Abstract
NETosis is a newly recognized mechanism of programmed neutrophil death. It is characterized by a stepwise progression of chromatin decondensation, membrane rupture, and release of bactericidal DNA-based structures called neutrophil extracellular traps (NETs). Conventional ‘suicidal’ NETosis has been described in pathogenic models of systemic autoimmune disorders. Recent in vivo studies suggest that a process of ‘vital’ NETosis also exists, in which chromatin is condensed and membrane integrity is preserved. Techniques to assess ‘suicidal’ or ‘vital’ NET formation in a specific, quantitative, rapid and semiautomated way have been lacking, hindering the characterization of this process. Here we have developed a new method to simultaneously assess both ‘suicidal’ and ‘vital’ NETosis, using high-speed multi-spectral imaging coupled to morphometric image analysis, to quantify spontaneous NET formation observed ex-vivo or stimulus-induced NET formation triggered in vitro. Use of imaging flow cytometry allows automated, quantitative and rapid analysis of subcellular morphology and texture, and introduces the potential for further investigation using NETosis as a biomarker in pre-clinical and clinical studies.
INTRODUCTION
The year 2014 marked the 10th anniversary of the initial description of neutrophil extracellular traps (NETs), a meshwork of chromatin fibers decorated with antimicrobial proteins ejected into the extracellular space to kill or immobilize microbes1. Since then, significant interest has emerged with regards to the role of NET formation as a key mechanism in host defense against microbes. In addition, the putative role of NETs in the induction of autoimmune responses, thrombosis, endothelial cell death and tissue damage is the focus of investigation by many research groups2, 3.
Since its original description, it has become apparent that NET formation is a heterogeneous process. The initial description of NET formation, designated as NETosis, was described as a ‘suicidal’ process distinct from apoptosis and necrosis4. A hallmark of early stage ‘suicidal’ NETosis is the nuclear translocation of the azurophilic granule proteins neutrophil elastase (NE) and myeloperoxidase (MPO), followed by histone degradation leading to chromatin decondensation5, 6. As such, the measurement of decondensed nuclei has been used as one of the hallmarks to quantify neutrophils that are undergoing NET formation. Later events in ‘suicidal NETosis’ include the development of cell lysis and cell membrane rupture, indicating that NETs emerge from dying neutrophils. The classical stimulus that induced ‘suicidal’ NETosis is phorbol myristate acetate (PMA) after a 4-hour incubation period4, a process that depends on production of cellular oxidants.
In addition to this cell death process, an additional mechanism of NET formation was recently described in which the extrusion of chromatin can also occur through an oxidant-independent mechanism termed ‘vital’ NETosis, whereby NETs are released, leaving behind functional anuclear cells7. Initial descriptions of ‘vital’ NETosis in vivo showed the cell nucleus changing from polymorphonuclear to spherical, with NETs then emerging in a localized area of the neutrophil surface through vesicular release. In vivo studies revealed that condensed DNA passed through the cytoplasm without lysing membranes8. Indeed, anuclear neutrophils are a common finding in human abscesses due to bacterial infection7. As such, ‘vital NETosis’ is described as ‘NETing during patrolling’, as these neutrophils undergoing NET formation simultaneously crawl in vivo, as quantified using intravital microscopy 7–9. This phenomenon is difficult to quantify in vitro, as the 2-D nature of slides or coverslips likely impair the identification of these “crawling” NETing neutrophils.
Methods to detect NETosis have been based on classical microscopy analysis requiring that cells are attached to a slide or coverslip. Identification is typically based on the classical appearance of “beads-on-a-string” captured with conventional fluorescence microscopy. While this technique reveals the endpoint of nuclear extrusion and can assess extracellular coexpression of nuclear material with granular proteins through immunolabeling, it does not easily lend itself to objective quantification and introduces possible sampling bias (see Supplemental Figure 1). Due to the relatively low throughput and subjective nature of conventional microscopy, the results obtained from different laboratories are difficult to compare and the results are not significantly quantitative. Furthermore, the process can be laborious and time-consuming. More automated assays currently used (such as the Sytox green plate assay) lack specificity, as externalization of DNA cannot be equated to NETosis. Also, as mentioned above, the resulting alteration of cellular morphology likely affects nuclear motility causing the failure to easily visualize ‘vital’ NETosis in vitro. While some on these limitations may be overcome by a recent protocol using dual channel fluorescence staining and image segmentation, there are still concerns regarding discrimination between enlarged nuclei from NETosis and mitotic or apoptotic events 10, 11. Therefore, a high-throughput, objective, and quantitative approach to imaging cells undergoing NETosis is required to further study this novel neutrophil process.
Multispectral Imaging Flow Cytometry (MIFC) combines features of fluorescence microscopy and flow cytometry by providing unbiased acquisition and analysis of large number of images from cells in suspension. CCD camera technology and time-delayed integration (TDI) allows high-speed, high resolution image capture in multiple wavelengths simultaneously12. MIFC has been widely used in cell studies including nuclear-cytoplasmic translocation13, quantification of apoptosis based on the changes in nuclear morphology14 and assessment of autophagy15. A morphology-based method for measuring NETosis has not yet been reported using this novel technology.
Here we present a novel and rapid image-based method that employs MIFC to study and quantify ‘suicidal’ and ‘vital’ NETosis by using transmitted light (Brightfield), side-scatter (SSC) and multiple fluorescence images of cellular components.
RESULTS
‘Suicidal’ NETosis is defined and quantified with imaging flow cytometry
We established a streamlined method for high-speed imaging in a fluid stream, followed by semi-automated image analysis to discover features characteristic of neutrophils undergoing ‘suicidal’ and ‘vital’ NETosis. We studied neutrophils obtained from peripheral blood from healthy controls, as well as distinct neutrophil subsets isolated from patients with systemic lupus erythematosus (SLE), an autoimmune disease characterized by an enhanced percentage of granulocytes undergoing NET formation. Overall, the morphological features observed in healthy control and lupus neutrophils were similar and representative images from lupus patients are used in the figures.
We began by examining the size and staining intensity and texture of neutrophil nuclei before and 4 hours after treatment with PMA. While untreated cells displayed normal multi-lobular nuclei, PMA treatment led to a subset of neutrophils displaying enlarged nuclei with less pronounced lobulation and diffuse staining intensity (Fig 1a). The average area of nuclei increased from 40 μm2 for the unstimulated cells to 125 μm2. This so-called ‘nuclear decondensation’ was quantified using the Bright Detail Intensity (BDI) feature, identifies areas of peak fluorescence intensity after subtraction of background fluorescence. The feature used measured bright intensity spots with a radius of 7 pixels or fewer, known as BDI R7, within the IDEAS software. Decondensed nuclei are identified by low BDI and high area, while normal nuclei showed high or variable BDI and low area (Fig 1b). The total Intensity of the Hoechst nuclear stain was similar between normal and decondensed nuclei, however the latter showed slightly lower SSC Intensity (Figure 1b), perhaps due to diffusion of granular proteins throughout the decondensed nuclei. Similar morphological changes were observed in a small percentage of neutrophils exposed in vitro to LPS for 1 hour (Fig. 2). To address whether MPO may be relocating to DNA, as has been described for ‘suicidal’ NETosis, a feature called Similarity Score was then calculated to quantify the degree of nuclear translocation of MPO, using the Hoechst nuclear stain to identify the nuclear region (Fig. 2b). The median Similarity Score for the unstimulated cells was found to be around −0.12, indicating an anti-correlation between MPO and Hoechst images, and therefore the absence of nuclear localization. In contrast, the median Similarity Score for the gated sub-population with decondensed nuclei was +1.0, indicating a positive correlation between the MPO and Hoechst images, and therefore increased co-localization of MPO and Hoechst had occurred. As an additional stimulus, we treated neutrophils with the calcium ionophore A23187, an antibiotic that induces NET formation and increases cytoplasmic Ca2+ level. As shown in Supplemental Figure 2, this stimulus primarily induced features of ‘suicidal’ NETosis.
Figure 1. Quantification of suicidal NETosis using flow-based imaging and image analysis.

a) Quantitative analysis of nuclear decondensation. Left panels show representative images from untreated (Un) or PMA-treated (PMA) neutrophils. Eight individual cells are shown by multispectral imaging of Brightfield (BF), side-scatter (SSC), and DNA staining (Hchst). Representative nuclei (yellow boxes) are detailed in the right panels where the nuclear mask (green) used for calculation of Nuclear Area (upper image) and Bright Detail Intensity (BDI, lower image) are shown for Untreated and PMA-treated samples. Values for Nuclear Area and BDI for the individual represe ntative images are shown inset in yellow text. b) A region containing cells with high nuclear area and low BDI is shown in red (left dotplots) for Untreated (upper panels) or PMA-treated (lower panels) samples. Arrows indicate the data points of the single representative cells shown in a). Cells in the gated region (red dots) are shown backgated onto dotplots of SSC and Hoechst Intensity (right dotplots). Scale bars are 7μm2
Figure 2. Myeloperoxidase (MPO) colocalization with DNA in decondensed nuclei following LPS stimulation.
a) Eight representative cells with normal (upper panels) or decondensed (lower panels) nuclei from untreated (Un) or LPS-treated (LPS) neutrophils are shown, with BF, MPO, Hoechst (Nuc), and an overlay of Hoechst and MPO (merge) images indicated. Scale bars are 7μm. b) Quantification of the Nuclear Area and BDI is shown in the dotplots for Untreated (top) and LPS-treated (bottom) samples. Nuclei with normal Nuclear Area and BDI are gated in blue, and those with high Nuclear Area and low BDI are gated in red. Right histogram overlay shows negative correlation of MPO and DNA localization for normal nuclei (blue trace) but positive correlation of MPO and DNA localization in decondensed nuclei (red trace) by log-transformed Pearson’s correlation coefficient (Similarity).
Overall, this new method defines ‘suicidal’ NETotic cells with features of large nuclear area and low BDI, as well as colocalization of MPO with the DNA.
A novel morphology is identified as ‘vital NETosis’ in vitro
In addition to the expected nuclear decondensation described above, we also observed a striking phenotype in neutrophils treated in vitro with LPS for 1 hour. Rather than a normal round cell shape, approximately 50% of the neutrophils were elongated, with large membrane blebs visible at one pole, and cell contents, including nucleus and granules (by SSC) at the other (Fig 3a). Notably, the chromatin remained condensed and multi-lobular, and displayed nuclear area and BDI similar to unstimulated neutrophils (Fig 3d and 2c). In order to quantify the observed morphological change, we used the Fisher’s Discriminate Ratio (RD) optimization tool within the IDEAS software (so-called ‘Feature Finder’). This tool allows comparison of a battery of default and user-defined image analysis algorithms to determine which of those best differentiates two input populations. In our case, we hand-selected images of cells displaying the normal expected neutrophil morphology (Truth Set 1, see Supplemental Fig. 3), and those of cells displaying the elongated morphology including the membrane blebs (Truth Set 2, see Supplemental Figure 4). The greatest RD value, 1.49, was observed for Aspect Ratio of the Brightfield image, which is calculated as the minor axis/major axis, as illustrated in Figure 3b and Supplemental Figure 5. Because the elongated phenotype displayed one cell pole with a high degree of pixel intensity and contrast, and the opposite pole with a very low intensity, low contrast membrane bleb, a feature was calculated to compare the Centroid XY position for the Brightfield Image versus the Intensity-weighted Brightfield Image. This feature, termed ‘Delta Centroid XY BF, BF Intensity’, will be referred to henceforth simply as Delta Centroid XY (see Fig. 3b and Supplemental Fig 6). The RD value for Delta Centroid was also high at 1.43, therefore the two features were used in a bivariate plot of Truth Populations 1 and 2 to reveal clear separation of these two populations (Fig. 3b). The same features were then used to plot at least 20,000 cells of untreated and LPS-treated samples, and we observed that while only ~8% of cells from the untreated group fell into the ‘elongated’ gate, ~50% of cells from the LPS-treated group had this phenotype. Notably, the Nuclear Area and Hoechst BDI values of the elongated cells were similar to those of normal neutrophil nuclei (Fig. 3d), implying that these cells are not undergoing apoptosis.
Figure 3. Observation and quantification of morphology change following LPS stimulation.

a) Six representative BF images of Untreated and LPS-treated samples are shown. b) Hand-selected images of cells with normal, round appearance (magenta dots) and those of elongated cells with membrane protrusions (blue dots) are plotted using the image analysis features that best distinguish these morphologies, determined by Fisher’s Discriminate Ratio (RD); BF Aspect Ratio and Delta Centroid XY BF, BF Intensity. Images to the right of the dotplots illustrate how these measurements are made. c) Eight representative images of cells in BF, SSC, Hoechst (Nuc) and an overlay of those images (merge) are displayed for the normal, round morphology of Untreated cells and for the elongated morphology observed following LPS-treated cells. Feature values for Aspect Ratio and Delta Centroid XY are displayed in blue text on the BF and merge images, respectively. d) Dotplot showing the appearance of cells with low Aspect Ratio and high Delta Centroid XY (red dots) after LPS treatment. Cells with elongated morphology (region outlined in cyan) are shown backgated onto the dotplot of Nuclear Area versus Hoechst Bright Detail Intensity (cyan spots), and have those features in common with normal neutrophil nuclei (see Figure 1b).
MPO did not appear to colocalize with DNA in the cells displaying the elongated phenotype and the mean Similarity Score comparing MPO and DNA pixel overlap in elongated cells was −0.5, indicating the absence of translocation of MPO to the nucleus compared to +1.5 in ‘suicidal’ NETosis (Fig. 4a). Intriguingly, cell-free chromatin was visualized and identified with low SSC and high intensity of Hoechst (Supplemental Fig. 7). Vital NETosis has been proposed to be a ROS independent-process. Furthermore, preincubation with Diphenyleneiodonium (DPI), an inhibitor of ROS production, did not significantly change the % of cells displaying features of vital NETosis after 1 hour of LPS stimulation (43 % for DPI vs. 45% for vehicle), consistent with previous observations that ‘vital” NETosis is an oxidant-independent process7. Taken together, our observations suggest that the elongated cells are likely undergoing a process that extrudes nuclei, leaving anuclear cells behind with intact membrane, which underlies the definition of vital NETosis.
Figure 4. Myeloperoxidase (MPO) does not colocalize with DNA in elongated cells after LPS treatment.

Nine representative individual elongated cells are shown to demonstrate anti-correlation between DNA and MPO (Hchst/MPO) in this phenotype. Cell surface and internalized sialic acid and N-acetylglucosamine are labeled with Wheat Germ Agglutinin (WGA) in order to visualize the plasma membrane. Anti-correlation between DNA and MPO (Similarity MPO, Hoechst) in elongated cells (blue trace), is shown relative to the positive correlation between DNA and MPO among decondensed nuclei (red trace), and the anti-correlation between those in normal nuclei (green trace). b) Three individual cells are shown exhibiting only WGA staining and very little BF contrast, Hoechst, and MPO positivity. These cells also displayed very low or no SSC. Scale bars are 7μm.
In contrast to treatment with PMA or LPS, treatment with staurosporine induced predominant apoptosis in neutrophils, exemplified by classic nuclear condensation and fragmentation, and increased refractive index (measured by Brightfield contrast) (Supplemental Figure 8). No features of ‘suicidal’ or ‘vital’ NETosis were observed after a 6-hour staurosporine treatment. Similar results were observed when we used another apoptosis inducer (camptothecin, supplemental figure 8). To further confirm that the cells identified as undergoing vital NETosis did not represent apoptotic cells, neutrophils were pretreated with the antiapoptotic pan-caspase inhibitor (z-VAD-FMK) or vehicle, prior to stimulation with LPS for an additional hour. As shown in Figure 5, the percentage of cells displaying ‘suicidal’ of ‘vital’ NETosis characteristics after LPS stimulation did not change in the presence of a pan-caspase inhibitor, supporting that the elongated cell morphology did not represent apoptotic cells. Overall, the nuclear shape and brightfield image are significantly different in elongated cells compared with staurosporine-induced apoptotic cells, supporting that vital NETosis can be distinguished from apoptosis using this approach.
Figure 5. Features of ‘vital” NETosis are not inhibited by pan-caspase inhibitors.
Neutrophils were pre-treated with the pan-caspase inhibitor Z-VAD-FMK (20 mM) for 30 minutes, followed by stimulation with LPS for an additional hour. Results represent mean % of cells undergoing ‘vital’ or ‘suicidal’ NETosis in the presence of LPS+/− DMSO or vehicle.
Lupus neutrophil subsets are prone to NETosis
Previous studies in neutrophils from SLE patients had characterized two distinct subsets; one, obtained from the red blood cell pellet after density gradient methods and called here normal-density neutrophils, displayed phenotype and function more similar to neutrophils isolated from healthy controls. The second subset, isolated from PBMC factions using Ficoll-density gradient, was named low-density granulocyte (LDG) and is characterized by prominent enhancement in the capacity to form NETs ex vivo in the absence of added stimulation. We proceeded to study whether LDGs and normal-density lupus neutrophils differed in the display of ‘vital” and/or ‘suicidal” NETosis features when compared to control neutrophils.
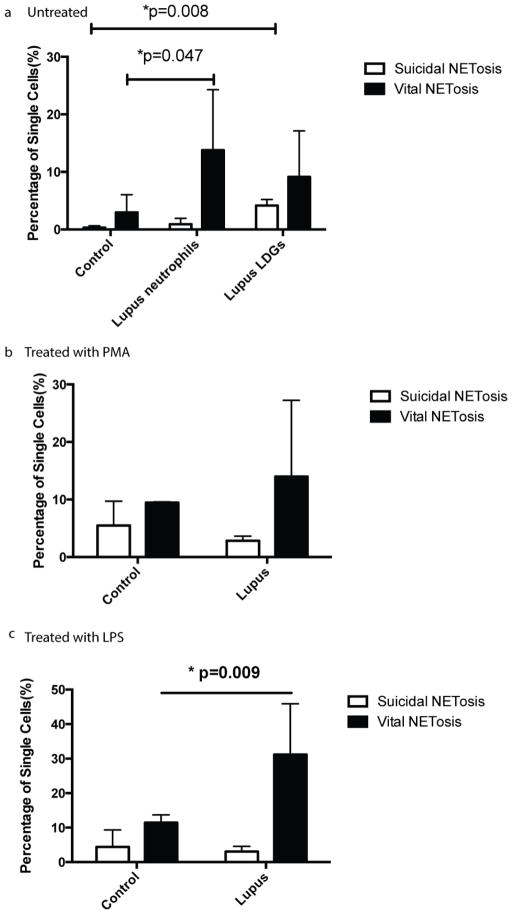
Circulating normal-density neutrophils in lupus patients demonstrated enhanced vital NETosis (~13%) ex vivo, in the absence of exogenous stimulus, as compared with healthy control unstimulated neutrophils (~3%)(Fig. 6). The lupus LDG subset displayed features of ‘suicidal’ NETosis in a significantly higher percentage of cells (~4%) in the absence of added stimulation, when compared to healthy control neutrophils (~0.3%). As previously shown by other group, these percentages did not change when additional stimulation was added to these cells (PMA or LPS), indicating that these cells have achieved maximum induction in vivo (for ‘suicidal’ NETosis 4± 0.5% for no stimulus, 6±0.8% for PMA and 5±0.5% for LPS; for ‘vital’ NETosis, 18±2% for no stimulus, 21±7% for PMA and 20±10% for LPS; results represent mean±SD of 2 independent experiments). This supports previous observations that have quantified NET formation by extrusion of nuclear material mixed with granular proteins by conventional microscopy, in which LDGs were found to be primed in vivo to release NETs and therefore do not require further in vitro stimulation to show enhanced capacity to form these structures 16, 17. In the case of normal-density lupus neutrophils incubated with LPS for 1h, features of ‘vital’ NETosis were present in 30% of these cells, when compared to 11% of healthy control neutrophils exposed to the same stimulus (Fig. 6). In contrast, no significant differences were observed between normal-density lupus and control neutrophils treated with PMA in rates of ‘suicidal’ NETosis. Overall, these results indicate that MIFC can be used as a rapid method to compare rates of NETosis between neutrophils isolated from patients with various disease states.
Figure 6. Comparisons between induction of ‘vital’ versus ‘suicidal’ NETosis features in control and lupus neutrophil subsets.
Untreated neutrophils from healthy control, normal and low density neutrophils from lupus patients (a,n=6), treated with PMA for 4 hours (b,n=3) or LPS for 1 hour (c,n=5) were stained with Hoechst 33342. Suicidal and Vital NETosis rate was quantified using the method described above.
DISCUSSION
Different mechanisms of cell death are often defined by characteristic changes in nuclear morphology. We now show that analysis of nuclear imagery features including size, texture and relative subcellular location, provided by the IDEAS software, allows objective and quantitative definition of apoptosis, ‘suicidal’ and ‘vital’ NETosis (Table I). Two features were found to identify apoptotic nuclei: decreased nuclear area and increased Bright Detail Intensity (intensity of localized bright spots (peaks of intensity) of a given pixel radius). In contrast, decondensed nuclei, a prerequisite for ‘suicidal NETosis’, are characterized by increased nuclear area and decreased Bright Detail Intensity. The hallmark of ‘vital’ NETotic neutrophils is multi-lobular/condensed nuclei and features suggesting mobility. Both nuclear polarization and membrane blebbing may contribute to cell mobility. Here we found that Delta Centroid XY (the difference between the Centroid XY of the Brightfield image and the Intensity-weighted Centroid XY of the Brightfield image) correlates with polarization of nuclei and membrane blebs. Therefore, Imaging flow cytometry allows the detection of both qualitative and quantitative differences in cellular functions between 2D and 3D environments. It may also be ideally suited to perform high throughput screening to test drugs and compounds that affect ‘vital’ or ‘suicidal’ NETosis.
Table 1.
Comparison of features of normal, apoptotic, suicidal and vital NETotic neutrophils using MIFC
| Normal | Suicidal NETosis | Vital NETosis | Apoptosis | |
|---|---|---|---|---|
| Bright Field | Spherical, | Spherical | Elongated with a polarized bleb | Spherical with multiple small blebs, high refractive index |
| Nuclear morphology | Condensed, lobulated | Decondensed, nonlobulated | Condensed, Lobulated, polarized within the cell | Condensed, fragmented DNA |
| MPO colocalization with DNA | No | Yes | No | No |
Our observations also further support heterogeneous morphology associated to NET-inducing stimuli. Non-apoptotic nuclear blebs, which lack apoptotic markers and nuclear fragmentation, have recently been found to play a central role of in cell migration18. Our findings are in line with those studies, as apoptotic markers were absent in the elongated cells we observed during ‘vital’ NETosis, while nuclear fragmentation, the most important hallmark of apoptosis, was not observed at the time points studied (data not shown). Furthermore, we could not inhibit ‘vital’ NETosis features using a pan-caspase inhibitor, indicating that this process is independent of caspases and therefore unlikely to represent apoptosis.
Intriguingly, Yipp et. al have reported that ‘vital’ NETosis occurs in vivo during “crawling” in response to Gram-positive bacteria. The polarized blebbing of vital NETotic neutrophils with elongated nuclei we visualized in vitro may resemble the in vivo process of neutrophils simultaneously “chasing” microbes while NETing, so-called mobile ‘vital’ NETosis19, 20. Certain conditions, including 3D environments, may be crucial for the formation of blebs, which likely explains why this specific process had not been systematically reported in cells attached to a slide or coverslip. Therefore, imaging within a fluid stream may serve as a powerful approach to study motility-related cellular functions, due to its capacity to capture images of cells in suspension, in which blebs appear to be maintained and quantifiable. Using apoptotic stimuli (staurosporine), neutrophils with condensed/fragmented nuclei in the center of cells showed both lower area and high Bright Detail Intensity. Furthermore, small blebs were visualized on the surface of neutrophils representing classical apoptotic morphology. In contrast, calcium ionophore preferentially induced suicidal NETosis. This could be explained by a flood of Ca2+ that could block actin remodeling, which is required for bleb formation in “mobile” ‘vital’ NETosis 21,22
One limitation of this technique is that it images the cells currently undergoing NETosis, and may miss those that have already lysed. While extracellular DNA is still detected by this method, it likely underestimates late phases of NETosis. This likely explains while on a regular slide visualized by conventional microscopy, the percentage of NETotic cells appears to be higher than those quantified by Imagestream. This technique may also miss a few events that are undergoing ‘vital’ NETosis, if the axis of ‘elongation’ is in the same plane as the cell relative to the camera, the event would look round instead of elongated. This probably only accounts for a very small percentage, as laminar flow causes most elongated events to orient vertically in the fluid stream.
In summary, we describe a novel image-based method that employs MIFC to study features of ‘suicidal’ and ‘vital’ NETosis in neutrophils from healthy controls exposed to distinct in vitro stimuli, as well as in neutrophils subsets isolated from patients with autoimmune diseases. Furthermore, this technique allowed for robust distinction between nuclear changes observed during NET formation and those observed during apoptosis.
METHODS
Neutrophil isolation
Peripheral blood was obtained from healthy controls and from SLE patients who fulfilled the American College of Rheumatology SLE classification criteria23. Healthy controls and SLE patients were recruited through a protocol approved by NIH Institutional Review Board. Neutrophils were isolated from freshly drawn blood as described previously24. Briefly, whole blood was spun on a Ficoll-density gradient (GE Healthcare) and neutrophils were isolated from the red-blood cell layer via dextran-sedimentation using dextran sedimentation. Lupus low-density granulocytes (LDGs) were purified as described by our group25.
Induction and observation of NETosis in liquid 3D culture
A total of 1 × 106 neutrophils in 1ml RPMI 1640 without phenol red with 1μg/mL of LPS were cultured in 1.5ml Eppendorf tubes with cap open for 1h or with PMA (20 nM) for 4 hours at 37°C with 5% CO2. Cells were treated with Calcium Ionophore A23187 (25 μM, EMD Chemical) for 1 hour to induce NETosis. Staurosporine (1 μM, Sigma-Aldrich) or Camptothecin (6μM, BD Biosciences) were used to induce apoptosis. Cell surface and internalized sialic acid and N-acetylglucosamine were labeled with Wheat Germ Agglutinin (WGA, Abcam) in order to visualize the plasma membrane. For some conditions, cells were preincubated with the pan-caspase inhibitor Z-VAD-FMK (20 mM Promega), with the ROS inhibitor DPI (Sigma) or vehicle (DMSO) before stimulation with LPS. Cells were centrifuged at 500 rcf for 5 min and re-suspended in 100ul of 2%PFA containing 1:1000 diluted Hoechst. For MPO staining, after blocking with 0.2% porcine skin gelatin (Sigma) in PBS for 30 min, cells were incubated with polyclonal rabbit anti-human myeloperoxidase (MPO, Dako) diluted in blocking buffer for 1h at 37°C, washed with PBS, and dye-conjugated secondary antibody solutions was added for 30 min (Alexa-555-conjugated donkey anti-rabbit IgG, Life Technologies). Images were acquired on the ImageStream Multispectral Imaging Flow Cytometer (Amnis, part of EMD Millipore, Billerica, MA), using the 60X magnification objective which provides a numerical aperture (NA) of 0.9, and a pixel dimension of 0.3 m × 0.3 m. A core diameter of 7 μm was uesd in order to miximize in-focus events. The 375nm excitation laser was used at an output power of 1 to 5 mW depending on the intensity of staining. Objects having a minimum cross-sectional area of 50 m2 and a maximum of 600 m2 were collected in order to avoid acquiring debris or cellular aggregates. Typical files contained imagery for 20,000 cells. Cell imagery was analyzed using the requisite software IDEAS, version 6.1. Cells in best focus were selected using the feature Brightfield (BF) Gradient RMS, a measurement of image contrast that excludes out-of-focus events. Doublets, aggregates, and dead cells and debris were excluded by using SSC Intensity and Hoechst Intensity, and all analyses were restricted to single cells 13.
Statistics
Statistical analysis was performed using Student’s t test, and data were analyzed using GraphPad Prism software version 6. Results are presented as the mean ± SEM.
Supplementary Material
Highlights.
A novel image-based quantitative method for the characterization of NETosis is described.
Features to distinguish vital from suicidal NETosis are investigated
Distinctive features between apoptosis and NETosis are described.
Acknowledgments
This study was supported by the Intramural Research Program at NIAMS/NIH. We thank Jason S. Knight for helpful discussions. Darin K. Fogg is employed by EMD Millipore Corporation.
Footnotes
The other authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science (New York, NY ) 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 2.Knight JS, et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circulation research. 2014;114:947–956. doi: 10.1161/CIRCRESAHA.114.303312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knight JS, et al. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Annals of the rheumatic diseases. 2014 doi: 10.1136/annrheumdis-2014-205365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs TA, et al. Novel cell death program leads to neutrophil extracellular traps. The Journal of cell biology. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. The Journal of cell biology. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metzler KD, Goosmann C, Lubojemska A, Zychlinsky A, Papayannopoulos V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell reports. 2014;8:883–896. doi: 10.1016/j.celrep.2014.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yipp BG, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nature medicine. 2012;18:1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pilsczek FH, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. Journal of immunology (Baltimore, Md : 1950) 2010;185:7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 9.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nature reviews. Immunology. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 10.Brinkmann V, Goosmann C, Kuhn LI, Zychlinsky A. Automatic quantification of in vitro NET formation. Frontiers in immunology. 2012;3:413. doi: 10.3389/fimmu.2012.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knight JS, et al. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. The Journal of clinical investigation. 2013;123:2981–2993. doi: 10.1172/JCI67390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basiji DA, Ortyn WE, Liang L, Venkatachalam V, Morrissey P. Cellular image analysis and imaging by flow cytometry. Clinics in laboratory medicine. 2007;27:653–670. viii. doi: 10.1016/j.cll.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George TC, et al. Quantitative measurement of nuclear translocation events using similarity analysis of multispectral cellular images obtained in flow. Journal of immunological methods. 2006;311:117–129. doi: 10.1016/j.jim.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Henery S, et al. Quantitative image based apoptotic index measurement using multispectral imaging flow cytometry: a comparison with standard photometric methods. Apoptosis: an international journal on programmed cell death. 2008;13:1054–1063. doi: 10.1007/s10495-008-0227-4. [DOI] [PubMed] [Google Scholar]
- 15.de la Calle C, Joubert PE, Law HK, Hasan M, Albert ML. Simultaneous assessment of autophagy and apoptosis using multispectral imaging cytometry. Autophagy. 2011;7:1045–1051. doi: 10.4161/auto.7.9.16252. [DOI] [PubMed] [Google Scholar]
- 16.Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? The Journal of cell biology. 2012;198:773–783. doi: 10.1083/jcb.201203170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knight JS, Carmona-Rivera C, Kaplan MJ. Proteins derived from neutrophil extracellular traps may serve as self-antigens and mediate organ damage in autoimmune diseases. Frontiers in immunology. 2012;3:380. doi: 10.3389/fimmu.2012.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paluch EK, Raz E. The role and regulation of blebs in cell migration. Current opinion in cell biology. 2013;25:582–590. doi: 10.1016/j.ceb.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobelli J, et al. Confinement-optimized three-dimensional T cell amoeboid motility is modulated via myosin IIA-regulated adhesions. Nature immunology. 2010;11:953–961. doi: 10.1038/ni.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auffray C, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science (New York, NY ) 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 21.Crespin M, Vidal C, Picard F, Lacombe C, Fontenay M. Activation of PAK1/2 during the shedding of platelet microvesicles. Blood coagulation & fibrinolysis: an international journal in haemostasis and thrombosis. 2009;20:63–70. doi: 10.1097/MBC.0b013e32831bc310. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann K, Sperling K, Olins AL, Olins DE. The granulocyte nucleus and lamin B receptor: avoiding the ovoid. Chromosoma. 2007;116:227–235. doi: 10.1007/s00412-007-0094-8. [DOI] [PubMed] [Google Scholar]
- 23.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and rheumatism. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 24.Clark RA, WM Nauseef Isolation and functional analysis of neutrophils. Current Protocols in Immunology. 2005;(Unit 7):23. doi: 10.1002/0471142735.im0723s19. [DOI] [PubMed] [Google Scholar]
- 25.Denny MF, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. Journal of immunology (Baltimore, Md : 1950) 2010;184:3284–3297. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.