Abstract
Rationale
The role of Parkin in hearts is unclear. Germ-line Parkin knockout mice have normal hearts, but Parkin is protective in cardiac ischemia. Parkin-mediated mitophagy is reportedly either irrelevant, or a major factor, in the lethal cardiomyopathy evoked by cardiac myocyte-specific interruption of Drp1-mediated mitochondrial fission.
Objective
To understand the role of Parkin-mediated mitophagy in normal and mitochondrial fission-defective adult mouse hearts.
Methods and Results
Parkin mRNA and protein were present at very low levels in normal mouse hearts, but were upregulated after cardiac myocyte-directed Drp1 gene deletion in adult mice. Alone, forced cardiac myocyte Parkin overexpression activated mitophagy without adverse effects. Likewise, cardiac myocyte-specific Parkin deletion evoked no adult cardiac phenotype, revealing no essential function for, and tolerance of, Parkin-mediated mitophagy in normal hearts. Concomitant conditional Parkin deletion with Drp1 ablation in adult mouse hearts prevented Parkin upregulation in mitochondria of fission-defective hearts, also increasing 6 week survival, improving ventricular ejection performance, mitigating adverse cardiac remodeling, and decreasing cardiac myocyte necrosis and replacement fibrosis. Underlying the Parkin knockout rescue was suppression of Drp1-induced hyper-mitophagy, assessed as ubiquitination of mitochondrial proteins and mitochondrial association of autophagosomal p62/SQSTM1 and processed LC3. Consequently, mitochondrial content of Drp1-deficient hearts was preserved. Parkin deletion did not alter characteristic mitochondrial enlargement of Drp1-deficient cardiac myocytes.
Conclusions
Parkin is rare in normal hearts and dispensable for constitutive mitophagic quality control. Ablating Drp1 in adult mouse cardiac myocytes not only interrupts mitochondrial fission, but markedly upregulates Parkin, thus provoking mitophagic mitochondrial depletion that contributes to the lethal cardiomyopathy.
Keywords: Parkin, mitophagy, mitochondrial fission, mitochondria disease cardiomyopathy, mitochondria, mouse, heart
INTRODUCTION
Mitochondria of adult cardiac myocytes are seemingly static, without apparent organelle fission and fusion. Nevertheless, interruption of mitochondrial fusion or fission pathways in cardiac myocytes provokes lethal cardiomyopathies 1. To resolve this paradox we proposed functioning of mitochondrial fusion and fission factors in mitophagy 2. Thus, mitofusin (Mfn) 2 co-mediates mitochondrial fusion with Mfn1 3, but is also a mitochondrial receptor for the mitophagy effector, Parkin 4, 5. A role for mitochondrial fission in mitophagy is less well defined. Three independent reports of cardiac myocyte-specific ablation of pro-fission dynamin related protein 1 (Drp1) described cardiomyopathy and abnormal mitophagy 6–8, but the underlying mechanisms are controversial; mitophagy was variously described as decreased 7, increased 8, or initiated independent of Parkin, but interrupted 6. Conclusions about Parkin-mediated mitophagy in the heart are uncertain because the germ-line Parkin knockout mouse has no baseline cardiac phenotype 9–11.
A different approach is required to define Parkin’s role in normal and mitochondrial fission-defective hearts. Here we determined how positive and negative regulation of cardiac myocyte Parkin impacts the in vivo mouse heart and interrogated the role of Parkin-mediated mitophagy in the cardiomyopathy provoked by cardiac myocyte-specific ablation of Drp1. In normal hearts Parkin-stimulated mitophagy is well tolerated and largely dispensable. However, Parkin is upregulated, stimulates mitophagy, and contributes to loss of mitochondria and cardiomyopathy in hearts defective in mitochondrial fission. These results reveal Parkin to be a stress-inducible factor with the potential to help or hurt the heart, depending on functional integrity of the mitophagy-mitochondrial dynamism interactome.
METHODS
Detailed Methods are available in the Online Data Supplement.
RESULTS
Parkin is upregulated in mitochondrial fission-defective mouse hearts
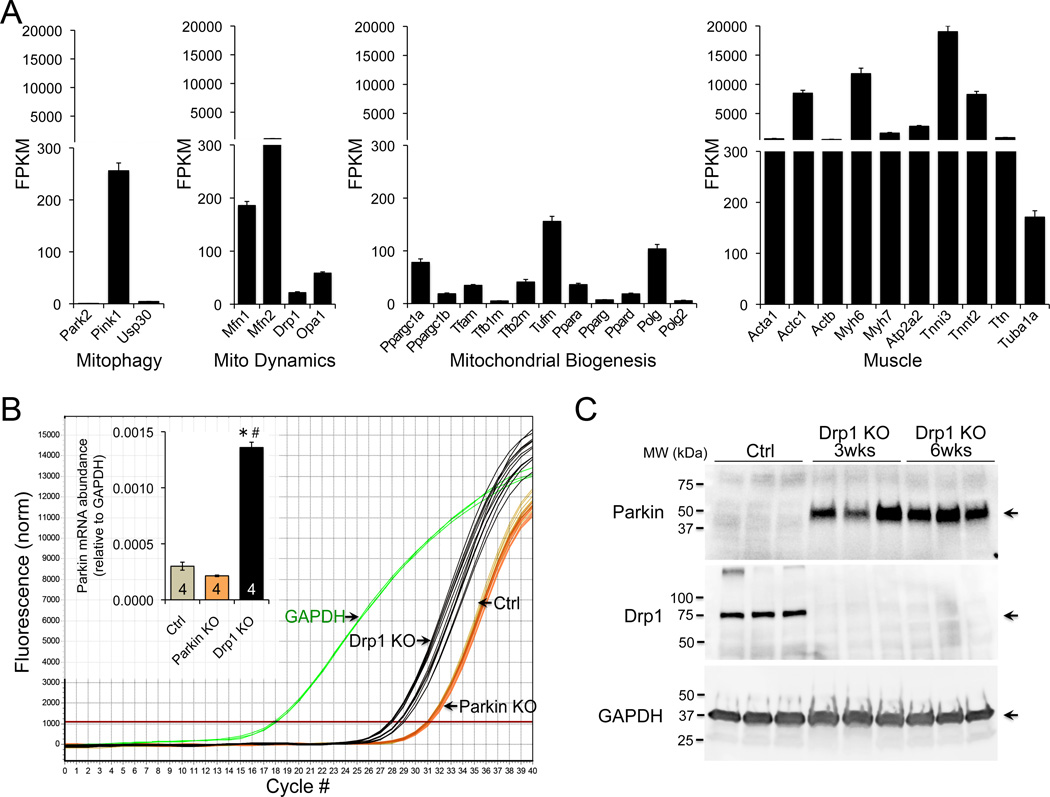
Interrupting mitochondrial fission by cardiac myocyte-specific Drp1 ablation induces cardiomyopathy with loss of mitochondria (Online Figure I) 8. Parkin localization to, and mitophagy of, mitochondria in Drp1-deficient cultured MEFs 8 suggested a role for Parkin. However, Parkin protein was not abundant in normal hearts (Online Figure II) 4 and Parkin mRNA levels equate to fewer than one transcript per cell (Figure 1A). These low levels of Parkin challenge the notion that Parkin causes hyper-mitophagy in cardiac Drp1-deficiency unless Parkin is upregulated therein. Indeed, we observed ~5-fold greater Parkin mRNA levels (Figure 1B), and anti-Parkin immunoblotting revealed massively increased myocardial Parkin (Figure 1C) after Drp1 ablation. Thus, myocardial Parkin protein increases out of proportion to Parkin-specific mRNA in hearts rendered defective for mitochondrial fission.
Figure 1. Parkin expression in normal and Drp1-deficient hearts.
A. RNA Sequencing of Parkin (Park2) and related factors in 25 normal adult mouse hearts. B. RT-qPCR of Parkin mRNA expression in normal, cardiac Parkin knockout (KO), and cardiac Drp1 KO hearts. C. Immunoblot analysis of myocardial Parkin after conditional cardiac myocyte-directed Drp1 ablation; each lane is a different mouse heart.
Activating Parkin-mediated mitophagy is not intrinsically damaging to normal mouse hearts
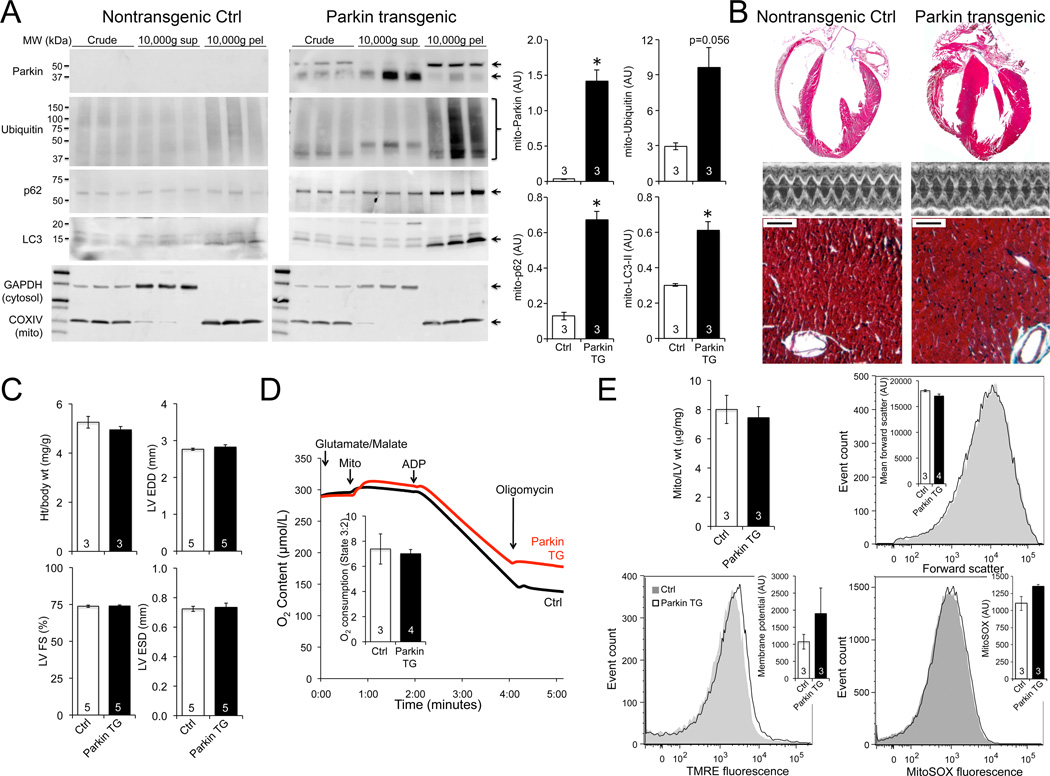
To determine how increased Parkin impacts hearts we transgenically expressed human Parkin in mouse cardiac myocytes. As Parkin evokes mitophagy by translocating to, and poly-ubiquitinating membrane proteins of, mitochondria 12,13, we measured mitochondrial Parkin content and protein ubiquitination. Parkin was plentiful in the mitochondria-enriched 10,000 ×g pellet fraction of Parkin transgenic hearts, and associated with mitochondrial protein poly-ubiquitination (Figure 2A). The autophagosomal protein LC3 and its docking protein p62/SQSTM1 were likewise increased in mitochondria, indicating activation of mitophagy (Figure 2A). When followed to 30 weeks of age there was no evidence for cardiac enlargement, contractile dysfunction (Figures 2B and 2C; Online Figure III), or any adverse mitochondrial effect of Parkin (Figure 2D and 2E). Although heart phenotypes may emerge over greater time, increasing Parkin activated mitophagy without provoking pathology in otherwise normal young adult mouse hearts.
Figure 2. Cardiac Parkin overexpression.
A. Immunoblot analyses of Parkin and mitophagy markers in mitochondria-enriched (10,000 ×g pellet; mito-) and -depleted (10,000 ×g supernatant) cardiac fractions; parallel blots were run and probed simultaneously. B–C. Cardiac phenotyping in 30 week old Parkin transgenic (TG) mice: B. Representative hearts, echocardiograms, and Masson’s trichrome histology; bar scale is 50 µm. C. Quantitative data. D. Isolated mitochondrial respiration. E. Mitochondrial abundance and flow cytometry studies. Grey curve is representative Ctrl; black is cardiac Parkin transgenic.
Parkin ablation has minimal effects on adult mouse hearts
The above results are consistent with observations that Parkin overexpression in other cell types is tolerated, even protective 14. Accordingly, Parkin upregulation cannot be the exclusive cause of dilated cardiomyopathy after cardiac myocyte Drp1 ablation. To test if Parkin upregulation is a contributory factor, while minimizing the potential for compensatory upregulation of Parkin-independent mitophagy pathways that occurs with germ-line Park2 deletion 15, 16, we created cardiac myocyte-specific Parkin knockout mice.
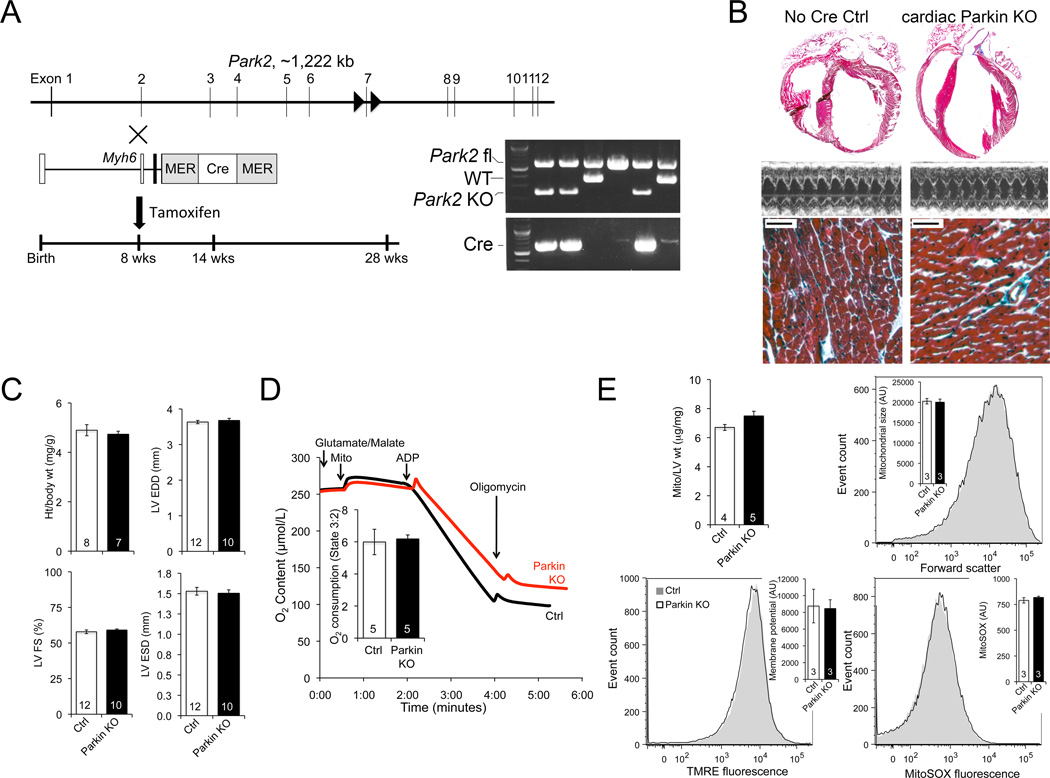
Mice with Lox-P sites flanking exon 7 of the Park2 (Parkin) gene 17 were obtained from Lexicon Pharmaceuticals and bred to myh6-driven MER-Cre-MER (Figure 3A). Tamoxifen was administered to 8 week old mice for cardiac myocyte-specific Park2 recombination. Cardiac Parkin deficient mice exhibited no change in cardiac size, heart weight corrected for body weight, or left ventricular contractile function over 20 weeks (Figures 3B and 3C; Online Figure IV). Mitochondrial appearance, respiration, and abundance were normal (Figures 3D and 3E; Online Figure V). Likewise, flow cytometry of cardiac mitochondria revealed normal organelle size (forward scatter), polarization status (TMRE fluorescence), and ROS production (MitoSOX fluorescence) (Figure 3E). Whereas absence of cardiac Parkin could prove to be detrimental with increasing age, within the current 20 week study it was not.
Figure 3. Cardiac myocyte-specific Parkin ablation.
A. Schematic of ablation strategy; inset shows PCR demonstrating Cre-mediated Park2 gene recombination. B–C. Cardiac phenotyping in 28 week old mice: B. Representative hearts, echocardiograms, and Masson’s trichrome histology; bar scale is 50 µm. C. Quantitative data. D. Isolated mitochondrial respiration. E. Mitochondrial abundance and flow cytometry studies. Grey curve is representative Ctrl; black is cardiac Parkin KO.
Concomitant ablation of Parkin delays the cardiac phenotype caused by Drp1 deficiency
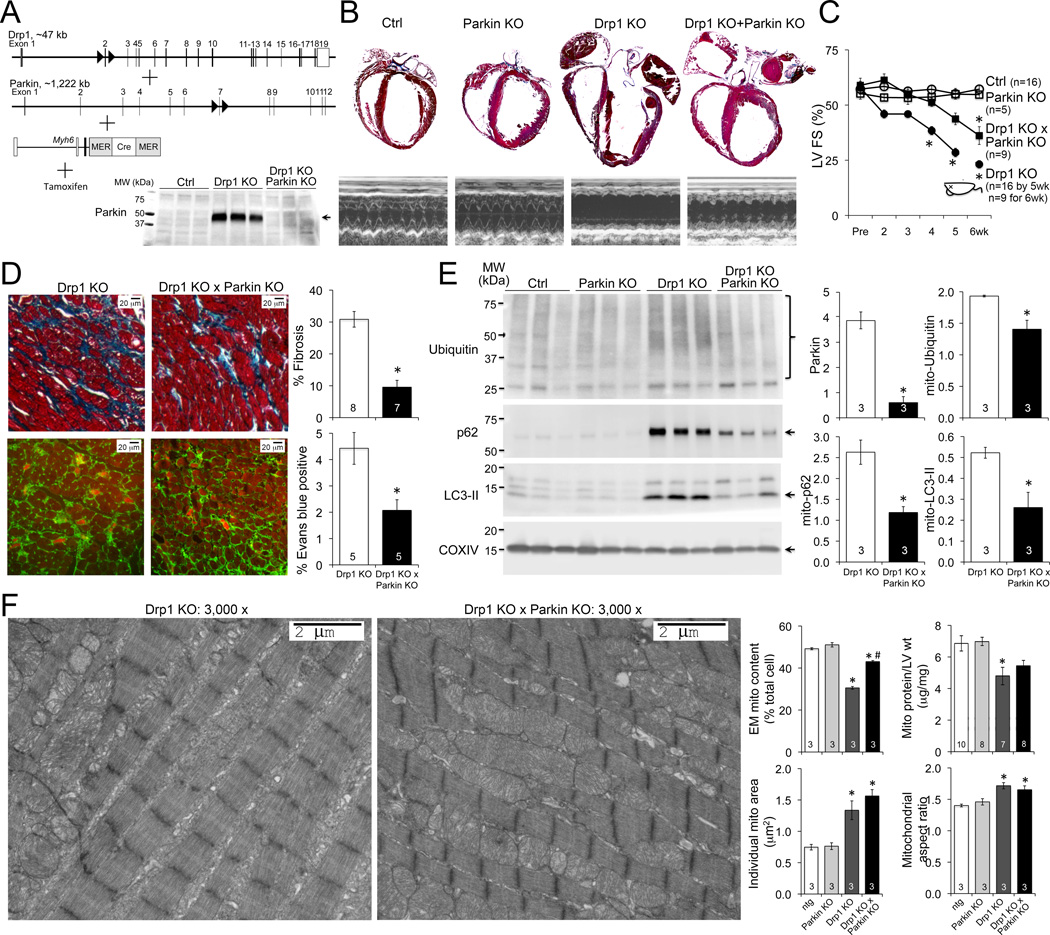
As cardiac Parkin ablation had no deleterious effects on otherwise normal hearts we assessed the scale of Parkin upregulation in Drp1-deficiency by concomitantly deleting the Drp1 and Parkin genes in adult mouse hearts (Figure 4A). As reported 8 Drp1 ablation caused lethal dilated cardiomyopathy. Concurrent Parkin ablation increased 6 week survival of cardiac Drp1-deficient mice, improving contractile function and protecting against adverse ventricular remodeling (Figures 4B and 4C; Online Figure VI), likely by suppressing cardiac myocyte necrosis and replacement fibrosis (Figure 4D). Parkin ablation moderated hyper-mitophagy in Drp1-deficient hearts as assessed by mitochondrial protein ubiquitin content and mitochondria-association of p62/SQSTM1 and LC3-II (Figure 4E). The loss of mitochondria seen late after Drp1 ablation was consequently ameliorated (Figure 4F). As previously described 8, mitochondrial function was normal in Drp1 deficient hearts, and was unaffected by concomitant Parkin deletion (Online Figure VII).
Figure 4. Parkin deficiency delays cardiomyopathy and mitigates mitophagy in Drp1 deficiency.
A. Schematic depiction of combined conditional cardiac Drp1/Parkin deletion strategy; inset shows normalization of Parkin levels in combined KO hearts. B. Representative hearts and M-mode echocardiograms 6 weeks after gene ablation. C. Time-dependent change in LV fractional shortening; dead mouse icon indicates LV FS data for ~50% surviving cardiac Drp1 KO mice at 6 wks. D. Masson’s trichrome (top) and in vivo Evan’s blue (bottom) stained cardiac sections; quantitative data to the right. E. Immunoblotting of mitophagy factors in mitochondrial fractions; quantitative data to the right; each lane is a different mouse heart. F. Representative transmission electron microscopy (EM). Quantitative metrics for mitochondria are to the right. *P<0.05 versus identically treated controls; #P<0.05 versus Drp1 KO.
DISCUSSION
These results help resolve ambiguous results relating to mitochondrial quality control in mammalian hearts. Parkin-mediated mitophagy acts as a stress-reactive pathway to remove damaged mitochondria as originally proposed by Gottlieb 18, but appears unnecessary to routinely maintain mitochondrial quality. Absence of a housekeeper function can explain normal hearts in mice systemically lacking Parkin 11.
Our work shows that Parkin-mediated hyper-mitophagy can contribute to cardiomyopathy, here induced by interrupting Drp1-mediated mitochondrial fission. In a perinatal cardiac Drp1-deficient model Sesaki and colleagues observed mitophagy that was not prevented by germ-line Parkin deletion6. Our results do not address Parkin’s role in perinatal hearts, but strongly implicate Parkin in mitophagy induced by interrupting mitochondrial fission in adult hearts. The cardiac myocyte-specific Parkin-deficient and -overexpressing models developed for this project should prove useful for rigorous interrogations of Parkin-mediated mitophagy and mitochondrial clearance after other forms of cardiac stress, helping to better define the roles of Parkin-dependent and ROS-stimulated Parkin-independent mitochondrial quality control pathways.
These results provide in vivo support for Shirihai’s concept that mitophagy and Drp1 are inextricably linked through the mechanism of asymmetrical mitochondrial fission 19. This has ramifications for ongoing therapeutic efforts directed toward inhibiting mitochondrial fission in cardiac disease 20, as inhibition of Drp1 may concomitantly stimulate Parkin-mediated mitochondrial removal, ultimately reducing mitochondrial content.
Supplementary Material
Novelty and Significance.
What Is Known?
Parkin is highly expressed in hearts.
Parkin-mediated mitophagy eliminates unhealthy mitochondria segregated via asymmetrical mitochondrial fission.
The role of Parkin-mediated mitophagy in hearts with defective mitochondrial fission is controversial.
What New Information Does This Article Contribute?
Parkin is not constitutively expressed at high levels in normal hearts, but is abundant when upregulated after stress.
In otherwise normal young hearts Parkin-mediated mitophagy is not intrinsically pathological.
Parkin-mediated mitophagy is induced and contributes to cardiomyopathy in mitochondrial fission-deficient hearts.
Mitochondrial quality is essential to cardiac homeostasis. Damaged mitochondria can be selectively eliminated via Parkin-mediated mitophagy, but the role of Parkin in hearts is unclear. We showed that Parkin is expressed at very low levels in normal young adult hearts, but was induced in mitochondrial fission-deficient hearts. Forced activation of Parkin-mediated mitophagy was not intrinsically harmful, but it contributed to the cardiomyopathy evoked by mitochondrial fission deficiency. These findings uncover the interdependent relationship between Parkin-mediated mitophagy and mitochondrial fission, sounding a cautionary note for therapeutic efforts directed toward inhibiting mitochondrial fission factor Drp1.
ACKNOWLEDGEMENTS
Parkin floxed allele mice were obtained from, and used under an MTA with, Lexicon Pharmaceuticals.
SOURCES OF FUNDING
Supported by NIH HL59888 (GWD) and HL128071 (GWD and RNK) and an American Heart Association predoctoral fellowship award (MS).
Nonstandard Abbreviations and Acronyms
- Drp1
Dynamin-related protein 1
- LC3
microtubule-associated protein 1 light chain 3
- Park2
Parkin gene
- SQSTM1
Sequestosome 1
- TMRE
tetramethylrhodamine ethyl ester
- MER
modified estrogen receptor
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Song M, Dorn GW., 2nd Mitoconfusion: noncanonical functioning of dynamism factors in static mitochondria of the heart. Cell Metab. 2015;21:195–205. doi: 10.1016/j.cmet.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorn GW, 2nd, Kitsis RN. The mitochondrial dynamism-mitophagy-cell death interactome: multiple roles performed by members of a mitochondrial molecular ensemble. Circ Res. 2015;116:167–182. doi: 10.1161/CIRCRESAHA.116.303554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 4.Song M, Chen Y, Gong G, Murphy E, Rabinovitch PS, Dorn GW., 2nd Super-suppression of mitochondrial reactive oxygen species signaling impairs compensatory autophagy in primary mitophagic cardiomyopathy. Circ Res. 2014;115:348–353. doi: 10.1161/CIRCRESAHA.115.304384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Dorn GW., 2nd PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA, Chen W, Hoke A, Dawson VL, Dawson TM, Gabrielson K, Kass DA, Iijima M, Sesaki H. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J. 2014;33:2798–2813. doi: 10.15252/embj.201488658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, Abdellatif M, Sadoshima J. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res. 2015;116:264–278. doi: 10.1161/CIRCRESAHA.116.303356. [DOI] [PubMed] [Google Scholar]
- 8.Song M, Mihara K, Chen Y, Scorrano L, Dorn GW., 2nd Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab. 2015;21:273–285. doi: 10.1016/j.cmet.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Von Coelln R, Thomas B, Savitt JM, Lim KL, Sasaki M, Hess EJ, Dawson VL, Dawson TM. Loss of locus coeruleus neurons and reduced startle in parkin null mice. Proc Natl Acad Sci U S A. 2004;101:10744–10749. doi: 10.1073/pnas.0401297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez FA, Palmiter RD. Parkin-deficient mice are not a robust model of parkinsonism. Proc Natl Acad Sci U S A. 2005;102:2174–2179. doi: 10.1073/pnas.0409598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubli DA, Zhang X, Lee Y, Hanna RA, Quinsay MN, Nguyen CK, Jimenez R, Petrosyan S, Murphy AN, Gustafsson AB. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J Biol Chem. 2013;288:915–926. doi: 10.1074/jbc.M112.411363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin SM, Youle RJ. PINK1- and Parkin-mediated mitophagy at a glance. J Cell Sci. 2012;125:795–799. doi: 10.1242/jcs.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rana A, Rera M, Walker DW. Parkin overexpression during aging reduces proteotoxicity, alters mitochondrial dynamics, and extends lifespan. Proc Natl Acad Sci U S A. 2013;110:8638–8643. doi: 10.1073/pnas.1216197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piquereau J, Godin R, Deschenes S, Bessi VL, Mofarrahi M, Hussain SN, Burelle Y. Protective role of PARK2/Parkin in sepsis-induced cardiac contractile and mitochondrial dysfunction. Autophagy. 2013;9:1837–1851. doi: 10.4161/auto.26502. [DOI] [PubMed] [Google Scholar]
- 16.Bhandari P, Song M, Chen Y, Burelle Y, Dorn GW., 2nd Mitochondrial contagion induced by Parkin deficiency in Drosophila hearts and its containment by suppressing mitofusin. Circ Res. 2014;114:257–265. doi: 10.1161/CIRCRESAHA.114.302734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson's disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C, Andres AM, Ratliff EP, Hernandez G, Lee P, Gottlieb RA. Preconditioning involves selective mitophagy mediated by Parkin and p62/SQSTM1. PLoS One. 2011;6:e20975. doi: 10.1371/journal.pone.0020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Twig G, Elorza A, Molina AJ, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.