Summary
Background
Methadone is an effective treatment for opioid dependence. When people who are receiving methadone maintenance treatment for opioid dependence are incarcerated in prison or jail, most US correctional facilities discontinue their methadone treatment, either gradually, or more often, abruptly. This discontinuation can cause uncomfortable symptoms of withdrawal and renders prisoners susceptible to relapse and overdose on release. We aimed to study the effect of forced withdrawal from methadone upon incarceration on individuals’ risk behaviours and engagement with post-release treatment programmes.
Methods
In this randomised, open-label trial, we randomly assigned (1:1) inmates of the Rhode Island Department of Corrections (RI, USA) who were enrolled in a methadone maintenance-treatment programme in the community at the time of arrest and wanted to remain on methadone treatment during incarceration and on release, to either continuation of their methadone treatment or to usual care—forced tapered withdrawal from methadone. Participants could be included in the study only if their incarceration would be more than 1 week but less than 6 months. We did the random assignments with a computer-generated random permutation, and urn randomisation procedures to stratify participants by sex and race. Participants in the continued-methadone group were maintained on their methadone dose at the time of their incarceration (with dose adjustments as clinically indicated). Patients in the forced-withdrawal group followed the institution’s standard withdrawal protocol of receiving methadone for 1 week at the dose at the time of their incarceration, then a tapered withdrawal regimen (for those on a starting dose >100 mg, the dose was reduced by 5 mg per day to 100 mg, then reduced by 3 mg per day to 0 mg; for those on a starting dose ≤100 mg, the dose was reduced by 3 mg per day to 0 mg). The main outcomes were engagement with a methadone maintenance-treatment clinic after release from incarceration and time to engagement with methadone maintenance treatment, by intention-to-treat and as-treated analyses, which we established in a follow-up interview with the participants at 1 month after their release from incarceration. Our study paid for 10 weeks of methadone treatment after release if participants needed financial help. This trial is registered with ClinicalTrials.gov, number NCT01874964.
Findings
Between June 14, 2011, and April 3, 2013, we randomly assigned 283 prisoners to our study, 142 to continued methadone treatment, and 141 to forced withdrawal from methadone. Of these, 60 were excluded because they did not fit the eligibility criteria, leaving 114 in the continued-methadone group and 109 in the forced-withdrawal group (usual care). Participants assigned to continued methadone were more than twice as likely than forced-withdrawal participants to return to a community methadone clinic within 1 month of release (106 [96%] of 110 in the continued-methadone group compared with 68 [78%] of 87 in the forced-withdrawal group; adjusted hazard ratio [HR] 2·04, 95% CI 1·48–2·80). We noted no differences in serious adverse events between groups. For the continued-methadone and forced-withdrawal groups, the number of deaths were one and zero, non-fatal overdoses were one and two, admissions to hospital were one and four; and emergency-room visits were 11 and 16, respectively.
Interpretation
Although our study had several limitations—eg, it only included participants incarcerated for fewer than 6 months, we showed that forced withdrawal from methadone on incarceration reduced the likelihood of prisoners re-engaging in methadone maintenance after their release. Continuation of methadone maintenance during incarceration could contribute to greater treatment engagement after release, which could in turn reduce the risk of death from overdose and risk behaviours.
Introduction
The illicit use of heroin and, increasingly in the past decade, misuse of prescription opioid analgesics are serious medical and public health problems.1,2 Methadone maintenance is a highly effective treatment for opioid addiction and has been included in WHO’s Model List of Essential Medicines since 2005.3 During the past 50 years, methadone maintenance treatment for opioid dependence has proved to reduce illicit opioid use4 and its negative results, including crime,5 mortality,6 overdose, and HIV risk behaviours.8 The natural history of opioid dependence, especially in the era of the so-called war on drugs, often results in incarceration.9 Once individuals become associated with the criminal justice system and prison, especially when the situation encompasses the chronic relapsing disease of addiction, they typically continue to be reincarcerated many times, even after criminal activity has ceased or has reduced substantially.10 In the USA, about 10% of people receiving methadone maintenance treatment are incarcerated annually.11 With more than 300 000 citizens receiving methadone treatment,12 this estimate equates to about 30 000 individuals per year who enter prison or jail receiving methadone. On incarceration in the USA, nearly 90% of people on prescribed methadone are forced to stop or taper off this treatment.11 This pervasive practice of summarily discontinuing an approved and effective therapy in correctional settings seems to be unique among medical treatments.
Discontinuation of metha done—by definition an interruption in treatment—often occurs in pre-trial detention, before determination of guilt or innocence, and results in the predictable discomfort of withdrawal symptoms. Methadone with drawal compounds psychological distress and has been implicated as a suicide trigger in the initial weeks of incarceration.13,14 Cessation of methadone maintenance also results in loss of opioid tolerance. Released prisoners are especially susceptible to drug-related death, with the risk of fatal overdose in the first 2 weeks after release, which is three to eight times greater than that during other periods at liberty,15 and 129 times higher than in the general population.16 An absence of opioid tolerance is a probable contributor to this increase in risk.15 The implications of forced methadone withdrawal in incarcerated prisoners have never been studied in a randomised trial. Therefore, our aim was to assess the effects of continued methadone maintenance versus forced withdrawal from methadone in incarcerated prisoners on re-engagement with community metha done maintenance treatment in the first month after release from incarceration.
Methods
Study design and participants
We did a randomised, open-label, controlled trial in the Rhode Island Department of Corrections, RI, USA. This study was approved by the Institutional Review Board (including a prisoner representative) of the Miriam Hospital in Providence, RI, and the Rhode Island Department of Corrections Medical Research Advisory Group. Because the study was done with prisoners, a vulnerable population, the study was also reviewed and approved by the US Federal Office for Human Research Protections. Participants were male and female inmates of the Rhode Island Department of Corrections. We recruited inmates receiving methadone treatment through the institution’s existing staff, with information sheets and word of mouth. To be eligible, inmates had to be enrolled in a Rhode Island methadone maintenance-treatment programme at the time of incarceration, be willing to be randomly assigned to either study group, speak English or Spanish, and want to remain on methadone maintenance treatment during incarceration and after release. Participants who had already started a tapered withdrawal regimen were ineligible to enrol in this study because of concern about possible coercion. Participants who had already started a tapered withdrawal regimen were ineligible to enrol in this study because of concern that the physical discomfort of already-started withdrawal symptoms might constitute undue influence, and make them more likely to give consent to participate in the study as a way to get methadone and alleviate the withdrawal symptoms. Pregnant women and inmates with HIV infection were excluded because the policy of the Rhode Island Department of Corrections is to offer to maintain these inmates on methadone. Participants were informed that if they were randomly assigned to continue methadone but had to receive disciplinary action resulting in segregation, they would be transferred to the standard forced-withdrawal protocol as per the institution’s mandate.
Participants were eligible for inclusion only if they were to be incarcerated for more than 1 week and less than 6 months; however, identification of whom would meet this criterion at the time of enrolment was not always possible. All participants gave written informed consent.
Randomisation and masking
After enrolment, we randomly assigned participants (1:1) using a computer-generated random permutation to either continued methadone maintenance treatment or usual care (forced tapered withdrawal from methadone). We did the randomisation procedure independently of the enrolment and consent processes. Field staff enrolled participants at the Department of Corrections. After obtaining participants’ consent, the field staff member returned to the study office, where the random assignment was obtained from a separate staff member who had no direct contact with participants. The same field staff member responsible for enrolling the participant was responsible for follow-up in the community after their release. More men than women were incarcerated at the time of our study, and few patients of racial minorities were in methadone clinics in Rhode Island;17 therefore, we used urn randomisation procedures to stratify individuals on the basis of sex and race. The advantages of urn randomisation are that it can effectively balance groups even with several stratifying covariates, with a low risk of experimenter bias or manipulation.18
Procedures
Participants in the continued-methadone group were maintained on their methadone dose at the time of incarceration, with dose adjustments made as clinically indicated. Participants receiving a stable dose were typically continued on that same dose. For participants whose doses were being adjusted at the time of incarceration, or with symptoms caused by doses that were too low or too high, adjustments were made in accordance with standard practices, usually in conjunction with their home clinic.19 Participants who were assigned to tapered forced withdrawal from methadone completed the institution’s standard protocol of continuation of methadone at their entry dose for week 1 of incarceration, then a tapered withdrawal regimen (a starting dose of >100 mg was reduced by 5 mg per day to 100 mg, then reduced by 3 mg per day to 0 mg; a starting dose of ≤100 mg was reduced by 3 mg per day to 0 mg). Participants in the forced-withdrawal group could therefore still be receiving a daily dose of methadone at the time of release, dependent on the length of their incarceration and starting dose. Before their release, all participants met with study staff who assisted them with arranging transportation and scheduling of their first methadone clinic appointment after release. For participants who did not have health coverage or who had insufficient funds to pay for their treatment, the study paid for 10 weeks of post-release methadone treatment. To our knowledge, such financial support is not the usual standard of care anywhere in the USA.
Outcomes
The main outcomes were engagement with a methadone maintenance-treatment clinic after release from incarceration and time to engagement with methadone maintenance treatment. Other outcomes were use of opioids or use of any other illicit drug use, entry to a drug treatment programme, HIV risk behaviours, reincarceration, and health-care costs. The adverse events measured were the occurrence of death, overdoses, hospital admissions, and visits to a hospital emergency room.
Statistical analysis
We planned to enrol 450 participants in the trial to achieve statistical power of 0·80 (α error=0·05, two-tailed) and detect an effect size of 0·30. To test associations between receipt of methadone while incarcerated and treatment entry after release, we did intention-to-treat and as-treated analyses. The intention-to-treat analysis included all eligible participants in the study as randomised. The as-treated analysis included all eligible participants in the study by their methadone status on the day before their release, either receiving any dose of methadone or not receiving methadone. This analysis was done because participants in the forced-withdrawal group could still be receiving some amount of methadone just before their release if they had not yet completed the department’s withdrawal protocol.
At enrolment, all participants gave written consent for the research team to access their methadone records at community clinics to assess post-release methadone treatment engagement. Data for time to re-enrolment in community methadone programmes were extracted from clinic records. We assessed substance use with the Addiction Severity Index20 and Timeline Follow Back method for 1 month data about drug relapses. Additionally, we obtained data for HIV risk behaviours, treatment for misuse of opioids or other substances, health-care use, and overdose. Other outcomes were measured through participant self-reports in face-to-face interviews.
For both the intention-to-treat and as-treated analyses, we plotted Kaplan-Meier curves of the primary outcome, the time to presentation at a methadone treatment clinic after release from incarceration, and applied the log-rank test of equality. We used Cox proportional hazards modelling to further explore predictors of post-release treatment entry. We assessed each predictor variable for its bivariate association with treatment entry, and variables with p<0·20 were used in the multivariable models.21 We did not do any further reduction of covariates to allow for comparison between the intention-to-treat and as-treated models. We tested the proportional hazards assumption for each variable in the multivariable model by including an interaction between the variable and log (time) in the model. No variables in either multivariable model violated the proportional hazards assumption.
We analysed secondary outcomes with the χ2 test to assess for differences between study groups.
With a health-care payer perspective, we based costs on drug administration fees for methadone and direct medical care costs for physician, and on ambulatory, emergency, and hospital care. We used the Mann- Whitney-Wilcoxon test for non-parametric data to test for differences in cost. The timeline was 30 days to match the primary clinical outcome. We calculated the total care costs and the incremental cost-effectiveness ratio with effectiveness as the proportion of individuals enrolled in methadone maintenance treatment post-release within 30 days converted to quality-adjusted life years: cost of forced withdrawal minus cost of methadone continuation divided by forced withdrawal from methadone on incarceration minus continuation with methadone on incarceration. We did a sensitivity analysis, taking into account societal costs and savings.
To estimate the uncertainty in the incremental cost-effectiveness, we generated a bootstrap estimate of the incremental cost-effectiveness ratio.22–24 Incremental cost-effectiveness ratios that were less than US$50 000–100 000 per quality-adjusted life-year saved were thought to be cost effective (appendix). Analyses were done with the Stata 13 and SAS 9.2 programmes.
The study was periodically reviewed by a data safety monitoring board every 6 months for the first 2 years of recruitment, then once per year until the study ended. This trial is registered with ClinicalTrials.gov, number NCT01874964.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
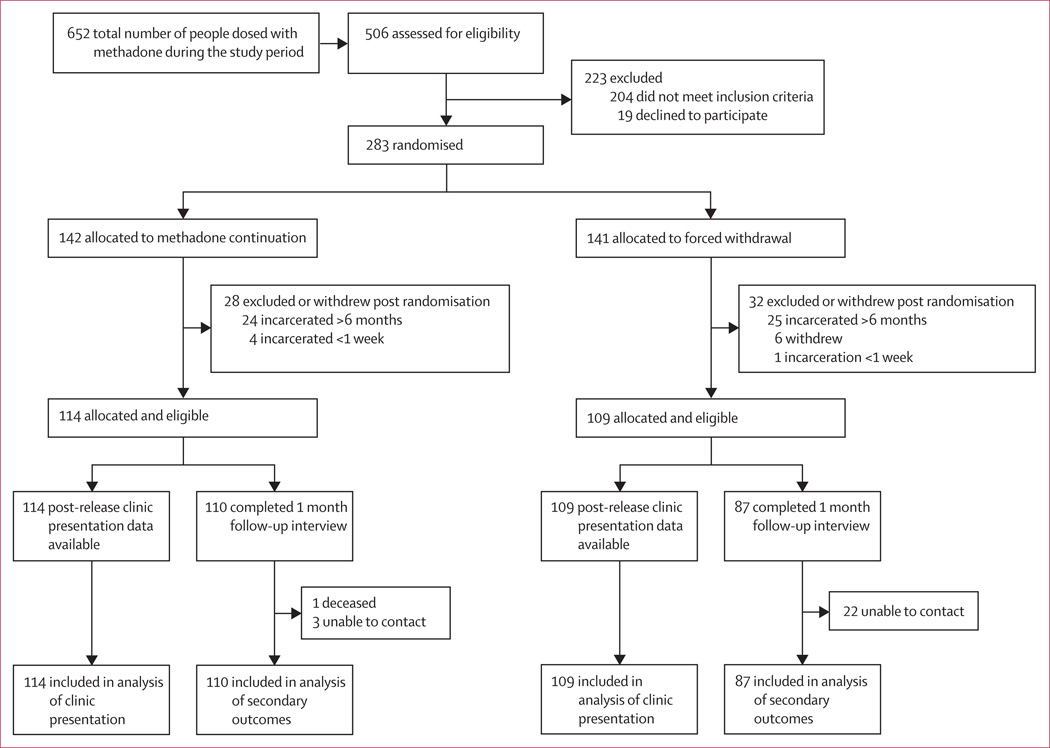
Between June 14, 2011, and April 3, 2013, 652 inmates were given methadone at the Rhode Island Department of Corrections (figure 1). Of these, 506 (78%) were assessed for participation in the trial, and 283 of them were randomly assigned, 142 to continued methadone and 141 to forced withdrawal from methadone. 28 participants from the continued-methadone group, and 32 from the forced-withdrawal group, were excluded after random assignment because they did not fit the eligibility criteria, leaving 114 participants in the continued-methadone group and 109 in the forced-withdrawal group. Table 1 shows participant details in the two groups. Overall, participants were mostly male and either white or non-Hispanic. We noted no differences between groups.
Figure 1. Trial profile.
Table 1.
Baseline characteristics of patients
| Continued methadone (n=114) |
Forced withdrawal (n=109) |
Total (n=223) |
|
|---|---|---|---|
| Sex | |||
| Male | 87 (76%) | 86 (79%) | 173 (78%) |
| Female | 27 (24%) | 23 (21%) | 50 (22%) |
| Ethnic origin | |||
| White | 93 (81%) | 88 (81%) | 181 (81%) |
| Black | 3 (3%) | 6 (6%) | 9 (4%) |
| Other | 18 (16%) | 15 (14%) | 33 (15%) |
| Non-Hispanic | 97 (85%) | 95 (87%) | 192 (86%) |
| Hispanic | 17 (15%) | 14 (13%) | 31 (14%) |
| Age at baseline (years) | 33 (8·0) 30 (27–54) |
36 (8·7) 33 (29–40) |
34 (8·4) 32 (27–40) |
| Number of years in education | |||
| Did not finish high school | 48 (42%) | 41 (37%) | 89 (40%) |
| Finished high school | 41 (36%) | 40 (37%) | 81 (36%) |
| College or higher education | 25 (22%) | 28 (26%) | 53 (24%) |
| Self-reported positive hepatitis C status | 36 (32%) | 48 (44%) | 84 (38%) |
| Duration of incarceration (days) | 56 (47) | 56 (42) | 56 (45) |
| 42 (17–76) | 45 (16–80) | 44 (17–78) | |
| Methadone use (weeks) | 156 (164); n=112 104 (28–224) |
239 (280); n=109 156 (52–312) |
197 (232); n=221 112 (32–260) |
| Methadone dose | |||
| Most recent methadone dose (mg) | 92 (52); n=111 80 (57–115) |
95 (67); n=106 80 (51–110) |
93 (60); n=217 80 (55–110) |
| Maintenance dose (mg) | 98 (47); n=94 87·5 (60–115) |
93 (49); n=89 80 (60–110) |
96 (48); n=183 80 (60–115) |
| Detox status before incarceration* | 6 (5%) | 12 (11%) | 18 (8%) |
| Drug use | |||
| Heroin use (years) | 8 (7); n=97 6 (2–11) |
9 (7); n=97 8 (3–12) |
8 (7); n=194 7 (3–12) |
| Use of other opioids (years) | 8 (7); n=82 5 (2–10) |
8 (5); n=67 6 (3–10) |
8 (6); n=149 6 (3–10) |
| Previous use of injectable drugs | 88 (77%) | 91 (86%) | 179 (80%) |
| Addiction Severity Index drug subscale score at baseline | 0·23 (0·13) 0·22 (0·10–0·33) |
0·27 (0·13) 0·28 (0·15–0·38) |
0·25 (0·14) 0·25 (0·13–0·36) |
Data are n (%), mean (SD), or median (IQR).
Participants with a clinical status of detox before their incarceration were undergoing methadone withdrawal.
For the primary outcome of post-release methadone treatment entry, administrative data were available for all participants. 88% of participants attended a follow-up interview 1 month after their release. The follow-up rate was higher in the methadone continuation group (96%) than in the forced-withdrawal group (80%); p=0·0003. Of participants assigned to continued methadone, 111 (97%) of 114 attended a community methadone clinic within 1 month of release, compared with 77 (71%) of 109 of those assigned to forced withdrawal (p<0·0001). Participants assigned to continued methadone were more than twice as likely than forced-withdrawal participants to return to a community methadone clinic within 1 month of release (106 [96%] of 110 in the continued-methadone group compared with 68 [78%] of 87 in the forced-withdrawal group (table 2).
Table 2.
Cox proportional hazards models of time to clinic presentation
| Single covariate HR (95% CI) | Multifactorial HR (95% CI) | ||
|---|---|---|---|
| Intention to treat | As treated | ||
| Continuing methadone in the intention-to-treat population | 2·22 (1·62–3·03) | 2·04 (1·48–2·80) | ·· |
| Receiving methadone maintenance treatment before release in the as-treated population | 6·83 (4·30–10·85) | ·· | 6·61 (4·00–10·91) |
| Sex | |||
| Male | 0·91 (0·65–1·23) | ·· | ·· |
| Female | Reference | ·· | ·· |
| Race | |||
| White | Reference | ·· | ·· |
| Black or African-American | 0·56 (0·23–1·37) | ·· | ·· |
| Hispanic ethnic origin | 1·05 (0·70–1·58) | ·· | ·· |
| Other | 1·05 (0·71–1·56) | ·· | ·· |
| Age* | 0·94 (0·86–1·03) | 1·01 (0·91–1·12) | 0·98 (0·88–1·09) |
| Duration of incarceration† | 0·93 (0·89–0·97) | 0·94 (0·90–0·97) | 1·01 (0·97–1·05) |
| Years of heroin use* | 0·91 (0·82–1·01) | 0·98 (0·86–1·11) | 0·99 (0·87–1·12) |
| Addiction Severity Index drug subscale score at baseline | 1·22 (0·88–1·69) | ·· | ·· |
| Detox status before incarceration‡ | 0·57 (0·31–1·06) | 0·74 (0·39–1·39) | 0·83 (0·44–1·56) |
| Methadone dose before incarceration§ | 1·02 (0·99–1·04) | 1·02 (0·99–1·04) | 1·01 (0·99–1·03) |
| Self-reported positive hepatitis C status | 0·82 (0·61–1·11) | ·· | ·· |
Models done in intention-to-treat and as-treated populations. Race classification ‘other’ includes participants of Asian, Native American, and many other racial classifications, those who reported Hispanic ethnic origin, and those who did not endorse any racial classification. HR=hazard ratio.
Hazard ratio for a 5 year increase in predictor variable.
Hazard ratio for a 10 day increase in duration of incarceration.
Participants with a clinical status of detox before incarceration were completing methadone withdrawal before incarceration.
Hazard ratio for a 10 mg increase in methadone dose received before incarceration.
Because of the nature of the withdrawal protocol, 45 participants randomised to forced withdrawal were released before completing the withdrawal programme (and therefore received methadone up until the day of their release, table 3). Furthermore, three participants in the continued-methadone group completed methadone withdrawal before release. Two were removed from methadone treatment by the institution for disciplinary reasons, and one participant chose to be withdrawn. When data were analysed by methadone status at release (receiving or not receiving methadone), 156 (100%) of those receiving methadone at that time presented to a community methadone clinic within 1 month of release, compared with 32 (48%) of 67 not receiving methadone (p<0·0001).
Table 3.
Clinical outcomes measured at 1 month after release from incarceration.
| Continued methadone |
Forced methadone withdrawal |
p value | |
|---|---|---|---|
| Dosed with methadone on day before release | 111 (97%) of 114 | 45 (41%) of 109 | 0·0001 |
| Drug use at 1 month | |||
| Opioids | 9 (8%) of 110 | 16 (18%) of 87 | 0·033 |
| Any other drugs | 70 (64%) of 110 | 66 (76%) of 87 | 0·065 |
| Drug treatment | |||
| Detox programme | 2 (2%) of 110 | 1 (1%) of /87 | 0·703 |
| Prescribed buprenorphine | 1 (1%) of 110 | 2 (2%) of 87 | 0·429 |
| Outpatient drug-free programme | 8 (7%) of 110 | 11 (13%) of 87 | 0·205 |
| Residential treatment programme | 13 (12%) of 110 | 5 (6%) of 87 | 0·142 |
| In methadone treatment programme | 106 (96%) of 110 | 68 (78%) of 87 | 0·0001 |
| In any treatment programme | 107 (97%) of 110 | 73 (84%) of 87 | 0·0001 |
| HIV risk behaviours | |||
| Use of injectable illegal drugs | 19 (17%) of 109 | 28 (32%) of 87 | 0·016 |
| Unprotected sex | 72 (91%) of 79 | 62 (74%) of 84 | 0·160 |
| Reincarcerated | 12 (11%) of 109 | 8 (9%) of 87 | 0·677 |
| Adverse events | |||
| Deaths* | 1 (1%) of 114 | 0 (0%) of 109 | ·· |
| Overdoses (non-fatal) | 1 (1%) of 110 | 2 (2%) of 86 | 0·423 |
| Admissions to hospital | 1 (1%) of 110 | 4 (5%) of 87 | 0·102 |
| Visits to emergency room | 11 (10%) of 110 | 16 (18%) of 87 | 0·089 |
Analyses were done in Stata and p values were calculated using the Pearson χ2 test.
One death occurred (a fatal overdose; details in main text). All results are based on self-reports except methadone dosing before release, and the fatality.
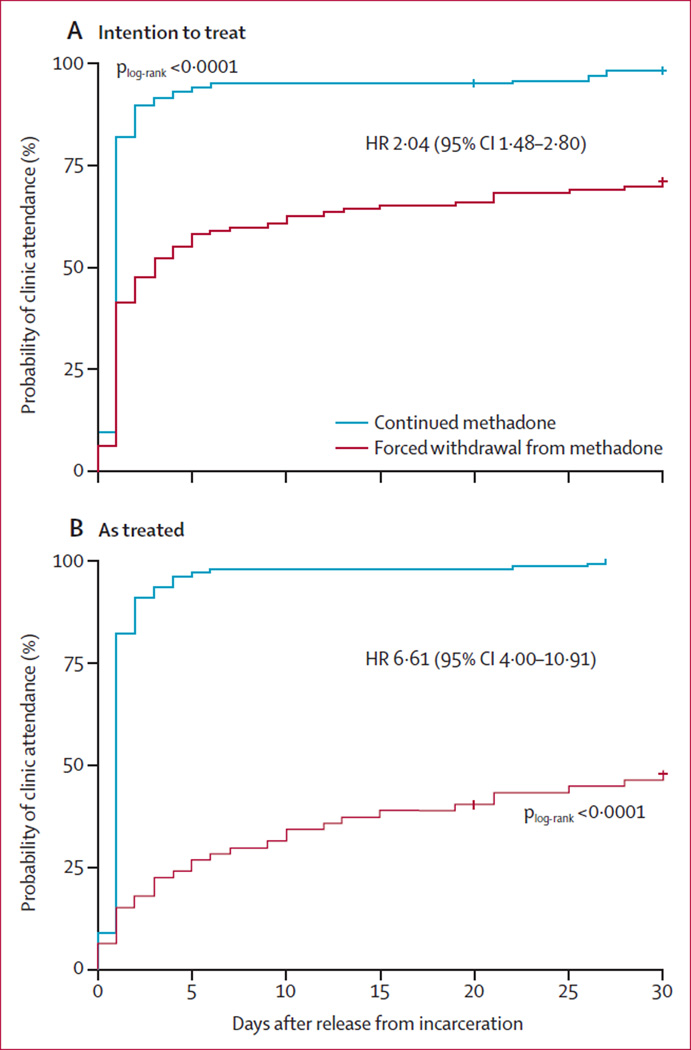
Participants assigned to continued methadone were significantly more likely than those assigned to forced withdrawal to attend methadone treatment on release from incarceration (figure 2A, plog-rank<0·0001). Each additional 10 days of incarceration was associated with a 6% decrease in the likelihood of attending a methadone treatment clinic after release (). Compared with the intention-to-treat analysis, the effect of receiving methadone while incarcerated on post-release treatment entry was increased in the as-treated analysis (figure 2B, p<0·0001). Participants who received methadone up until their day of release were nearly seven times more likely than those not receiving methadone to get methadone treatment after their release (table 2). Receipt of methadone before release was the only factor associated with post-release treatment entry (table 3).
Figure 2. Probability of attending a methadone clinic in (A) the intention-totreat and (B) the as-treated populations.
Data are for 1 month follow-up after particpants’ release from incarceration.
More than half of participants reported any drug use in the month after release (table 3), and opioid use was higher in participants in the forced-withdrawal group than in the continued-methadone group. In both groups, the most common method of treatment for drug use was methadone. Injected drug-related HIV risk behaviours occurred more frequently in those assigned to forced withdrawal. Self-reported occurrences of unprotected sex were high in both groups, whereas self-reported reincarceration was slightly lower in the forced-withdrawal group than in the methadone group.
Participants self-reported three non-fatal overdoses in the first month after their release, one in the continued-methadone group and two in the forced-withdrawal group (table 3). Similar numbers of emergency room visits took place in both groups. One participant from the continued-methadone group died 12 days after release from incarceration from an overdose (intoxication from cocaine, methadone, and quetiapine). This participant had attended the methadone clinic after release but had not presented for dosing for 9 days before death. Although rates of death from overdose are higher straight after release from incarceration than at other times, the total number in our study was not more than that noted in other similar studies. No unexpected adverse events occurred.
Continued-methadone treatment resulted in higher methadone treatment costs that were offset by savings in costs for physician and medical care after release, resulting in a reduced 30 day total cost (table 4). Because continued methadone treatment during incarceration also resulted in a greater probability of attendance at a methadone clinic after release, it dominated in deterministic analyses by being less expensive and more effective than forced withdrawal. The sensitivity analysis showed that continued-methadone treatment instead of forced withdrawal reduced costs by $19 per individual with a 21% likelihood of being cost saving, and was optimum for societal willingness to pay thresholds of more than $70 000 on the cost-effectiveness analysis frontier. When we incorporated societal costs (but excluded savings from avoiding HIV or transmission of viral hepatitis), continued-methadone treatment reduced costs by $1632 per individual, with a 47% likelihood of being cost saving, and was always optimum for the cost-effectiveness analysis frontier in the sensitivity analysis.
Table 4.
Cost-associated outcomes measured at 1 month after release from incarceration
| Continued methadone (US$) |
Forced withdrawal from methadone (US$) |
p value* | |
|---|---|---|---|
| Intention to treat | |||
| Methadone treatment† | $362 | $225 | 0·0001 |
| Physician‡ | $6·60 | $8·80 | 0·793 |
| Medical care§ | $211 | $372 | 0·894 |
| Total | $609 | $637 | 0·0001 |
| Treatment as received on release | |||
| Methadone treatment† | $403 | $147 | 0·0001 |
| Physician‡ | $6·81 | $9·65 | 0·388 |
| Medical care§ | $257 | $365 | 0·420 |
| Total | $667 | $521 | 0·0001 |
Mann-Whitney-Wilcoxon test for non-parametric data.
Costs reported by the centre for methadone-dispensation costs.
Reimbursement from US Medicaid.
Estimates for costs from the hospital accounting system (not charges).
Discussion
Our study shows that prisoners receiving any methadone before release were seven times more likely than their untreated peers to present to a community methadone clinic within 30 days of release from incarceration. We also showed that forced withdrawal of methadone in short-term incarceration is associated with delays or prevention of re-engagement in methadone treatment after release from incarceration (panel).
The design of our study was complicated because we could not control the duration of incarceration, and thus many (41%) of participants who were assigned to stop methadone were released before completing the forced withdrawal programme. In Rhode Island, the standard practice is to gradually taper methadone; however, in most US jurisdictions, methadone is abruptly stopped on incarceration, which might lead to an even greater effect for those incarcerated for shorter times. Our results of the as-treated analysis lend support to this theory.
The forced withdrawal of methadone on incarceration and decrease in re-engagement in the community are of particular concern because of the heightened risk of death in the first weeks after release from incarceration.15 Cohort studies show that receipt of opioid pharmacotherapies in correctional settings and after release significantly reduces mortality both in custody and after release.40,41 Additionally, methadone during incarceration is associated with reduced drug use25 and diminished drug-related HIV risk behaviours.42 Continuation of methadone from incarcerated settings into the community has been associated with a reduced risk of reincarceration.43 Research from our group and others39,44 has shown that initiation of methadone during incarceration is also associated with improved engagement in methadone care after release. Therefore, to force prisoners and detainees who are enrolled in methadone maintenance programmes to withdraw from treatment runs counter to a large and methodologically rigorous body of evidence showing the public health and safety benefits associated with methadone maintenance treatment in correctional settings.
We noted that continued methadone treatment during incarceration resulted in reduced medical costs in the first 30 days after release and saved costs in a deterministic analysis, compared with forced withdrawal of methadone. The cost-effectiveness frontier analysis suggests that continued methadone would be preferred over the range of well accepted willingness-to-pay thresholds. This finding provides further justification for a change in policy to allow continued methadone maintenance on incarceration. Despite the need to assess the “efficacy of substitution drugs within the criminal justice system”,45 to our knowledge, no similar economic analysis has examined forced withdrawal versus continued methadone in the criminal justice system. For comparison, Connock and colleagues45 reported that methadone maintenance versus no drug therapy in the community had an incremental cost-effectiveness of ℒ13 697 per quality-adjusted life-year gained, and Barnett46 reported an incremental cost-effectiveness ratio of $5915 per life-year gained. Another study47 has also shown the cost benefits of continued maintenance therapy after release in terms of reduced mortality.
Forced withdrawal of methadone treatment in correctional settings is unusual in developed countries. In most of western Europe, the UK, Canada, and most Australian jurisdictions, people entering correctional facilities while receiving prescribed opioid pharmacotherapies are allowed to continue methadone while incarcerated, and often could start such treatment during incarceration if it is clinically indicated.48,49 Such an approach is in accordance with the internationally recognised principle of equivalence of care,48,50 which states that incarcerated people are entitled to the same standard of health care as is available in the surrounding community. Furthermore, in the USA, to not provide medically necessary care is regarded as cruel and unusual punishment in violation of the US Constitution, and, although rare, some correctional jurisdictions have had to pay legal settlements to individuals involuntarily withdrawn from methadone maintenance treatment.51
Our sample size was limited by the substantial challenges in initiating and undertaking this study, which is not uncommon for research in correctional settings.52 In addition to the inability to control the length of incarceration, this study was done in a single institution in a state where people in methadone maintenance programmes are predominantly white.17 Both are factors that might restrict the generalisability of our results. For obvious reasons this study included only patients who wanted to continue on methadone; however, we noted that more than 92% of people assessed wanted to do so. This study included only participants incarcerated for fewer than 6 months, and thus does not address the question of methadone treatment for prisoners with longer incarcerations. Finally, some participants might have entered a treatment programme in another state.
This study generally did not include individuals known to have HIV infection because, under the policy of the Rhode Island Department of Corrections, people with HIV who are receiving methadone maintenance on entry to custody are exempt from forced withdrawal. However, this exemption is not the case in most US states. The retention of HIV-positive individuals in methadone treatment programmes in turn improves retention in HIV care.53,54 This retention might have positive implications not only for these individuals’ health, but also, in view of the much increased risk of HIV transmission by individuals who are not on HIV treatment, for public health and health-care costs.
We chose to remove the variable of insurance coverage for methadone treatment in the first 10 weeks by offering treatment to all participants who did not otherwise have coverage. Regional variability in insurance coverage of methadone and associated costs could somewhat restrict the generalisability of our findings. However, under the US Affordable Care Act, many more people released from prisons and jails could be eligible for health insurance, rendering costs less of an issue.55,56
Data from this trial and others substantiate that stopping methadone treatment during incarceration leads to reduced and delayed re-engagement in methadone treatment in the community.44 In the USA, with the exception of Riker’s Island jail in New York City,57only a few of the estimated 30 000 people incarcerated while receiving methadone each year continue to get this treatment during incarceration. The correctional policies that force withdrawal from methadone on incarceration not only lead to poorer health, public health, and public safety outcomes at raised expense, but also hamper the ability of communities to engage a challenging population with a highly effective treatment.58,59 The withdrawal symptoms of abrupt cessation from methadone maintenance, especially insomnia, can last for months, as opposed to withdrawal symptoms from heroin which typically resolve in a less than 1 week. Emerging evidence suggests that some people avoid entering methadone treatment in the community so that they do not have the protracted (compared with heroin) withdrawal from methadone in the event of incarceration.58,59
The period of incarceration is a public health opportunity to diagnose and engage people with opioid dependence with treatment. For those already receiving treatment who wish to continue after incarceration, the public health imperative is to continue methadone. Although evidence-based health care in correctional settings is hampered by logistical and political obstacles, these can be addressed through strong leadership, training, and education for health and custodial staff, and attention to safety and security issues.52 Our study shows that continuation of methadone treatment for people at the time of incarceration reduces medical costs in the first 30 days after release and hastens and increases the probability that they will return to methadone treatment on release, at a dangerous time when they would probably benefit the most from continuing methadone treatment.
Supplementary Material
Panel: Research in context.
Systematic review
Methadone maintenance is a highly effective treatment for opioid addiction and has been included in WHO’s Model List of Essential Medicines since 2005. 11 randomised controlled trials25–35 have assessed the efficacy of methadone maintenance in treating opioid dependence as compared with placebo or non-pharmacological therapy and showed the effectiveness of methadone maintenance therapy in reducing illicit opioid use and increasing retention in treatment.36 In prisons, where many individuals are addicted to opioids, WHO recommends the provision of buprenorphine or methadone maintenance as best practice for opioid agonist therapy and opioid withdrawal.37 Accordingly, many nations, including Iran, Australia, Canada, and most of the European Union, have made methadone maintenance therapy available in correctional facilities. By contrast, in most of the USA, the standard procedure is to discontinue methadone treatment for prisoners on incarceration.
We sought to compare the effects, including costs, of continued versus forced discontinuation of methadone maintenance on re-engagement with care after release from prison. We reviewed the scientific literature by searching PubMed, the Cochrane Database of Systematic Reviews, and Google Scholar for any original English language articles published up to October, 2014, with the search terms “methadone maintenance”, “opioid”, opiate”, “addiction”, “prison”, “jail”, “correction”, “incarc”, “forced withdrawal”, “detoxification”, “cost”, “effective”, “benefit”, and “utility”.
International research comparing the effects of continued methadone to forced cessation at incarceration on post-release treatment re-entry and outcomes has been non-existent. Studies of other methadone-related outcomes show consistent evidence of an association between methadone maintenance and other opioid-substitution therapy in correctional settings and increased post-release treatment entry and retention compared with no opioid-substitution therapy.38 Up to now in the USA, two randomised trials29,39 have assessed and shown the benefits to starting methadone treatment before release from incarceration, but these studies did not assess the effects of methadone continuation compared with forced cessation. Several studies have assessed the cost-effectiveness of methadone maintenance therapy in the treatment of opioid addiction, but none have compared the cost-effectiveness of forced withdrawal from methadone versus continued treatment.
Interpretation
We did the first randomised controlled trial to study the effects of continued versus interrupted methadone maintenance therapy at incarceration on re-engagement with treatment after release from prison. In the first month after release, those randomised to continue treatment were more than twice as likely to resume methadone treatment after release. Furthermore, continued methadone decreased medical costs in the first 30 days after release and was cost effective. These data suggest that, rather than force people to cease methadone maintenance on incarceration, efforts should be made to continue treatment, and, for those in whom it is indicated, initiate methadone before release, and make arrangements for follow-up treatment in the community. Continuation of methadone maintenance during incarceration could contribute to greater treatment engagement after release, which could in turn reduce the risk of death from overdose and risk behaviours.
Acknowledgments
We thank the medical and custodial staff of the Rhode Island Department of Corrections including Ashbel T Wall, Fred Vohr, Gordon Bouchard, Pat Threats, Brenda Abelli, Vincent Jacavone, Eric Lewis, J R Perez, Pauline Marcussen, Jeff Renzi, and Margaret Paquette; our research team Candelaria Barroso, Ricky Lugo, Charlotte Reels, Chandra Cannon, Maria Garcia, Skye Tirado, Christina Anastacio; Sophie Sprecht and the other study nurses; staff in all the methadone clinics in Rhode Island and, in particular, the participants in this study. This research was supported by grants from the National Institute on Drug Abuse (K24DA022112 and R01DA027211) and the Lifespan/Tufts/Brown Center for AIDS Research, from the National Institutes of Health, Centers for AIDS Research (grant P30-AI-42853). Sarah Larney is funded by a National Health and Medical Research Council Early Career Fellowship (APP1035149). The National Drug and Alcohol Research Centre at the University of New South Wales is supported by funding from the Australian Government under the Substance Misuse Prevention and Service Improvements Grants Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Funding
National Institute on Drug Abuse and the Lifespan/Tufts/Brown Center for AIDS Research from the National Institutes of Health.
Footnotes
Contributors JDR, NZ, SL, AN, MR, and MM did the literature search. JDR, MM, NZ, and JC contributed to the study design and conceptualisation. MM, JDR, and JC obtained the data. NZ, SL, JBW, MM, JR, LT, MR, and JC did the data analysis. JDR, SL, NZ, JBW, MM, and JC interpreted the data. SL, LT, NZ, MM, JBW, and JDR produced the figures. JDR, SL, JBW, NZ, MM, AN, MR, and JC drafted and revised the report.
Declaration of interests We declare no competing interests.
References
- 1.Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379:55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- 2.CDC grand rounds: prescription drug overdoses—a US epidemic. MMWR Morb Mortal Wkly Rep. 2012;61:10–13. [PubMed] [Google Scholar]
- 3.WHO. 14th Model list of essential medicines. Geneva: World Health Organization; 2005. [Google Scholar]
- 4.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3:CD002209. doi: 10.1002/14651858.CD002209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deck D, Wiitala W, McFarland B, et al. Medicaid coverage, methadone maintenance and felony arrests: outcomes of opioid treatment in two states. J Addict Dis. 2009;28:89–102. doi: 10.1080/10550880902772373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degenhardt L, Bucello C, Mathers BM, et al. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 2011;106:32–51. doi: 10.1111/j.1360-0443.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- 7.Kerr T, Fairbairn N, Tyndall M, et al. Predictors of non-fatal overdose among a cohort of polysubstance-using injection drug users. Drug Alcohol Depend. 2007;87:39–45. doi: 10.1016/j.drugalcdep.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 8.MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ. 2012;345:e5945. doi: 10.1136/bmj.e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boutwell AE, Nijhawan A, Zaller N, Rich JD. Arrested on heroin: a national opportunity. J Opioid Manag. 2007;3:328–332. doi: 10.5055/jom.2007.0021. [DOI] [PubMed] [Google Scholar]
- 10.Durose MR, Cooper AD, Snyder HN. Recidivism of prisoners released in 30 states in 2005: patterns from 2005 to 2010: US Department of Justice. 2014 http://www.bjs.gov/index.cfm?ty=pbdetail&iid=4986.
- 11.Fiscella K, Moore A, Engerman J, Meldrum S. Jail management of arrestees/inmates enrolled in community methadone maintenance programs. J Urban Health. 2004;81:645–654. doi: 10.1093/jurban/jth147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Survey of Substance Abuse Treatment Services (N-SSATS) Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. 2011 https://www.icpsr.umich.edu/icpsrweb/NAHDAP/studies/34539?q=%22substance+abuse+treatment%22. [Google Scholar]
- 13.Rivlin A, Ferris R, Marzano L, Fazel S, Hawton K. A typology of male prisoners making near-lethal suicide attempts. Crisis. 2013;34:335–347. doi: 10.1027/0227-5910/a000205. [DOI] [PubMed] [Google Scholar]
- 14.Humber N, Webb R, Piper M, Appleby L, Shaw J. A national case-control study of risk factors for suicide among prisoners in England and Wales [corrected] Soc Psychiatry Psychiatr Epidemiol. 2013;48:1177–1185. doi: 10.1007/s00127-012-0632-4. [DOI] [PubMed] [Google Scholar]
- 15.Merrall ELC, Kariminia A, Binswanger IA, et al. Meta-analysis of drug-related deaths soon after release from prison. Addiction. 2010;105:1545–1554. doi: 10.1111/j.1360-0443.2010.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Binswanger IA, Stern MF, Deyo RA, et al. Release from prison—a high risk of death for former inmates. N Engl J Med. 2007;356:157–165. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaller ND, Bazazi AR, Velazquez L, Rich JD. Attitudes toward methadone among out-of-treatment minority injection drug users: implications for health disparities. Int J Environ Res Public Health. 2009;6:787–797. doi: 10.3390/ijerph6020787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei LJ, Lachin JM. Properties of the urn randomization in clinical trials. Control Clin Trials. 1988;9:345–364. doi: 10.1016/0197-2456(88)90048-7. [DOI] [PubMed] [Google Scholar]
- 19.Anon. Medication-assisted treatment for opioid addiction in opioid treatment programs. Rockville: Substance Abuse and Mental Health Services Administration; 2005. [PubMed] [Google Scholar]
- 20.McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 21.Hosmer DW, Lemeshow S. Applied survival analysis: regression modelling of time-to-event data. New York: Wiley-Interscience; 1999. [Google Scholar]
- 22.Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ. 1997;6:327–340. doi: 10.1002/(sici)1099-1050(199707)6:4<327::aid-hec282>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 23.Desgagne A, Castilloux AM, Angers JF, LeLorier J. The use of the bootstrap statistical method for the pharmacoeconomic cost analysis of skewed data. Pharmacoeconomics. 1998;13:487–497. doi: 10.2165/00019053-199813050-00002. [DOI] [PubMed] [Google Scholar]
- 24.Ramsey S, Willke R, Briggs A, et al. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health. 2005;8:521–533. doi: 10.1111/j.1524-4733.2005.00045.x. [DOI] [PubMed] [Google Scholar]
- 25.Dolan K, Shearer J, MacDonald M, Mattick RP, Hall W, Wodak A. A randomised controlled trial of methadone maintenance treatment versus wait list control in an Australian prison system. Drug Alcohol Depend. 2003;72:59–65. doi: 10.1016/s0376-8716(03)00187-x. [DOI] [PubMed] [Google Scholar]
- 26.Dole VP, Robinson JW, Orraca J, Towns E, Searcy P, Caine E. Methadone treatment of randomly selected criminal addicts. N Engl J Med. 1969;280:1372–1375. doi: 10.1056/NEJM196906192802502. [DOI] [PubMed] [Google Scholar]
- 27.Gruber VA, Delucchi KL, Kielstein A, Batki SL. A randomized trial of 6-month methadone maintenance with standard or minimal counseling versus 21-day methadone detoxification. Drug Alcohol Depend. 2008;94:199–206. doi: 10.1016/j.drugalcdep.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunne LM, Grönbladh L. The Swedish methadone maintenance program: a controlled study. Drug Alcohol Depend. 1981;7:249–256. doi: 10.1016/0376-8716(81)90096-x. [DOI] [PubMed] [Google Scholar]
- 29.Kinlock TW, Gordon MS, Schwartz RP, O’Grady K, Fitzgerald TT, Wilson M. A randomized clinical trial of methadone maintenance for prisoners: results at 1-month post-release. Drug Alcohol Depend. 2007;91:220–227. doi: 10.1016/j.drugalcdep.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman RG, Whitehill WB. Double-blind comparison of methadone and placebo maintenance treatments of narcotic addicts in Hong Kong. Lancet. 1979;2(8141):485–488. doi: 10.1016/s0140-6736(79)91550-2. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz RP, Jaffe JH, Highfield DA, Callaman JM, O’Grady KE. A randomized controlled trial of interim methadone maintenance: 10-month follow-up. Drug Alcohol Depend. 2007;86:30–36. doi: 10.1016/j.drugalcdep.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 32.Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000;283:1303–1310. doi: 10.1001/jama.283.10.1303. [DOI] [PubMed] [Google Scholar]
- 33.Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Dose-response effects of methadone in the treatment of opioid dependence. Ann Intern Med. 1993;119:23–27. doi: 10.7326/0003-4819-119-1-199307010-00004. [DOI] [PubMed] [Google Scholar]
- 34.Vanichseni S, Wongsuwan B, Choopanya K, Wongpanich K. A controlled trial of methadone maintenance in a population of intravenous drug users in Bangkok: implications for prevention of HIV. Int J Addict. 1991;26:1313–1320. doi: 10.3109/10826089109062163. [DOI] [PubMed] [Google Scholar]
- 35.Yancovitz SR, Des Jarlais DC, Peyser NP, et al. A randomized trial of an interim methadone maintenance clinic. Am J Public Health. 1991;81:1185–1191. doi: 10.2105/ajph.81.9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Degenhardt L, Charlson F, Mathers B, et al. The global epidemiology and burden of opioid dependence: results from the global burden of disease 2010 study. Addiction. 2014;109:1320–1333. doi: 10.1111/add.12551. [DOI] [PubMed] [Google Scholar]
- 37.WHO. Geneva: World Health Organization; 2009. Guidelines for the psychosocially assisted pharmacological treatment of opioid dependence. [PubMed] [Google Scholar]
- 38.Hedrich D, Alves P, Farrell M, Stover H, Moller L, Mayet S. The effectiveness of opioid maintenance treatment in prison settings: a systematic review. Addiction. 2012;107:501–517. doi: 10.1111/j.1360-0443.2011.03676.x. [DOI] [PubMed] [Google Scholar]
- 39.McKenzie M, Zaller N, Dickman SL, et al. A randomized trial of methadone initiation prior to release from incarceration. Subst Abus. 2012;33:19–29. doi: 10.1080/08897077.2011.609446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Degenhardt L, Larney S, Kimber J, et al. The impact of opioid substitution therapy on mortality post-release from prison: retrospective data linkage study. Addiction. 2014;109:1306–1317. doi: 10.1111/add.12536. [DOI] [PubMed] [Google Scholar]
- 41.Larney S, Gisev N, Farrell M, et al. Opioid substitution therapy as a strategy to reduce deaths in prison: retrospective cohort study. BMJ Open. 2014;4:e004666. doi: 10.1136/bmjopen-2013-004666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larney S. Does opioid substitution treatment in prison reduce injecting-related HIV risk behaviours? A systematic review. Addiction. 2010;105:216–223. doi: 10.1111/j.1360-0443.2009.02826.x. [DOI] [PubMed] [Google Scholar]
- 43.Larney S, Toson B, Burns L, Dolan K. Effect of prison-based opioid substitution treatment and post-release retention in treatment on risk of re-incarceration. Addiction. 2012;107:372–380. doi: 10.1111/j.1360-0443.2011.03618.x. [DOI] [PubMed] [Google Scholar]
- 44.Gordon MS, Kinlock TW, Schwartz RP, O’Grady KE. A randomized clinical trial of methadone maintenance for prisoners: findings at 6 months post-release. Addiction. 2008;103:1333–1342. doi: 10.1111/j.1360-0443.2008.002238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Connock M, Juarez-Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1–171. iii–iv. doi: 10.3310/hta11090. [DOI] [PubMed] [Google Scholar]
- 46.Barnett PG. The cost-effectiveness of methadone maintenance as a health care intervention. Addiction. 1999;94:479–488. doi: 10.1046/j.1360-0443.1999.9444793.x. [DOI] [PubMed] [Google Scholar]
- 47.Gisev N, Larney S, Kimber J, et al. Determining the impact of opioid substitution therapy upon mortality and recidivism among prisoners: a 22-year data linkage study. Sydney: University of New South Wales; 2014. [Google Scholar]
- 48.Larney S, Dolan K. A literature review of international implementation of opioid substitution treatment in prisons: equivalence of care? Eur Addict Res. 2009;15:107–112. doi: 10.1159/000199046. [DOI] [PubMed] [Google Scholar]
- 49.Harm Reduction International. The global state of harm reduction 2012: towards an integrated response. [accessed March 31, 2015]; http://www.ihra.net/files/2012/07/24/GlobalState2012_Web.pdf.
- 50.WHO. Copenhagen: World Health Organization; 2007. Health in prisons: a WHO guide to the essentials in prison health. [Google Scholar]
- 51.Boucher R. The case for methadone maintenance treatment in prisons. V Law Rev. 2003;27:453–482. [Google Scholar]
- 52.McKenzie M, Nunn A, Zaller ND, Bazazi AR, Rich JD. Overcoming obstacles to implementing methadone maintenance therapy for prisoners: implications for policy and practice. J Opioid Manag. 2009;5:219–227. doi: 10.5055/jom.2009.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet. 2010;376:355–366. doi: 10.1016/S0140-6736(10)60832-X. [DOI] [PubMed] [Google Scholar]
- 54.Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med. 2012;156:817–833. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cuellar AE, Cheema J. As roughly 700 000 prisoners are released annually, about half will gain health coverage and care under federal laws. Health Aff (Millwood) 2012;31:931–938. doi: 10.1377/hlthaff.2011.0501. [DOI] [PubMed] [Google Scholar]
- 56.Rich JD, Chandler R, Williams BA, et al. How health care reform can transform the health of criminal justice-involved individuals. Health Aff (Millwood) 2014;33:462–467. doi: 10.1377/hlthaff.2013.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harris A, Selling D, Luther C, et al. Rate of community methadone treatment reporting at jail reentry following a methadone increased dose quality improvement effort. Subst Abus. 2012;33:70–75. doi: 10.1080/08897077.2011.620479. [DOI] [PubMed] [Google Scholar]
- 58.Fu JJ, Zaller ND, Yokell MA, Bazazi AR, Rich JD. Forced withdrawal from methadone maintenance therapy in criminal justice settings: a critical treatment barrier in the United States. J Subst Abuse Treat. 2013;44:502–505. doi: 10.1016/j.jsat.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitchell SG, Kelly SM, Brown BS, et al. Incarceration and opioid withdrawal: the experiences of methadone patients and out-of-treatment heroin users. J Psychoactive Drugs. 2009;41:145–152. doi: 10.1080/02791072.2009.10399907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.