carrillo JR, Herrero MA, Fontana A, de Mata Muñoz J. Comb. Chem. High Throughput Screening. 2007;10:163–169. doi: 10.2174/138620707780126679. [DOI] [PubMed] [Google Scholar]
- 23b.Díaz-Ortiz A, de La Hoz A, Alcázar J, Carrillo JR, Herrero MA, Fontana A, de Mata Muñoz J, Prieto P, de Cózar A. Comb. Chem. High Throughput Screening. 2011;14:109–116. doi: 10.2174/138620711794474088. [DOI] [PubMed] [Google Scholar]
- 24a.Motorina IA, Fowler FW, Grierson DS. J. Org. Chem. 1997;62:2098–2105. doi: 10.1021/jo9614046. [DOI] [PubMed] [Google Scholar]
- 24b.De Borggraeve WM, Rombouts FJR, Verbist BMP, Van der Eycken EV, Hoornaert GJ. Tetrahedron Lett. 2002;43:447–449. [Google Scholar]
- 25a.Cossío F, Díaz-Ortiz A. unpublished results.
- 25b.Salmoria GV, Dall′Oglio E, Zucco C. Tetrahedron Lett. 1998;39:2471–2474. [Google Scholar]
- 25c. A mixture of 4-fluoronitrobenzene (0.7 g, 5 mmol) and piperidine (0.425 g, 5 mmol) in DMSO (20 mL) was irradiated at 300 W (190 °C) for 2 min in a Discover microwave reactor (Method A) or heated in a thermostatized oil bath at 190 °C for 2 min (Method B). In both cases, the reaction mixture reached boiling. The reaction mixture was immediately poured into water (200 mL) to obtain 4-piperidinonitrobenzene as a yellow–orange solid. The precipitate was isolated by filtration, washed, dried and analyzed by 1kkH NMR to verify its purity. Each experiment was carried out five times to obtain the average */ ratio reported.
- 26.Mayo KG, Nearhoof EH, Kiddle JJ. Org. Lett. 2002;4:1567–1570. doi: 10.1021/ol025789s. [DOI] [PubMed] [Google Scholar]
- 27.Garbacia S, Desai B, Lavastre O, Kappe CO. J. Org. Chem. 2003;68:9136–9139. doi: 10.1021/jo035135c. [DOI] [PubMed] [Google Scholar]
- 28.Stuerga D. In: Microwaves in Organic Synthesis, Vol. 1. 3. de la Hoz A, Loupy A, editors. Weinheim: Wiley-VCH; 2012. pp. 3–56. rd Chapter 1, [Google Scholar]
- 29a.Brummond KM, Chen D. Org. Lett. 2005;7:3473–3475. doi: 10.1021/ol051115g. [DOI] [PubMed] [Google Scholar]
- 29b.Shen Q, Hammond GB. J. Am. Chem. Soc. 2002;124:6534–6535. doi: 10.1021/ja0263893. [DOI] [PubMed] [Google Scholar]
- 30.Díaz-Ortiz A, Díez-Barra E, de la Hoz A, Loupy A, Petit A, Sánchez L. Heterocycles. 1994;38:785–792. [Google Scholar]
- 31.Sasaki S, Ishibashi N, Kuwamura T, Sano H, Matoba M, Nisikawa T, Maeda M. Bioorg. Med. Chem. Lett. 1998;8:2983–2986. doi: 10.1016/S0960-894X(98)00541-1. [DOI] [PubMed] [Google Scholar]
- 32.Gómez MV, Aranda AI, Moreno A, Cossío FP, de Cózar A, Díaz-Ortiz Á, de la Hoz A, Prieto P. Tetrahedron. 2009;65:5328–5336. [Google Scholar]
- 33.Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Montgomery J A Jr, Vreven T, Kudin K N, Burant J C, Millam J M, Iyengar S S, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson G A, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J E, Hratchian H P, Cross J B, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Ayala P Y, Morokuma K, Voth G A, Salvador P, Dannenberg J J, Zakrzewski V G, Dapprich S, Daniels A D, Strain M C, Farkas O, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Ortiz J V, Gui Q, Baboul A G, Clifford S, Gioslowski J, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin R L, Fox D J, Keith T, Al-Laham M A, Peng C Y, Nanayakkara A, Challacombe PM, Gill M W, Johnson B, Chen W, Wong M W, Gonzalez C, Pople J A. Gaussian 03, Revision C.1 Gaussian, Inc., Pittsburgh PA (USA) 2003.
- 34.Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Scalmani G, Barone V, Mennucci B, Petersson G A, Nakatsuji H, Caricato M, Li X, Hratchian H P, Izmaylov A F, Bloino J, Zheng G, Sonnenberg J L, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T Jr. , Montgomery J A, Peralta J E, Ogliaro F, Bearpark M, Heyd J J, Brothers E, Kudin K N, Staroverov V N, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant J C, Iyengar S S, Tomasi S J, Cossi M, Rega N, Millam N J, Klene M, Knox J E, Cross J B, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev OO, Austin A J, Cammi R, Pomelli C, Ochterski J W, Martin R L, Morokuma K, Zakrzewski V G, Voth G A, Salvador P, Dannenberg J J, Dapprich S, Daniels A D, Farkas O, Foresman J B, Ortiz J V, Cioslowski J, Fox D J. Gaussian 09, Revision B.1 Gaussian, Inc., Wallingford CT (USA) 2009.
- 35.Parr RG, Yang W. Density-Functional Theory of Atoms and Molecules. New York: Oxford University Press; 1994. [Google Scholar]
- 36a.Kohn W, Becke AD, Parr RGJ. Phys. Chem. 1996;100:12974–12980. [Google Scholar]
- 36b.Becke AD. J. Chem. Phys. 1993;98:5648–5652. [Google Scholar]
- 36c.Becke AD. Phys. Rev. A. 1988;38:3098–3100. doi: 10.1103/physreva.38.3098. [DOI] [PubMed] [Google Scholar]
- 37a.Ditchfield R, Hehre WJ, Pople JA. J. Chem. Phys. 1971;54:724–728. [Google Scholar]
- 37b.Hehre WJ, Ditchfield R, Pople JA. J. Chem. Phys. 1972;56:2257–2261. [Google Scholar]
- 37c.Hariharan PC, Pople JA. Theor. Chim. Acta. 1973;28:213–222. [Google Scholar]
- 37d.Hariharan PC, Pople JA. Mol. Phys. 1974;27:209–215. [Google Scholar]
- 37e.Gordon MS. Chem. Phys. Lett. 1980;76:163–168. [Google Scholar]
- 38a.Goldstein E, Beno B, Houk KN. J. Am. Chem. Soc. 1996;118:6036–6043. [Google Scholar]
- 38b.Wiest O, Montiel DC, Houk KN. J. Phys. Chem. A. 1997;101:8378–8388. [Google Scholar]
- 38c.García JI, Martinez-Merino V, Mayoral JA, Salvatellla L. J. Am. Chem. Soc. 1998;120:2415–2420. doi: 10.1021/ja003695c. [DOI] [PubMed] [Google Scholar]
- 38d.Paddon-Row MN, Moran D, Jones GA, Sherburn MS. J. Org. Chem. 2005;70:10841–10853. doi: 10.1021/jo051973q. [DOI] [PubMed] [Google Scholar]
- 39a.Schwerdtfeger P, Dolg M, Schwarz WH, Bowmaker GA, Boyd PDW. J. Chem. Phys. 1989;91:1762–1774. [Google Scholar]
- 39b.Andrae D, Haubermann U, Dolg M, Stoll H, Preuβ H. Theor. Chim. Acta. 1990;77:123–141. [Google Scholar]
- 39c.Bergner A, Dolg M, Küchle W, Stoll H, Preuβ H. Mol. Phys. 1993;80:1431–1441. [Google Scholar]
- 40a.Miertus S, Scrocco E, Tomasi J. Chem. Phys. 1981;55:117–129. [Google Scholar]
- 40b.Mennucci B, Tomasi J. J. Chem. Phys. 1997;106:5151–5158. [Google Scholar]
- 40c.Cammi R, Mennucci B, Tomasi J. J. Phys. Chem. A. 2000;104:5631–5637. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary


