Abstract
Rationale
Oxidative stress is an important contributing factor in a number of human pathologies ranging from atherosclerosis to cancer progression; however, the mechanisms underlying tissue protection from oxidation products are poorly understood. Oxidation of membrane phospholipids, containing the polyunsaturated fatty acid DHA, results in the accumulation of an end product, 2-(ω-carboxyethyl)pyrrole (CEP), which was shown to have pro-angiogenic and pro-inflammatory functions. While CEP is continuously accumulated during chronic processes such as tumor progression and atherosclerosis, its levels during wound healing return to normal when the wound is healed, suggesting the existence of a specific clearance mechanism.
Objective
To identify the cellular and molecular mechanism for CEP clearance.
Methods and Results
Here we show that macrophages are able to bind, scavenge, and metabolize carboxyethylpyrrole derivatives of proteins but not structurally similar ethylpyrrole derivatives, demonstrating the high specificity of the process. F4/80hi and M2-skewed macrophages are much more efficient at CEP binding and scavenging compared to F4/80lo and M1-skewed macrophages. Depletion of macrophages leads to increased CEP accumulation in vivo. CEP binding and clearance are dependent on two receptors expressed by macrophages, CD36 and TLR2. While knockout of each individual receptor results in diminished CEP clearance, the lack of both receptors almost completely abrogates macrophages’ ability to scavenge CEP derivatives of proteins.
Conclusions
Our study demonstrates the mechanisms of recognition, scavenging, and clearance of pathophysiologically active products of lipid oxidation in vivo, thereby contributing to tissue protection against products of oxidative stress.
Keywords: Oxidized lipids, CEP, macrophage, wound healing, angiogenesis, CD36, TLR2
INTRODUCTION
Oxidative stress is linked to the pathogenesis of age-related and chronic diseases, including atherosclerosis, cancer, etc.1–3 While well-controlled oxidative response has a protective role during acute inflammation and subsequent tissue repair, excessive oxidation exerts detrimental effects on tissues, thereby contributing to a number of pathologies.4 Reactive oxygen species (ROS) generated during inflammation modify a wide spectrum of targets resulting in accumulation of oxidized metabolic products.2 Among substrates highly prone to oxidation are polyunsaturated fatty acids (PUFA). Therefore, the formation and accumulation of lipid peroxidation (LPO) products is an inevitable consequence of oxidative stress in vivo.5–7 During the considerable lifespan of humans, repeated oxidative stress combined with a virtually limitless source of substrates, exemplified by PUFAs of plasma membranes, might ultimately result in accumulation of high levels of potentially harmful metabolic byproducts.7, 8 The imbalance between the production and removal of harmful substances leads to impaired homeostasis.9
One PUFA, docosahexaenoate (DHA), gives rise to products of oxidative fragmentation that contain (E)-4-hydroxy-7-oxohept-5-enoic acid (HOHA), which, in turn, reacts with proteins or ethanolamine phospholipids, thereby generating 2-(ω-carboxyethyl)pyrrole (CEP) containing protein or lipid derivatives, see detail in 10. Due to abundance of DHA, high levels of CEP in vivo can be regarded as a sign of substantial oxidative stress.10–13 Indeed, CEP-containing products were found in a number of inflammation- and age-related human pathologies.10, 14–16 CEP- modified proteins10, 13, 17, 18 and phospholipids,15 were shown to be elevated in patients with age-related macular degeneration (AMD) using both immunoassays19, 20 and LC-MS/MS.15 CEP levels are significantly elevated within the vessel wall of aging animals and in tumors with substantial inflammation.14 Recent studies identified a number of CEP activities, including pro-angiogenic14, 15 and pro-inflammatory21, 22 functions. Stimulation of angiogenesis was shown to be mediated by TLR2 on endothelial cells14, 15 independently of vascular endothelial growth factor (VEGF) signaling.14, 23 CEP was reported to stimulate macrophages to express a number of pro-inflammatory cytokines.21, 22, 24 CEP adducts also promote platelet activation, granule secretion, and aggregation in vitro and thrombosis in vivo.25 Collectively, CEP accumulation might contribute to a number of pathologies. Therefore, it is important to establish whether or not the highly active products of LPO can be further metabolized and cleared from the tissues.
Indeed, CEP level appears to be finely regulated in the physiological context, as it is dramatically increased at the early stage of wound healing, but returns to normal when the wound has healed.14 It appears that after revascularization, CEP has been either degraded or metabolized. Although many macromolecules have fast rates of turnover, this mechanism does not seem to be applicable to CEP, where the pyrrole moiety is stabilized by aromaticity. Alternatively, scavenger cells, such as macrophages, might be involved in this process.9, 20, 26,27 Macrophages are highly heterogeneous, but can be generally placed into two major subtypes, pro-inflammatory M1 and anti-inflammatory M2; the latter are more efficient in scavenging activity, due to higher expression of scavenger and mannose receptors.26, 27 Peritoneal macrophages are often divided into two populations, F4/80hi and F4/80lo macrophages.28, 29 F4/80lo macrophages promote immune response through antigen presentation, whereas F4/80hi macrophages are specialized for scavenging mainly through phagocytosis.28
In this study we show that CEP, but not structurally similar compounds, is specifically recognized and scavenged by macrophages; CD36, and, to a lesser extent, TLR2, are directly involved in this process. Deficiency in receptor-mediated CEP clearance causes CEP accumulation and increased angiogenesis in wounds.
METHODS
Expanded Methods are presented in the Online Data Supplement.
RESULTS
Differential accumulation patterns for CEP modifications in vivo
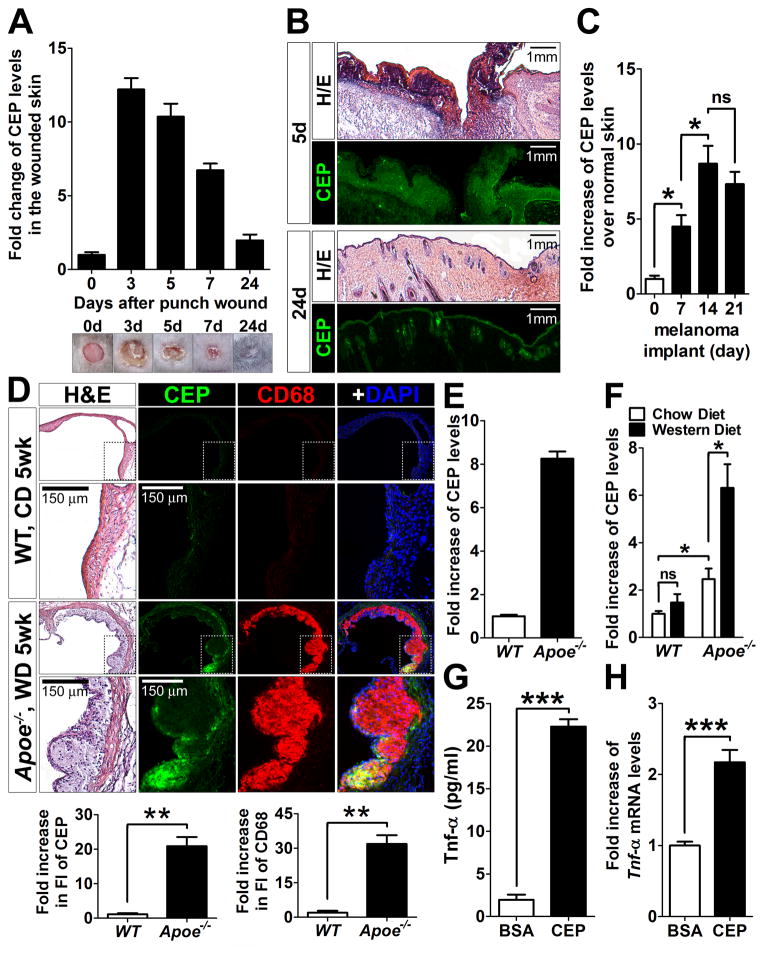
We first analyzed the pattern of CEP accumulation in wounds on the back of 8-week-old mice for a month. While CEP was barely detectable in normal skin, its levels were dramatically increased in 3-day-old wounds, following a bell shaped curve at later time points and returning to the normal levels at day 24 post-injury, when wounds were almost completely healed (Figure 1A). Histological analyses revealed that CEP was highly localized in the scabs, granulation tissues, and hypertrophic epidermal wound edges at day 5, whereas at day 24, it was only present in the epidermis and hair follicles (Figure 1B). The transient nature of CEP presence in wounds and its rapid downregulation after completion of angiogenesis and the healing process indicate an existence of a specific mechanism of CEP clearance.
Figure 1. Regulation of CEP levels in vivo.
A. The presence of CEP-containing modifications in vivo during wound healing (n=5, top). The wounded skins were collected at the indicated times, homogenized, and then each supernatant was used for ELISA. CEP concentration was normalized by total protein concentration. Bottom: Representative images of wounds on the mouse back skins at the times indicated (n=5) are shown. B. Hematoxylin-eosin and CEP immunofluorescence staining of the wounded skins at day 5 (5d) and 24 (24d). Note that CEP is stained on the scabs, granulation tissues, and hypertrophic epidermal wound edges at day 5, whereas CEP is slightly stained in the epidermis and hair follicles at day 24. C. CEP accumulation in implanted B16-F10 murine melanomas was measured by ELISA. Melanomas were collected at day 7, 14, and 21 after subcutaneous injections of B16-F10 murine melanoma cells (7.0 × 105 cells per injection) into mice (n=4). D. Hematoxylin-eosin and fluorescence staining for CEP, CD68 (macrophages) and DAPI (nuclei) of the aorta of Apoe−/− mice (fed with western diet for 5 weeks) and age- and gender-matched WT control mice (fed with chow diet). Note that CEP was positively stained only in atherosclerotic lesions (n=3). Bottom: quantification of CEP and CD68 immunofluorescence of WT and Apoe−/− mice. Fold increase over WT control is shown. E. Plasma levels of CEP from the same Apoe−/− and WT mice (D), measured by ELISA (n=3). Fold increase over WT control is shown. The absolute concentration of CEP in Apoe−/− mice was 70–100× 10−12 M based on CEP standard, see Methods). F. Plasma levels of CEP from Apoe−/− and WT mice, which were fed with a chow or western diet for 16 weeks, were measured by ELISA. Fold increase over WT control on chow diet is shown. G. Upregulation of TNF-α, measured by ELISA, in thioglycolate-elicited mouse peritoneal macrophages upon treatment with 10 μM of CEP-BSA for 4 hours. H. Upregulation of Tnf-α gene, measured by qPCR, in R-MPMs upon treatment with 10 μM of CEP-BSA for 4 hours. Fold increase over BSA-treated cells is shown.
Since wound healing is a physiological process that restores original tissue integrity and functionality, we next assessed CEP accumulation in chronic pathological processes associated with continuous oxidative stress, such as tumor progression and atherosclerotic lesion development.4, 30 CEP levels in melanomas were continuously increasing during tumor progression at 7, 14 and 21 days post implantation, while the tumor volume was increased by more than 60-fold within 2 weeks (Figure 1C and Online Figure I). Likewise, CEP was present at high levels in atherosclerotic lesions of Apoe−/− mice fed with a western diet (WD) as compared to a control, in which there was no positive signal for CEP (Figure 1D). Moreover, the plasma CEP levels in these Apoe−/− mice were increased over 8-fold as compared to controls, demonstrating that CEP presence in vivo was not limited to the atherosclerotic lesions but was a rather systemic response (Figure 1E). Plasma CEP levels in Apoe−/− mice were higher than those of wild-type (WT) mice even without a WD, however, a WD substantially augmented the oxidative processes resulting in at least 2 fold increase of CEP levels in blood of Apoe−/− mice (Figure 1F). After 18 weeks on a WD, CEP levels within the atherosclerotic lesions of Apoe−/− mice remained continuously high (Online Figure II). Thus, the transient nature of CEP presence during wound healing is in contrast to its continuous accumulation in pathologies associated with inflammation and oxidative stress, implying the existence of a specific mechanism responsible for CEP degradation.
Besides the pro-angiogenic effect of CEP (Online Figure III and 14, 23), it has a pro-inflammatory role.21, 22, 24 Indeed, in the presence of CEP, macrophages upregulated a pro-inflammatory cytokine, TNF-α, both at a protein (Figure 1G) as well as at an mRNA level (Figure 1H). Together with previous observations, this suggests that high levels of CEP might augment chronic pathological processes and emphasizes an importance of the timely removal of this end-product of LPO.
CEP binding to macrophages
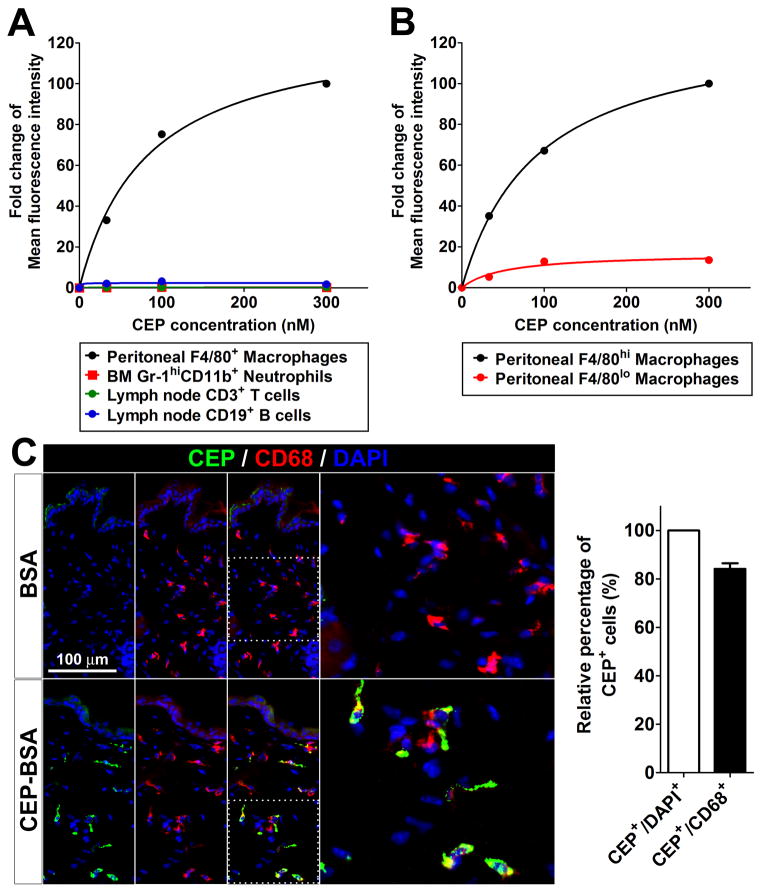
To identify the cell type responsible for CEP clearance, we first tested the ability of various immune cells, such as R-MPMs, bone marrow neutrophils, and lymph node T and B cells to bind to CEP directly. Based on FACS analysis, macrophages bound to CEP-BSA in a concentration-dependent manner, achieving saturation at 200 nM (Figure 2A). Then, we observed two distinct populations of macrophages according F4/80 expression: F4/80lo and F4/80hi, and measured CEP binding by FACS. F4/80hi macrophages bound to CEP in a concentration-dependent manner and the maximum binding of CEP-BSA to this cell population was at least 10 times higher than to F4/80lo macrophages based on fluorescence intensity (Figure 2B). F4/80lo macrophages are a minor subset of R-MPMs, whereas over 80% of total R-MPMs are F4/80hi (Online Figure IVA). MHC-II expression is high on F4/80lo macrophages, which are known to serve as antigen-presenting cells in vitro, but very low on F4/80hi macrophages (Online Figure IVB). In contrast, scavenger receptors and pattern recognition receptors, such as CD36 and TLR2, respectively, are highly expressed on F4/80hi macrophages (Online Figure IVB), which are known to be involved in phagocytosis of apoptotic cells in vivo.28 The concentration-dependent manner of CEP-BSA binding with a clear saturation together with differential interaction with distinct cell populations suggests the involvement of specific cell surface receptors. To rule out the possibility that the binding might be mediated by BSA but not by the CEP moiety, FITC-conjugated BSA (FITC-BSA) was used as a control. FACS analysis showed that there were dose-dependently positive signals when CEP-BSA, but not FITC-BSA, was exposed to macrophages (Online Figure V).
Figure 2. CEP binding and scavenging by macrophages.
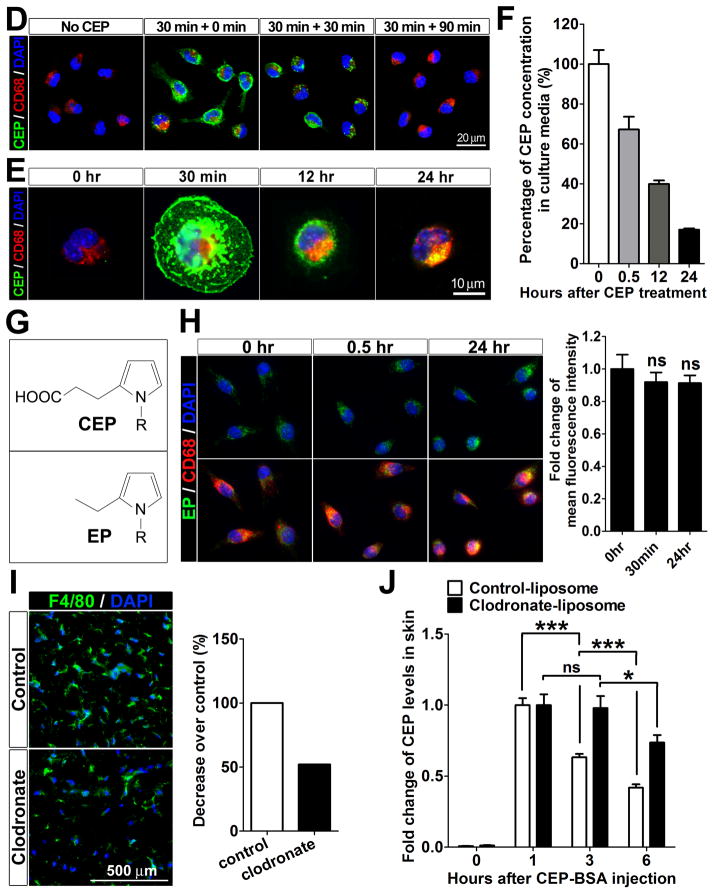
A and B. Flow cytometry analysis of CEP binding on primary blood cells, such as macrophages, neutrophils, T and B cells (A), and F4/80hi and F4/80lo macrophages (B), which were incubated on ice for 30 min with CEP-BSA at the concentrations indicated, after freshly isolated from mouse bone marrow (for neutrophils), lymph nodes (for T and B cells), and peritoneum (for macrophages and B cells). Mean fluorescence intensity was normalized to that of 300 nM CEP treatment, which was assigned a value of 100%. C. Immunofluorescence staining for CEP, CD68 and DAPI on the frozen sections of mouse back skins collected at 2 hours after intradermal injections of 10 μM CEP-BSA or an equal amount of BSA as a control. Right: CEP presence in cells was measured by quantification of CEP/DAPI staining, whereas CEP presence specifically in macrophages was assessed based on CEP/CD68 co-staining in tissues section (left). The value of CEP/DAPI co-staining was assigned 100%. D and E. Immunostaining for CEP, CD68 and DAPI on the primary R-MPMs is shown. Cells were incubated at 37 °C for the times indicated; then washed, culture media were changed with fresh media without CEP-BSA, and incubated for additional 0, 30, and 90 minutes after 30 minutes for pulse-treatment (D) or during the entire incubation (E) with 500 nM of CEP-BSA. F. CEP was quantified by ELISA before and after incubation with macrophages. After 500 nM CEP-BSA was added to the R-MPMs at 37 °C, the supernatants were then collected at the times indicated, measured by ELISA, and normalized to the CEP only-containing culture media without macrophages. Percentage of remaining CEP is shown. Initial concentration of CEP (time point 0) was assigned a value of 100%. G. Simplified chemical structures of CEP and EP. H. Detection of EP by immunofluorescence in primary R-MPMs. In parallel, the staining for CD68 and DAPI is shown. R-MPMs were incubated at 37 °C for the times indicated with 500 nM of EP-BSA. Note that certain levels of EP are endogenously present in cells, but both binding and scavenging are lacking. Right: quantification of EP fluorescence at the times indicated. Fold changes in EP staining are shown. Initial staining for EP (0 hr) was assigned a value of 1. I. Immunofluorescence of F4/80 (macrophage) and DAPI on the back skins of mice treated with control- or clodronate-liposome for 3 days. Right: quantification of F4/80 fluorescence based on tissue sections shown (left). Fold changes in macrophage count is shown. Macrophage count in mice treated with control antibody was assigned a value of 100%. J. Impaired CEP clearance in clodronate-treated mice (as in I). Skins were collected at the times indicated from control- or clodronate-liposome treated mice (n=3), after subcutaneous injections of 10 μM CEP-BSA. Concentration of CEP in homogenates was measured by ELISA. Fold changes in CEP levels are shown. CEP concentration in skin 1 hour post-injection was assigned a value of 1.
Next, we assessed whether exogenously provided CEP-BSA can interact with macrophages in vivo. Two hours after CEP injection, more than 80% of cells positive for CEP were also positive for CD68, a macrophage marker (Figure 2C). Thus, CEP is recognized by macrophages in vitro as well as in vivo.
Macrophages are able to specifically scavenge CEP but not similar protein modifications
To examine whether macrophages are able to scavenge and metabolize CEP, R-MPMs (that do not generate their own CEP) were incubated with exogenous CEP, washed and the presence of remaining metabolite in cells was monitored. Within the first 30 min of incubation, CEP was strongly present in R-MPMs, however, the CEP positive signal was gradually reduced at 30 min and finally disappeared at 90 min after media change, indicating that CEP was being scavenged by macrophages (Figure 2D). When macrophages were incubated with CEP for 24 hours without media change, all of the CEP had merged with CD68, known to be expressed in the lysosomes31, 32 (Figure 2E), implying that CEP was scavenged and possibly metabolized by macrophages. The rate of CEP scavenging by macrophages was monitored by measuring the concentration of CEP remaining in the culture medium by ELISA. As macrophages actively bound and scavenged CEP, the CEP levels reached at approximately 70% of the initial concentration within 30 minutes and continued decreasing to approximately 20% of the original CEP amount within 24 hours (Figure 2F).
To investigate the specificity of CEP recognition and clearance, we compared it with that of a structurally similar protein modification, ethylpyrrole (EP). This protein modification is not only structurally similar to CEP (only lacking the carboxy group as shown in Figure 2G), but it is cogenerated through an alternative oxidative cleavage of DHA to give 4-hydroxyhex-2-enal (HHE) followed by condensation of HHE with the ε-amino group of lysyl residues (Online Material and Figure VIA). We compared the interaction of EP-BSA and CEP-BSA with macrophages (Online Figure VIB). As shown in Figure 2H, EP-BSA exhibited no binding to macrophages, which were not able to scavenge it for as long as 24 hours. Therefore, it appears that the process of recognition and scavenging by macrophages is highly specific for CEP-containing derivatives and it is not applicable to similar derivatives such as EP-BSA modifications.
To demonstrate whether and how CEP is scavenged by macrophages in vivo, macrophages were depleted by clodronate treatment,33, 34 resulting in at least 50% decrease in the amount of resident macrophages in skin (Figure 2I). Exogenous CEP was subcutaneously injected into control and macrophage-depleted mice and amount of CEP in vivo was monitored. While CEP levels in control mice were decreased in a time-dependent manner reaching approximately 60% and 40% after 3 and 6 hours, respectively, there was a substantial delay in macrophage-depleted mice, where the levels were unchanged for at least 3 hours after CEP injection (Figure 2J). At the 6 hour time point, the remaining amount of CEP was about 2 times higher in macrophage-depleted mice as compared to controls (Figure 2J). Collectively, these results demonstrate that CEP, a stable end-product of DHA oxidation, is scavenged by macrophages ex vivo and in vivo.
M2-like macrophages efficiently bind to and scavenge CEP
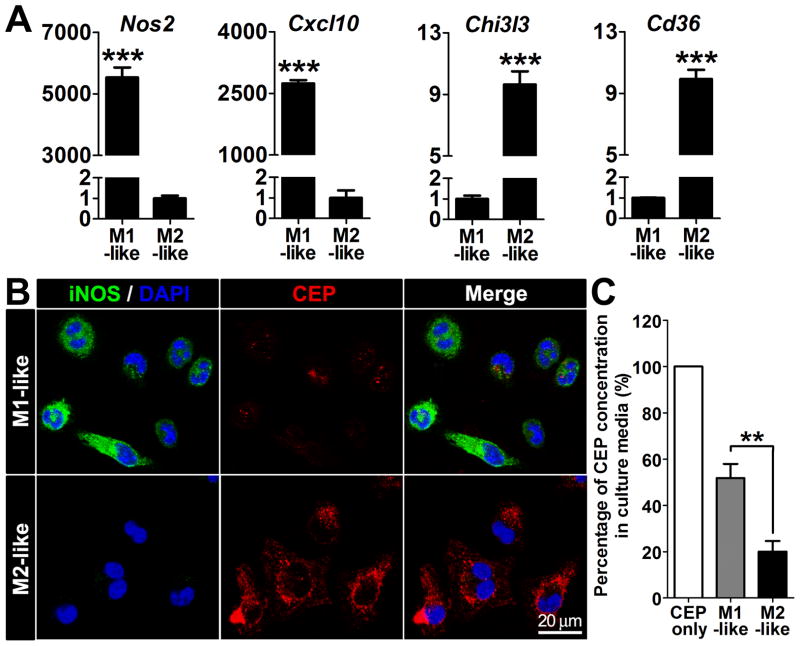
As macrophages are mainly classified into two subtypes, M1 and M2, we examined whether one of these two populations preferentially bind to and scavenge CEP. To polarize macrophages toward M1- or M2-phenotypes, R-MPMs were treated by IFN-γ and LPS or IL-4, respectively, and the polarization was verified using known M1- or M2-markers.27 While M1-markers, Nos2 and Cxcl10, were remarkably increased in the M1-like macrophages; M2-markers, Chi3l3 and Cd36, were markedly increased on the M2-like macrophages (Figure 3A). While M2-like macrophages were able to efficiently bind CEP, the M1-like macrophages exhibited relatively low binding (Figure 3B). CEP scavenging activity was also significantly lower for the M1-like macrophages compared to the M2-like macrophages (Figure 3C). These results indicate that both, CEP binding and scavenging, were primarily mediated by M2-like macrophages rather than by M1-like macrophages.
Figure 3. CEP binding and scavenging by M1- or M2-like macrophages.
A. Expression of M1 and M2 marker genes in polarized macrophages. Quantitative real-time RT-PCR analysis of representative M1 marker genes (Nos2 and Cxcl10) and M2 marker gene (Chi3l3 and Cd36) normalized by 18s, from R-MPMs is shown. B. Immunofluorescence of CEP, iNOS (M1 marker) and DAPI on M1-like and M2-like macrophages treated by 500 nM of CEP-BSA for 20 min. C. CEP levels that remained in the culture media. After 500 nM CEP-BSA was treated to the R-MPMs at 37 °C for 24 hours, the supernatants were collected. CEP levels were measured by ELISA and normalized to the CEP only-containing culture media without macrophages (this was assigned a value of 100%).
CEP demonstrates direct interactions with CD36 and TLR2
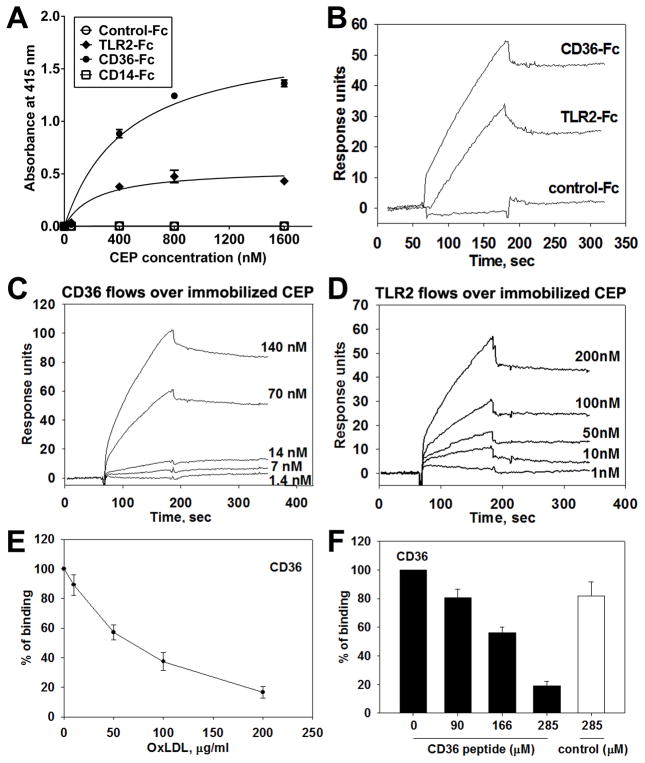
As CEP was shown to bind to TLR2 on endothelial cells to induce angiogenesis, we examined whether TLR2 on macrophages serves as a possible receptor for CEP. Since TLR2 is known to partner with other receptors, such as CD14 and CD36, these receptors were also considered. CD36 is highly expressed by both F4/80hi- and M2-like macrophages and is known to recognize a number of ligands, including modified lipoproteins, glycated proteins, and amyloid-forming peptides (Figure 3A, Online Figure IVD, and 35–37). Accordingly, we assessed CEP interactions with recombinant TLR2-Fc, CD36-Fc, and CD14-Fc chimeras in a solid phase immunoassay. Both, CD36 and TLR2, bound to CEP in a concentration-dependent manner, whereas binding to CD14-Fc and Fc fragment alone was negligible (Figure 4A). Then, the binding of CEP to CD36 and TLR2 was measured in real time by surface plasmon resonance (SPR) where CEP was injected and allowed to flow over immobilized CD36-Fc or TLR2-Fc (Figure 4B). The binding was confirmed by reversed SPR, where CD36-Fc or TLR2-Fc protein was injected at various concentrations and allowed to flow over immobilized CEP. Consistent with the results from Figure 4A, both proteins bound to CEP (Figure 4C and 4D). The dissociation constants (Kd) for CD36 and TLR2 with CEP were 6.53 nM and 11.8 nM, respectively. Since the functions of TLR2 depend on its association with either TLR6 or TLR1, where TLR6 is known to be involved in its partnership with CD36,38 we assessed the direct binding of binding of CEP to both TLRs using SPR. Although CEP interacted with both receptors (Online Figure VIIA–C), the binding to TLR6 was substantially higher than that to TLR1 and compatible to that to CD36. This was confirmed by ELISA (Online Figure VIID). CEP binding to Tlr6−/− macrophages and the consequent intake was diminished compared to WT cells as evidenced by ~30% decrease in average fluorescence intensity (Online Figure VIIE and F).
Figure 4. Direct binding between CEP and CD36 or TLR2.
A. ELISA-based binding assay using various concentrations of CEP-BSA (0 to 1600 nM) and Fc-conjugated chimeric proteins, such as TLR2-Fc, CD36-Fc, CD14-Fc. Binding to Fc-only served as a negative control (control-Fc). The absorbance values at 415 nm are shown. Values obtained using BSA-alone (as a coating substrate) were subtracted. B–D. Surface plasmon resonance (SPR) analysis of the binding between CEP and CD36 (B, C, E, and F), CEP and TLR2 (B and D). CD36-Fc, TLR2-Fc, and Fc-only were immobilized on CM5 chip and 2.5 μM CEP-HSA in HEPES buffer was flowed over the chip (B). CEP-HSA was immobilized on a CM5 chip and various concentrations of CD36-Fc (1.4 to 140 nM) (C) or TLR2-Fc (1 to 200 nM) (D) were flowed over the chip. Note that CEP demonstrates direct binding to CD36 and, to a lesser extent, TLR2. E and F. SPR analyses for CEP binding site on CD36. 70 nM of CD36-Fc were preincubated with various concentrations of copper oxidized LDL (10 to 200 μg/ml) and flowed over immobilized CEP-HAS. CEP binding to CD36 in the absence of OxLDL was assigned a value of 100% (E). Various concentrations of CD36 peptide (160SLINKSKSSMF170) or scrambled peptide (SIKVNLSQMKILNSI) were preincubated with 10 μM of CEP-HSA and flowed over immobilized CD36-Fc. CEP binding to CD36 in the absence of peptide was assigned a value of 100% (F).
To further characterize the binding site for CEP on CD36, we performed a competition assay using a known ligand for CD36, oxidized LDL (oxLDL).36 OxLDL competed with CEP in a dose dependent manner, indicating that CEP and oxLDL might share the same binding site on CD36 (Figure 4E). Indeed, 160SLINKSKSSMF170 peptide derived from an extracellular domain of human CD36 and known to be critical for oxLDL binding39 inhibited CEP-CD36 interaction in a dose-dependent manner (Figure 4F).
CEP scavenging depends on CD36 and TLR2
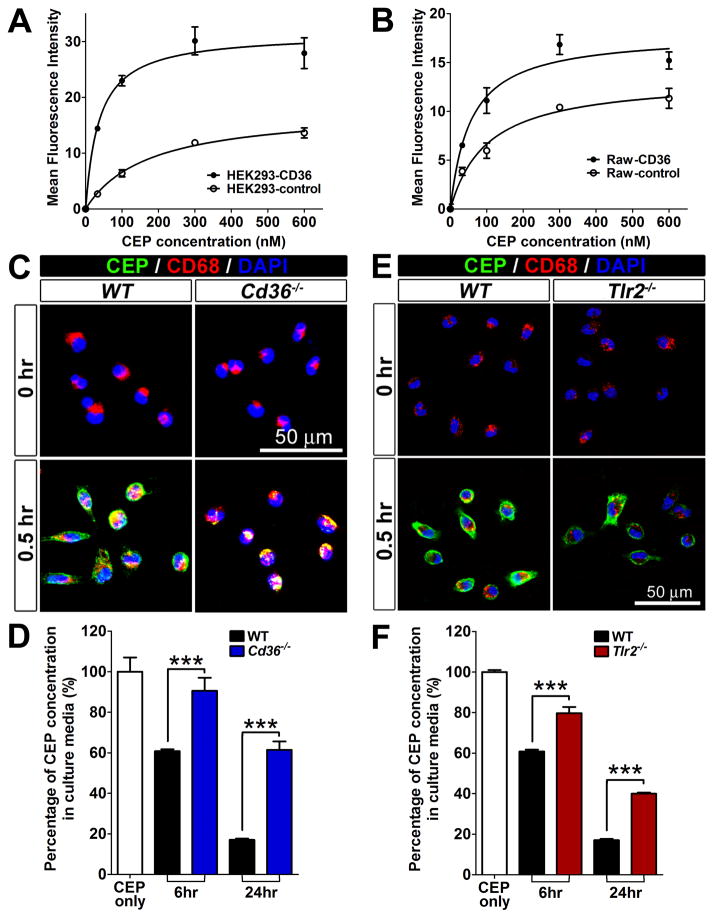
The physical binding of CEP to CD36 was further confirmed by two different cell-based binding assays using stable cell lines expressing CD36, Raw264.7-CD36 and HEK293-CD36 (Supplementary Figure 8). HEK293-CD36 cells bound about 3 times more to CEP than control cells (Figure 5A). A similar trend was observed using Raw264.7-CD36 cells (Figure 5B).40
Figure 5. CEP binding and scavenging by CD36 and TLR2.
A and B. Cell-based CEP binding assay using HEK293 (A) and Raw264.7 (B), which were stably expressing CD36. CEP binding to the cells was visualized by FACS after staining the cells with rabbit anti-CEP antibody and Alexa-488-conjugated anti-rabbit IgG secondary antibody. MFI are shown. C. Reduced CEP binding to CD36 null cells. Immunofluorescence of CEP, CD68 and DAPI on the primary WT and Cd36−/− R-MPMs, which were incubated at 37 °C with 500 nM CEP-BSA for 30 min. D. Reduced CEP scavenging by CD36 null cells. WT and Cd36−/− R-MPMs were treated with 500 nM CEP-BSA for 24 hours at 37 °C, then the supernatants were collected. CEP levels were measured by ELISA and residual CEP values are shown. The amount of CEP without added R-MPMs was assigned a value of 100%. E. Reduced CEP binding to TLR2 null cells. Immunofluorescence of CEP, CD68 and DAPI on the primary WT and Tlr2−/− R-MPMs, which were incubated at 37 °C with 500 nM CEP-BSA for 30 min. F. CEP scavenging by Tlr2−/− cells is reduced. WT and WT and Cd36−/− R-MPMs were treated with 500 nM CEP-BSA for 24 hours at 37 °C, then the supernatants were collected. CEP levels, measured by ELISA are shown. Amount of CEP without added R-MPMs was assigned a value of 100%.
Then, the role of CD36 in CEP clearance was tested using CD36-null (Cd36−/−) macrophages. When treated with CEP for 30 minutes, Cd36−/− macrophages bound substantially less CEP than WT macrophages (Figure 5C). Moreover, Cd36−/− macrophages were defective in their ability to scavenge CEP. While WT macrophages were able to metabolize about 40% of CEP in first 6 hours, CEP levels were not significantly changed in the presence of Cd36−/− macrophages (Figure 5D). After 24 hours WT macrophages were able to remove approximately 85%, whereas Cd36−/− macrophages metabolized only 40% of exogenously provided CEP (Figure 5D), indicating that CD36 on macrophages is essential for CEP binding and scavenging.
Similar experiments were conducted using Tlr2−/− macrophages. Compared to WT, Tlr2−/− macrophages bound less to CEP within 30 minutes (Figure 5E). Accordingly, CEP scavenging activity was also reduced in Tlr2−/− macrophages since after 24 hours, remaining amounts of CEP were approximately 15% for WT and 40% for Tlr2−/− macrophages (Figure 5F). These experiments demonstrate that similar to CD36, TLR2 deficiency affects CEP binding and scavenging by macrophages, although the role of CD36 appears to be more prominent.
CEP clearance is severely impaired in CD36/TLR2 DKO macrophages
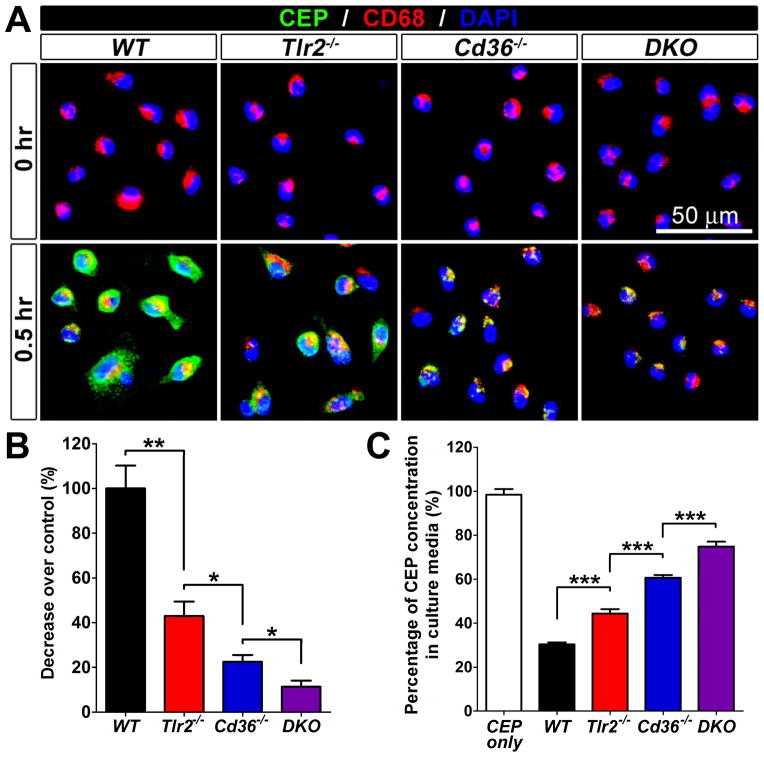
Since CD36 often serves as a co-receptor for TLR2,38, 41 we generated Cd36−/−;Tlr2−/− double knockout (DKO) mice to assess the cooperation between these receptors in CEP clearance by macrophages. We compared the ability of R-MPMs from WT, Cd36−/−, Tlr2−/−, and DKO mice to bind to and scavenge CEP. While both, Tlr2−/− and Cd36−/− macrophages bound only 60% and 75% less to CEP than WT, respectively, the binding to DKO macrophages was reduced by about 90% as compared to WT (Figure 6A and B). Likewise, the amount of CEP scavenged by WT, Tlr2−/−, Cd36−/− and DKO macrophages within 24 hours was 70%, 55%, 40% and 22%, respectively (Figure 6C). Together, the additive effect of Tlr2−/− and Cd36−/− deficiencies suggests that while these receptors are both important for CEP binding and clearance by macrophages, they might function independently and the role of CD36 was more prominent.
Figure 6. Additive effect of CD36 and TLR2 on CEP binding and scavenging.
A. Immunofluorescence of CEP, CD68 and DAPI using primary WT, Tlr2−/−, Cd36−/−, and Cd36−/−;Tlr2−/− DKO R-MPMs, which were incubated at 37 °C with 500 nM CEP-BSA for 30 min. B. Quantification of CEP binding based on fluorescence. Binding to WT macrophages was assigned a value of 100% (A). C. Tlr2−/−, Cd36−/−, and Cd36−/−;Tlr2−/− DKO R-MPMs were treated with 500 nM CEP-BSA for 24 hours at 37 °C, then the supernatants were collected. CEP levels were measured by ELISA and residual CEP values are shown. The amount of CEP without added R-MPMs was assigned a value of 100%.
Defective CEP clearance affects angiogenesis and wound closure in CD36 null mice
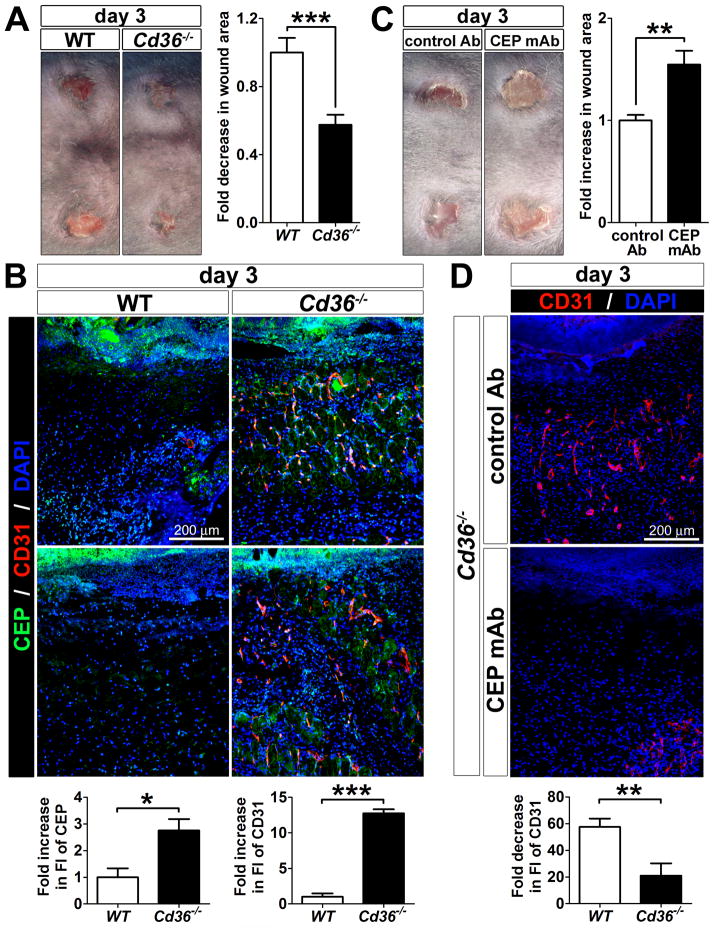
To assess the importance CEP presence and timely removal in tissues, we analyzed angiogenesis and wound healing in Cd36−/− mice. Punch wounds were made on the back skin of 8-week-old WT and Cd36−/− mice and monitored for 7 days. Surprisingly, at the early time points (day 3), wound closure was accelerated in Cd36−/− mice as compared to WT, albeit no longer significantly at day 7 (Figure 7A and Online Figure IXA), which might be due to delayed clearance of apoptotic cells also mediated by CD36.35 At the same time, CD31 immunostaining revealed that angiogenesis in Cd36−/− wounds also occurred earlier than in WT mice (day 3 vs. days 5–7, respectively) (Figure 7B and Online Figure IXB). Co-staining for CD31 and CEP revealed the presence of new blood vessels primarily in the CEP-positive area, suggesting that the faster angiogenesis might be due to increased levels of CEP in the Cd36−/− wounds (Figure 7B).
Figure 7. Defective CEP clearance in Cd36−/− wounds.
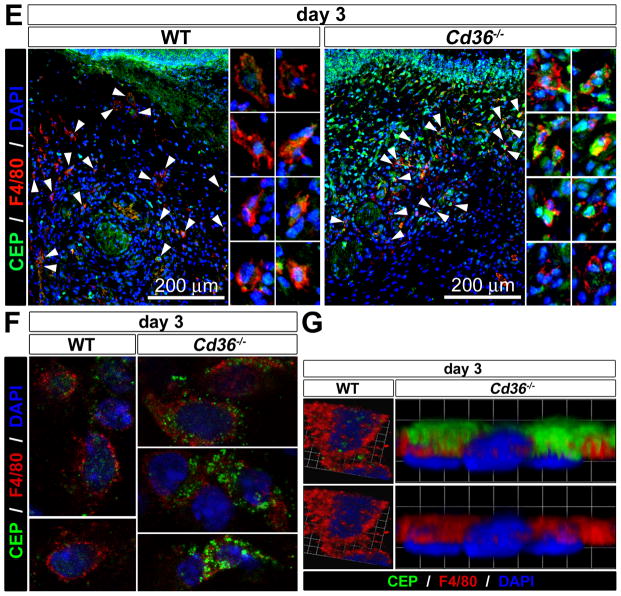
A. Representative images of wounds on the back skins of WT and Cd36−/− mice at day 3 (n=10). B. Two independent representative immunofluorescence staining for CEP, CD31 and DAPI in tissue sections of WT and Cd36−/− mice at day 3 after wounding (n=5). Bottom: quantification of CEP and CD31 fluorescence in tissue section of WT and Cd36−/− mice normalized to WT control is shown. C. Representative images of wounds on the back skins of Cd36−/− mice treated with either control- or CEP blocking antibody at day 3 (n=4). D. Immunofluorescence of CD31 and DAPI in skin of Cd36−/− mice treated with either control- or CEP blocking antibody at day 3 after wounding (n=4). Bottom: quantification of CD31 fluorescence normalized to WT control. E. Immunofluorescence of CEP, F4/80, and DAPI in tissues sections of WT and Cd36−/− mice at day 3 after wounding. Right: enlarged pictures of single macrophages (arrowheads). F and G. Multi-photon confocal images of CEP, F4/80, and DAPI staining of the single cells from wounded skins of WT and Cd36−/− mice at day 3 show reduced CEP levels in Cd36−/− skins. G. A cross-sectional image of WT cell and a side view of Cd36−/− cell were obtained from 3D reconstructions of multi-photon confocal z-stack images. Note that CEP localization differs between WT and Cd36−/− macrophages: it is seen in WT cells in cross-sectional images (inside of the cells) but not in other planes, whereas it’s presence was mainly extracellular in Cd36−/− cells.
To demonstrate the causative effect of CEP accumulation on the accelerated angiogenesis and wound closure in Cd36−/− mice, a mouse anti-CEP monoclonal antibody was introduced during wound healing to block the pro-angiogenic activity of CEP in vivo. The blockade of CEP activity in Cd36−/− mice substantially inhibited both the accelerated angiogenesis and wound closure, indicating a causative role for CEP accumulation in those processes (Figure 7C and 7D). Despite the comparable numbers of recruited macrophages, CEP clearance was less efficient in Cd36−/− wounds as compared to WT, based on F4/80 and CEP staining (Figure 7E). Analysis of the single cells in WT and Cd36−/− wounds at day 3 by a multi-photon confocal microscopy revealed that CEP was localized inside of the cells in WT, but remained on the cell membrane in Cd36−/− macrophages, implying defective scavenging (Figure 7F and 7G). Taken together, these results demonstrate that CD36 is absolutely required for CEP clearance in vivo. Cd36−/− mice exhibit increased CEP accumulation, which, in turn, mediates the accelerated angiogenesis and wound closure.
DISCUSSION
The key findings of this study are the following: First, a biologically active product of DHA oxidation, CEP, is continuously accumulated in pathologies exemplified by tumors and atherosclerotic lesions. During wound healing, however, CEP levels follow a bell shaped curve returning to normal low levels when the wound is healed. In contrast to the accepted paradigm, CEP modifications are not the final and non-degradable products of DHA oxidation, they can be removed by F4/80hi and/or M2-like macrophages ex vivo and in vivo. Second, this process is specific for CEP, since the structurally similar modification, EP, was neither bound to nor degraded by macrophages. Third, this scavenging function is mediated by CD36 in cooperation with TLR2/TLR6, but not another TLR2 co-receptor, CD14. Cd36−/−, Tlr2−/−, Tlr6−/− and especially Cd36−/−;Tlr2−/− DKO macrophages exhibit impaired abilities to bind to and scavenge CEP modifications. Forth, the impaired CEP clearance in Cd36−/− mice leads to accelerated angiogenesis and wound closure. Together, this provides a cellular and molecular mechanism controlling tissue levels of the highly biologically active LPO product, CEP, and establishes the pathophysiological importance of this process.
As an oxidation fragment of DHA, which is abundant in retina and brain,42 CEP has been first shown to accumulate in retinas of patients with AMD.16, 17, 43 Multiple studies from a number of laboratories demonstrated that CEP exerts both proangiogenic and proinflammatory activities potentially related to pathogenesis of AMD.10, 15, 16, 21–24, 44, 45 Moreover, the presence of CEP modifications as well as anti-CEP specific autoantibodies in plasmas of AMD patients were shown to serve as early signs of susceptibility to AMD,46 thereby underscoring the role of oxidized DHA products in this disease. Furthermore, more recent studies revealed the presence and biological significance of CEP modifications in other physiological and pathological conditions such as tumor growth, atherosclerosis progression and thrombosis.10, 14, 25 These studies emphasize an importance of the mechanism controlling CEP accumulation in tissues, and suggest that defective CEP clearance might play a causative role in pathologies. Indeed, here we show that delayed clearance of CEP in Cd36−/− mice causes accelerated angiogenesis in vivo. Thus, the receptor-mediated CEP scavenging might serve as a potential therapeutic target for conditions associated with excessive oxidative stress, such as AMD or atherothrombosis.
The receptor-mediated specific recognition and clearance of LPO products in tissues represents a novel host defense mechanism which differs from well-known processes of ROS neutralization by enzymatic and non-enzymatic antioxidants.7 Interestingly, besides CEP, an alternative cleavage of DHA can give rise to EP, a modification similar to CEP. The only structural difference between CEP and EP is a presence of a carboxyl group in CEP, which seems to be essential for its recognition by macrophages. Thus, macrophages do not merely recognize any modified or denatured proteins, but bind to and scavenge a class of well-defined chemical moieties, i.e. carboxyethylpyrroles, which might be present either on proteins10 or lipids.15
We demonstrate that macrophages are specifically responsible for CEP recognition and clearance. Our data suggest that macrophages might metabolize CEP in lysosomes after uptake, since CEP staining is merged with that of CD68, a lysosome marker.31, 32 This suggests that CEP, which is believed to be a stable end product of DHA oxidation can be further degraded. Moreover, differential CEP binding and scavenging activities of F4/80hi vs. F4/80lo and M1- vs. M2-like macrophages suggest that CEP clearance is mainly mediated by non-inflammatory macrophages, but not by activated macrophages.27–29 This is also supported by the results of in vivo wound healing experiments, where CEP is scavenged as early as at day 3 prior to the arrival of blood monocytes. The latter cells are known to express Ly-6C at high levels and F4/80 at low levels and to exhibit pro-inflammatory functions.47–49 Wound macrophages undergo a transition from a high to a low level of Ly-6C as wound healing progresses,50 supporting the idea that CEP clearance within the first 3 days is mainly mediated by resident macrophages. It appears that these macrophage populations might tip the balance toward either excessive or insufficient CEP levels, which, in turn, might define the spectrum and severity of angiogenic and inflammatory processes in vivo.
On macrophages, CEP binds to two main receptors, CD36 and TLR2, the latter has been shown to mediate activities of CEP-containing protein14, 45 as well as lipid15 modifications on other cell types. The fact that the defects of DKO macrophages were more severe than those of Cd36−/− or Tlr2−/− macrophages supports a possibility that CD36 and TLR2 play independent roles in CEP clearance. Interestingly, in contrast to many other ligands of TLR2, such as Pam3CSK4 and Lipoteichoic acid, this process does not involve CD14.51, 52 Instead, it depends on CD36, another TLR2 co-receptor, known to mediate TLR2/TLR6 rather than TLR2/TLR1 functions.52 In agreement with previous studies,38, 41 it appears that during the scavenging of CEP modifications, CD36 operates with TLR2/TLR6 heterodimers, whereas the CEP signaling seems to be dependent on TLR2/TLR1.15, 45 Also known as a scavenger receptor, CD36 recognizes misfolded proteins and specific lipid modifications,35 including intermediate products of DHA oxidation, which serve as precursors of CEP and are known to play a causative role in atherosclerosis and thrombosis.36, 53 Similar to TLR2, the recognition of CEP by CD36 is highly specific for carboxyalkylpyrroles since ethylpyrrole modifications are not recognized by either receptor. Therefore, CD36 might play a dual role in the metabolism of DHA oxidation products and in the pathogenesis of atherosclerosis in general, first, by mediating foam cell formation and thrombosis triggered by intermediate products of DHA oxidation, and, second, by facilitating the removal of the end-products of this oxidation pathway.
CD36, as a receptor for thrombospondin (TSP), has been shown to serve as a negative regulator of angiogenesis.54 This function might be context dependent, since TSP1/TSP2 double knockout mice were reported not to exhibit increased vascularity or accelerated wound healing when compared to WT controls.55 Moreover, both wound closure and leukocyte recruitment over a 9 day period after injury in Cd36−/− mice were shown to be comparable to that in WT.56 This is consistent with our study (Online Figure VIII). However, 3 days after injury, wounds of Cd36−/− mice had more CEP than WT, which, in turn, accelerated vascularization and wound closure. If CEP is neutralized, these differences were no longer apparent, thereby demonstrating a causative role for CEP in this process. It might be plausible that at the later time points other CD36-dependent functions, including removal of apoptotic cells by macrophages, might slow down the wound closure rate in Cd36−/− mice as compared to WT. Nevertheless, delayed CEP clearance in Cd36−/− mice leads to accelerated angiogenesis and faster wound closure during the early and very important phase of wound healing. In conclusion, the present study underscores the role of endogenous metabolites of LPO in pathophysiology, and demonstrates that the cellular processes naturally controlling their levels in tissues might serve as promising targets for the development of future therapeutic interventions.
Supplementary Material
Novelty and Significance.
What Is Known?
Reactive oxygen species generate a range of secondary toxic products from phospholipids, including Carboxy Ethyl Pyrroles (CEP).
CEP has been shown to promote vascularization and inflammation.
CEP is believed to be a final and stable product of phospholipid oxidation; implying that it could accumulate in tissues for years.
What New Information Does This Article Contribute?
Proteins modified by oxidized phospholipid products, CEP (Carboxy Ethyl Pyrrole) are only transiently present during wound healing, whereas they are continuously accumulated in atherosclerotic lesions and growing tumors.
These oxidation products are recognized, scavenged and metabolized by macrophages, which control CEP levels in tissues.
CEP, but not structurally similar modifications, bind to CD36 Toll like receptors 2 and 6 with high specificity.
Oxidative stress is a key determinant of cardiovascular diseases and aging. It results in tissue accumulation of chemically modified products with bot, physiological and pathological activities. While some of these products are essential for tissue repair processes, their excessive accumulation could lead to tissue injury. We found that CEP-modifications are recognized and metabolized by tissue macrophages. During wound healing, tissue macrophages are responsible for the clearance of CEP-modifications. This process is highly selective for CEP-products and it involves specific innate immunity receptors on macrophages. This detoxification process represents a novel natural defense mechanism against uncontrolled accumulation of oxidized chemicals.
Acknowledgments
We thank Nickolas Tomko for help with reagents, Dr. Maria Febbraio for providing Cd36−/− mice, Mira Tischenko for technical assistance with caring for mice and parts of histology, and Dr. Bethany A. Kerr and Emelye Crehore for assistance in editing the manuscript.
SOURCES OF FUNDING
This work was supported by an endowed chair fund and in part by a NIH grant HL071625 to T.V.B., an American Heart Association postdoctoral Fellowship 13POST14570001 to Y.W.K., by NIH grants DK102020 to V.P.Y., HL077213 to E.A.P., and EY016813 and GM021249 to R. G. S.
Nonstandard Abbreviations and Acronyms
- CAP
Carboxy(alkylpyrrole)
- CEP
2-(ω-carboxyethyl)pyrrole
- DHA
docosahexaenoic acid
- EP
Ethylpyrrole
- FACS
fluorescence activated cell sorting
- HOHA
(E)-4-hydroxy-7-oxohept-5-enoic acid
- PUFA
polyunsaturated fatty acids
- R-MPM
Resident mouse peritoneal macrophage
- ROS
Reactive oxygen species
- TLR
Toll like receptor
- VEGF
Vascular endothelial growth factor
- WD
Western diet
Footnotes
DISCLOSURES
The authors declare no competing financial interest.
References
- 1.Benz CC, Yau C. Ageing, oxidative stress and cancer: Paradigms in parallax. Nat Rev Cancer. 2008;8:875–879. doi: 10.1038/nrc2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim YW, Byzova TV. Oxidative stress in angiogenesis and vascular disease. Blood. 2014;123:625–631. doi: 10.1182/blood-2013-09-512749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim YW, West XZ, Byzova TV. Inflammation and oxidative stress in angiogenesis and vascular disease. J Mol Med (Berl) 2013;91:323–328. doi: 10.1007/s00109-013-1007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free radical biology & medicine. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Negre-Salvayre A, Auge N, Ayala V, Basaga H, Boada J, Brenke R, Chapple S, Cohen G, Feher J, Grune T, Lengyel G, Mann GE, Pamplona R, Poli G, Portero-Otin M, Riahi Y, Salvayre R, Sasson S, Serrano J, Shamni O, Siems W, Siow RC, Wiswedel I, Zarkovic K, Zarkovic N. Pathological aspects of lipid peroxidation. Free Radic Res. 2010;44:1125–1171. doi: 10.3109/10715762.2010.498478. [DOI] [PubMed] [Google Scholar]
- 6.Sies H. Oxidative stress: Oxidants and antioxidants. Exp Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 7.Gueraud F, Atalay M, Bresgen N, Cipak A, Eckl PM, Huc L, Jouanin I, Siems W, Uchida K. Chemistry and biochemistry of lipid peroxidation products. Free Radic Res. 2010;44:1098–1124. doi: 10.3109/10715762.2010.498477. [DOI] [PubMed] [Google Scholar]
- 8.Cutler RG, Mattson MP. The adversities of aging. Ageing Res Rev. 2006;5:221–238. doi: 10.1016/j.arr.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nature reviews. Immunology. 2013;13:621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 10.Salomon RG, Hong L, Hollyfield JG. Discovery of carboxyethylpyrroles (ceps): Critical insights into amd, autism, cancer, and wound healing from basic research on the chemistry of oxidized phospholipids. Chemical research in toxicology. 2011;24:1803–1816. doi: 10.1021/tx200206v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siddiqui RA, Harvey K, Stillwell W. Anticancer properties of oxidation products of docosahexaenoic acid. Chem Phys Lipids. 2008;153:47–56. doi: 10.1016/j.chemphyslip.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nature medicine. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renganathan K, Gu J, Rayborn ME, Crabb JS, Salomon RG, Collier RJ, Kapin MA, Romano C, Hollyfield JG, Crabb JW. Cep biomarkers as potential tools for monitoring therapeutics. PloS one. 2013;8:e76325. doi: 10.1371/journal.pone.0076325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.West XZ, Malinin NL, Merkulova AA, Tischenko M, Kerr BA, Borden EC, Podrez EA, Salomon RG, Byzova TV. Oxidative stress induces angiogenesis by activating tlr2 with novel endogenous ligands. Nature. 2010;467:972–976. doi: 10.1038/nature09421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Guo J, West X, Bid HK, Lu L, Hong L, Jang GF, Zhang L, Crabb JW, Linetsky MD, Salomon RG. Detection and biological activities of carboxyethylpyrrole ethanolamine phospholipids: A correlation with age-related macular degeneration. Chemical research in toxicology. 2014 doi: 10.1021/tx500216a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu X, Meer SG, Miyagi M, Rayborn ME, Hollyfield JG, Crabb JW, Salomon RG. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. The Journal of biological chemistry. 2003;278:42027–42035. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- 18.Renganathan K, Ebrahem Q, Vasanji A, Gu X, Lu L, Sears J, Salomon RG, Anand-Apte B, Crabb JW. Carboxyethylpyrrole adducts, age-related macular degeneration and neovascularization. Advances in experimental medicine and biology. 2008;613:261–267. doi: 10.1007/978-0-387-74904-4_30. [DOI] [PubMed] [Google Scholar]
- 19.Gu J, Pauer GJ, Yue X, Narendra U, Sturgill GM, Bena J, Gu X, Peachey NS, Salomon RG, Hagstrom SA, Crabb JW, Clinical G Proteomic AMDSG. Assessing susceptibility to age-related macular degeneration with proteomic and genomic biomarkers. Molecular & cellular proteomics: MCP. 2009;8:1338–1349. doi: 10.1074/mcp.M800453-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukhopadhyay S, Pluddemann A, Gordon S. Macrophage pattern recognition receptors in immunity, homeostasis and self tolerance. Advances in experimental medicine and biology. 2009;653:1–14. doi: 10.1007/978-1-4419-0901-5_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruz-Guilloty F, Saeed AM, Echegaray JJ, Duffort S, Ballmick A, Tan Y, Betancourt M, Viteri E, Ramkhellawan GC, Ewald E, Feuer W, Huang D, Wen R, Hong L, Wang H, Laird JM, Sene A, Apte RS, Salomon RG, Hollyfield JG, Perez VL. Infiltration of proinflammatory m1 macrophages into the outer retina precedes damage in a mouse model of age-related macular degeneration. International journal of inflammation. 2013;2013:503725. doi: 10.1155/2013/503725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cruz-Guilloty F, Saeed AM, Duffort S, Cano M, Ebrahimi KB, Ballmick A, Tan Y, Wang H, Laird JM, Salomon RG, Handa JT, Perez VL. T cells and macrophages responding to oxidative damage cooperate in pathogenesis of a mouse model of age-related macular degeneration. PloS one. 2014;9:e88201. doi: 10.1371/journal.pone.0088201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebrahem Q, Renganathan K, Sears J, Vasanji A, Gu X, Lu L, Salomon RG, Crabb JW, Anand-Apte B. Carboxyethylpyrrole oxidative protein modifications stimulate neovascularization: Implications for age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13480–13484. doi: 10.1073/pnas.0601552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doyle SL, Campbell M, Ozaki E, Salomon RG, Mori A, Kenna PF, Farrar GJ, Kiang AS, Humphries MM, Lavelle EC, O’Neill LA, Hollyfield JG, Humphries P. Nlrp3 has a protective role in age-related macular degeneration through the induction of il-18 by drusen components. Nature medicine. 2012;18:791–798. doi: 10.1038/nm.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panigrahi S, Ma Y, Hong L, Gao D, West XZ, Salomon RG, Byzova TV, Podrez EA. Engagement of platelet toll-like receptor 9 by novel endogenous ligands promotes platelet hyperreactivity and thrombosis. Circulation research. 2013;112:103–112. doi: 10.1161/CIRCRESAHA.112.274241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nature reviews. Immunology. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen HH, Tran BT, Muller W, Jack RS. Il-10 acts as a developmental switch guiding monocyte differentiation to macrophages during a murine peritoneal infection. J Immunol. 2012;189:3112–3120. doi: 10.4049/jimmunol.1200360. [DOI] [PubMed] [Google Scholar]
- 29.Ghosn EE, Cassado AA, Govoni GR, Fukuhara T, Yang Y, Monack DM, Bortoluci KR, Almeida SR, Herzenberg LA. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2568–2573. doi: 10.1073/pnas.0915000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010;163:257–268. doi: 10.1111/j.1365-2133.2010.09804.x. [DOI] [PubMed] [Google Scholar]
- 31.Saito N, Pulford KA, Breton-Gorius J, Masse JM, Mason DY, Cramer EM. Ultrastructural localization of the cd68 macrophage-associated antigen in human blood neutrophils and monocytes. Am J Pathol. 1991;139:1053–1059. [PMC free article] [PubMed] [Google Scholar]
- 32.Ramprasad MP, Terpstra V, Kondratenko N, Quehenberger O, Steinberg D. Cell surface expression of mouse macrosialin and human cd68 and their role as macrophage receptors for oxidized low density lipoprotein. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:14833–14838. doi: 10.1073/pnas.93.25.14833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: A new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng W, McCabe NP, Mahabeleshwar GH, Somanath PR, Phillips DR, Byzova TV. The angiogenic response is dictated by beta3 integrin on bone marrow-derived cells. J Cell Biol. 2008;183:1145–1157. doi: 10.1083/jcb.200802179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silverstein RL, Febbraio M. Cd36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Febbraio M, Hajjar DP, Silverstein RL, Hoff HF, Salomon RG, Hazen SL. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor cd36 and is enriched in atherosclerotic lesions. The Journal of biological chemistry. 2002;277:38517–38523. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- 37.Martin C, Chevrot M, Poirier H, Passilly-Degrace P, Niot I, Besnard P. Cd36 as a lipid sensor. Physiol Behav. 2011;105:36–42. doi: 10.1016/j.physbeh.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 38.Triantafilou M, Gamper FG, Haston RM, Mouratis MA, Morath S, Hartung T, Triantafilou K. Membrane sorting of toll-like receptor (tlr)-2/6 and tlr2/1 heterodimers at the cell surface determines heterotypic associations with cd36 and intracellular targeting. The Journal of biological chemistry. 2006;281:31002–31011. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- 39.Kar NS, Ashraf MZ, Valiyaveettil M, Podrez EA. Mapping and characterization of the binding site for specific oxidized phospholipids and oxidized low density lipoprotein of scavenger receptor cd36. The Journal of biological chemistry. 2008;283:8765–8771. doi: 10.1074/jbc.M709195200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiao L, Zou C, Shao P, Schaack J, Johnson PF, Shao J. Transcriptional regulation of fatty acid translocase/cd36 expression by ccaat/enhancer-binding protein alpha. The Journal of biological chemistry. 2008;283:8788–8795. doi: 10.1074/jbc.M800055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. Cd36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 42.Neuringer M, Anderson GJ, Connor WE. The essentiality of n-3 fatty acids for the development and function of the retina and brain. Annu Rev Nutr. 1988;8:517–541. doi: 10.1146/annurev.nu.08.070188.002505. [DOI] [PubMed] [Google Scholar]
- 43.Hollyfield JG, Salomon RG, Crabb JW. Proteomic approaches to understanding age-related macular degeneration. Advances in experimental medicine and biology. 2003;533:83–89. doi: 10.1007/978-1-4615-0067-4_11. [DOI] [PubMed] [Google Scholar]
- 44.Lu L, Gu X, Hong L, Laird J, Jaffe K, Choi J, Crabb J, Salomon RG. Synthesis and structural characterization of carboxyethylpyrrole-modified proteins: Mediators of age-related macular degeneration. Bioorganic & medicinal chemistry. 2009;17:7548–7561. doi: 10.1016/j.bmc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saeed AM, Duffort S, Ivanov D, Wang H, Laird JM, Salomon RG, Cruz-Guilloty F, Perez VL. The oxidative stress product carboxyethylpyrrole potentiates tlr2/tlr1 inflammatory signaling in macrophages. PloS one. 2014;9:e106421. doi: 10.1371/journal.pone.0106421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu J, Pauer GJ, Yue X, Narendra U, Sturgill GM, Bena J, Gu X, Peachey NS, Salomon RG, Hagstrom SA, Crabb JW, Clinical G Proteomic AMDSG. Proteomic and genomic biomarkers for age-related macular degeneration. Advances in experimental medicine and biology. 2010;664:411–417. doi: 10.1007/978-1-4419-1399-9_47. [DOI] [PubMed] [Google Scholar]
- 47.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 48.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nature reviews. Immunology. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brancato SK, Albina JE. Wound macrophages as key regulators of repair: Origin, phenotype, and function. Am J Pathol. 2011;178:19–25. doi: 10.1016/j.ajpath.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koh TJ, DiPietro LA. Inflammation and wound healing: The role of the macrophage. Expert Rev Mol Med. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akashi-Takamura S, Miyake K. Tlr accessory molecules. Current opinion in immunology. 2008;20:420–425. doi: 10.1016/j.coi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Lee CC, Avalos AM, Ploegh HL. Accessory molecules for toll-like receptors and their function. Nature reviews. Immunology. 2012;12:168–179. doi: 10.1038/nri3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Podrez EA, Byzova TV, Febbraio M, Salomon RG, Ma Y, Valiyaveettil M, Poliakov E, Sun M, Finton PJ, Curtis BR, Chen J, Zhang R, Silverstein RL, Hazen SL. Platelet cd36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nature medicine. 2007;13:1086–1095. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simantov R, Silverstein RL. Cd36: A critical anti-angiogenic receptor. Front Biosci. 2003;8:s874–882. doi: 10.2741/1168. [DOI] [PubMed] [Google Scholar]
- 55.Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (tsp1) dictates the course of wound healing in double-tsp1/tsp2-null mice. Am J Pathol. 2002;161:831–839. doi: 10.1016/S0002-9440(10)64243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-cd36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med. 2006;203:2613–2625. doi: 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.