Abstract
The double burden of under- and overnutrition profoundly affects human health globally. According to the World Health Organization, obesity and diabetes rates have almost doubled worldwide since 1980 and, in 2011, more than 40 million children less than 5 years of age were overweight. Ecologic factors, parental genetics and fitness, and the intrauterine environment significantly influence the likelihood of offspring developing the dysmetabolic diathesis of obesity. This report examines effects of these factors, including preconception, intrauterine and postnatal energy balance affecting programming of transgenerational transmission, and development of chronic diseases later in life—in particular, diabesity and its comorbidities.
Keywords: obesity, diabesity, nutrition, econutritional, epigenetics, microbiome, exercise, taste preferences, breast feeding
Introduction
Whereas (1) the practice of medicine pursues the reductionist goal of diagnosing single causes of disease, tractable by “magic bullets” on the flawed premise of organ-specific responses, receptors, and mechanisms; (2) public health generates “evidence-based” policy-driving population data and one-size-fits-all timid, ineffective dictates, applicable at their worst to less than 51% of a population; and (3) the promise of individualized precision medicine is increasingly challenged by cellular pluripotency, plasticity, and epigenetics, evident in the failures of current drug combinations, multi-modal therapies and staged approaches, the multitude of redundant, evolutionarily conserved mechanisms that defend maintenance of life-sustaining energy stores defies univariate solutions.
Like few other global epidemics threatening quality-adjusted life years, the chronic inflammatory, insulin-resistant, dysmetabolic overnutrition syndrome (commonly termed “obesity”) has resisted two centuries of attempts to curb its progress, by focusing on food and body size rather than metabolism. The clear and present dangers of maternal death and illness, fetal loss, malformations, chronic disease, lifelong suffering, and premature demise associated with obesity are unremitting in the face of compelling evidence and substantial progress elucidating mechanisms from mitochondrial to societal.
In this context, the conference “Early-Life Influences on Obesity: From Pre-Conception to Adolescence,” held on September 26, 2014 at the New York Academy of Sciences, was conceived to examine obesity from an ontological as well as an ecologic perspective, hoping to identify critical nodes amenable to “small miracles”1 resulting in beneficial “creeping normalcy” from grassroots to government, to find realistic solutions for a conflicted field of real and perceived stakeholders in education, transportation, business, and industry. Successful examples of cross-sectorial collaborations exist, as do barriers to scaling and replicating such initiatives. Ultimately, these are questions of measurement, health equity, sustainability, and leadership. Thus, the opening speaker, Mark L. Wahlqvist, framed the conflict between individuals’ health and obesifying ecosystems he had termed “econutritional,” while the final speaker, Nico Rizzo, addressed adolescent physical fitness, a determinant of chronic cardiometabolic diseases of later life, as well as transgenerational transmission of the dysmetabolic diathesis of diabesity. Presentations covered the diverse areas of unintended pregnancies, sperm RNA, patrilineal transmission of disease, dysbiosis in the human microbiome, gestational exercise and other intrauterine influences, breast feeding, appetitive behavior, metabolic mechanisms of physical activity, and adolescent exercise.
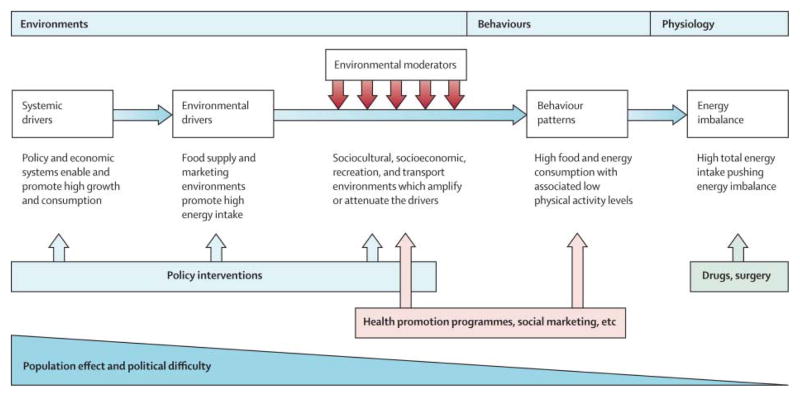
Readers should view the report from this one-day conference as a “tasting menu” where, however, each item is a meal in itself, each topic sufficiently rich to generate multi-day annual conferences, and each speaker is a chef. The goal was to bring together experts from different domains who do not usually participate in the same meetings or compete for the same grants, to generate innovative strategies for solving a seemingly intractable evolutionary problem (Fig. 1). Its scope, however, precludes attempts at being comprehensive.
Figure 1.
Obesity: global drivers and local environments. Reproduced from Ref. 107.
Preconception and generational effects of nutrition
Weight management in transitional economies: an ecosystem health dilemma
Mark L. Wahlqvist (Zhejiang University) discussed the dilemma of obesity and other weight-management issues in transitional economies. According to Wahlqvist, virtually every economy is in transition and the most vulnerable individuals sacrifice ecosystems for survival—for example, to obtain firewood to cook. Others may be environmentally rapacious for profit. Most current health pattern change, of which body compositional disorders are indicative, is attributable to the loss of sustainable ecosystems. The achievement of optimal weight for the majority requires ecological approaches. Effective health care practitioners will be required to prevent, diagnose, and manage adiposity and its consequences using ecological methods.
The ways and the circumstances in which we live are changing our energy acquisition and utilization requirements to the detriment of anatomy and physiology, not only intra- but also inter-generationally. The Food and Agricultural Organization (FAO) reports that, as agricultural productivity increases from the lowest to highest quartile, stunting and micronutrient deficiencies diminish while overweight and obesity increase, and its adverse consequences dominate.2 Prima facie, this looks like a problem of energy balance with more or less micronutrient intakes, especially iron, zinc, iodine, and vitamin A. But it is more than that, with problems of impaired energy regulation (IER), with its associated disorders of body composition and a limited diversity of sensory and nutritional inputs. Biodiversity and the use of biodiverse foods are essential for optimal health. That biodiversity extends to our heterogenomic prokaryotic microbiomes and to the extensive functions of molecules by which we sense our general and food environments. In other words, the overall problem is one of ecological disturbance which might be termed econutritional.3 A striking example of this is the United States, where the access to and consumption of fruit and vegetables by locality is inversely associated with obesity prevalence.4
The Organisation for Economic Co-operation and Development (OECD) has reported in 2014 that the global financial crisis of 2008–2009 “caused a surge in unhealthy eating as people switched to cheaper, higher calorie food” and that “as a result, rising poverty likely contributed to rising obesity”. The most vulnerable were found to be women and the poor. Of particular note, “fruit and vegetable consumption decreased as unemployment increased” in the United States. In the UK, 8.5% less was spent on food, but 4.5% more energy was consumed.5 In Australia, a cohort study of 7,787 people during and after the global financial crisis in relation to financial stress showed that, where present during 2008–2009, there was a 20% increase in the prevalence of obesity to 2010.6 Adiposity to some extent reflects the financial environment.
A complex biological dysfunction may be regarded as an ecosystem health disorder (EHD). The diagnosis and management of weight disorders requires an appreciation of their ecological context and as EHDs. Thus, while indices of body composition disorder may be represented by anthropometric or instrumental measures (e.g. impedance, DEXA), these belie primary causality and various pathogeneses, with a range of possible personal and societal interventions.
It is increasingly clear that, short of interventions such as bariatric or metabolic surgery for the very obese or metabolically ill, successful long-term arrest of progressive adiposity is difficult. But the risk of this eventuality can be much less where communities, localities, and food systems are conducive to the safe pursuit of walking and recreational activity, and encourage the appreciation, enjoyment, and societal role of food. The success of community-led programs to prevent obesity, such as EPODE (Ensemble, Prévenons l’Obésité Des Enfants – Together, Let’s Prevent Childhood Obesity) is testimony to this.7
Our dilemma, according to Wahlqvist, is how to promote and enable these ecosystem-based strategies for health at-large, even as such systems disappear and become dysfunctional. We must understand that the features required for competent energy regulation without disorders of body composition are environmental connectedness by regular movement to the point of healthful tiredness and natural wakefulness (like walking, cycling community dancing, tai chi) a wide range of sensory inputs (as in touch, sight, sound, smell, taste), physiological endocrine inputs into gut, integument, respiratory tract, reproductive tract and breast. These require not only appropriate personal behaviors, but also cooperative communities where the household, school, workplace and recreational settings reflect and support these attributes. The best prospects to arrest the growing epidemic of disorders of body composition and their attendant disordered metabolism are to be found where the approaches are ecologically sensitive and community based.
Intended and unintended pregnancies: the role of socioeconomic inequities
Lawrence B. Finer (Guttmacher Institute) discussed the importance of considering unintended pregnancy as a fundamental measure of a population’s reproductive health. Fundamentally, unintended pregnancies are reflective of an inability of women and couples to determine whether and when to have children. Unintended pregnancies are mostly mistimed pregnancies, in which a woman becomes pregnant earlier than intended, but they also include unwanted pregnancies in women who did not ever intend to become pregnant. Unintended pregnancies are associated with negative perinatal behaviors (less likely to obtain adequate prenatal care or to breastfeed) that can have negative health outcomes for infants and children.
In the United States, the rates of unintended pregnancies (51%)8 and unintended births (40%) (Finer and Zolna, unpublished) are much higher than in most other industrialized nations. In 2008 (the last year for which data are available), 51% of women of reproductive age had unintended pregnancies, and these occurred disproportionately among disadvantaged women. Striking economic and social disparities are seen in the rates of unintended pregnancies, with poor women, women with less education, unmarried women, and black and Hispanic women having more unintended pregnancies than their counterparts, with the disparities associated with income and education showing considerable growth between 2001 and 2008.8 Over this period, while the unintended pregnancy rate (as well as the overall pregnancy rate) decreased among teens, it increased among older women (above 25 years old).
According to Finer, 53% of unintended pregnancies occur where no method of birth control was used, while only 2% affect women using highly effective methods of birth control. In 2012, the American College of Obstetricians and Gynecologists released a position statement arguing that long-acting reversible contraception (LARC) methods, such as implants and intrauterine devices, “should be first-line recommendations for all women and adolescents.”9 Finer stated that, between 2006 and 2008, 5.5% of U.S. women reported current use of a LARC method, the highest observed since the early 1980s, although below the 14% worldwide LARC use rate.
Fundamentally, Finer concluded, reducing unintended pregnancy must include increasing overall contraceptive use—particularly highly effective methods (e.g., LARC)—and addressing the associated socioeconomic disparities.
Paternal contributions: the role of sperm RNA
Stephen A. Krawetz (Wayne State University School of Medicine) began his presentation with an overview of spermatogenesis, the differentiation process that yields mature, morphologically distinct sperm cells. He emphasized that sperm—an ultra-compact delivery system (a nucleus that is ~13-fold more compact than that of the oocyte)—contains the same amount of DNA as the oocyte, yet has room for the ~2 m of paternally derived centrosomes, sperm oocyte-activating factor, and RNAs. This, however, comes at a price, as a substantial portion, if not all, of the cytoplasm has been extruded during metamorphic transformation into a sperm. The majority of the RNA that remains appears fragmented, including the most abundant sperm RNA, rRNAs, which ensures transcriptionally and translationally inert sperm. However, within the sperm of fertile males, some RNAs (in addition to rRNAs) survive10 (e.g., ACSBG2, a transcript of unknown function (in this context)).
When considering the impact of the delivery of sperm RNAs, Krawetz argued, one must take into account the relative state of the contents, along with the timing of zygotic genome activation in order to understand the impact of the sperm at fertilization. For example, zygotic genome activation differ markedly in mouse and human. In mice, zygotic genome activation occurs just before the initial division, whereas human zygotic genome activation appears to occur around the 4- to 8-cell stage. The first division is critical in both species, and is likely modulated in part by two microRNAs, mi-RNA-34c and pri-miRNA-181c. The former is one of the most abundant miRNAs observed in human sperm,10,11 where it is perhaps required for the first division, although in mouse this is now contentious. At the two-cell stage, activation of the paternal pri-miRNA-181c by the oocyte likely targets destruction of CARM1 mRNA in one of the blastomeres to promote differentiation.10,12 In the remaining blastomere, the activity of CARM1 appears enhanced, as active chromatin marks within the OCT4 and SOX2 promoters are laid down. Their activation is essential to maintain cells in a pluripotent state, while the other cell differentiates.
Both examples clearly emphasize the significance of this paternal contribution to the initial steps of development. While they articulate the importance of sperm-derived RNAs in the early embryo, the question remains: what attributes do sperm-derived RNAs contribute to individual development?
Recent reports describing the effects of paternal diet or stress on the offspring have suggested that environmental stressors can predispose offspring to various metabolic syndromes.13,14 A role for paternal RNAs in the epigenetic transgenerational inheritance of these traits has been proposed. While this seems rather unusual, Krawetz observed, one could view this process as the organism “testing” a response before fixation within the genome. Also unanswered is how such testing is achieved, considering that a signal has to be transmitted—in this case RNAs—from the target to the testis and perhaps back. This has fueled speculation that, perhaps, signaling is mediated by exosomes.15
Krawetz summarized by saying that sperm are not just transporters of genomes, but also contributors of other essential genetic, epigenetic, and non-genetic information to offspring.
Transgenerational response to nutrition, early-life circumstances, and longevity
Nico S. Rizzo (Loma Linda University and Karolinska Institute) discussed recent developments in research of epigenetics and transgenerational responses to nutrition and early-life circumstances, which may identify novel pathways providing insight into the pathogenesis of chronic diseases.16,17
While the sequencing and detailed mapping of the genome is essential for a basic understanding of DNA, knowing the mechanisms required for expressing the complex genetic information is essential for understanding the manifestation or repression of disease patterns. Epigenetics—a relatively young field—describes the mitotically heritable state of gene expression potential, enabled by the mechanisms of DNA methylation, histone modification and chromatin marks.18 Epigenetics involves the investigation of both the mechanisms of heritable and non-heritable changes in gene expression that do not involve modifications of the DNA coding sequence.19 The study of inherited and environmentally-acquired epigenetic alterations is important to understand disease etiology and effects of health-altering environmental events across multiple generations.20
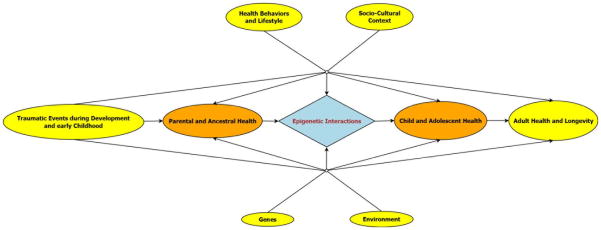
Transgenerational epigenetic research, continued Rizzo, focuses on measurable outcomes in the offspring generation that are related to pre-conception exposures in the parental—or further removed— ancestral generations.17 An inclusive investigation of possible transgenerational epigenetic pathways should take into account social patterning and cultural inheritance in order to disentangle secondary pathways that influence measurable health outcomes in offspring. The socio-cultural context, environment, acquired and transferred health behaviors, and lifestyles of ancestral and offspring generations need to be considered. Figure 2 provides a schematic overview of interactions and pathways that influence adult health and longevity in the offspring generation.
Figure 2.
Schematic diagram delineating the relationships and interactions between exposures and health outcomes in an inclusive transgenerational model.
Interesting paternal transgenerational effects in humans have been observed in the Överkalix cohort in northern Sweden.21,22 Meticulously maintained parish registries in this mainly agricultural region over more than a century recorded fluctuations in crop yields between years of abject famine and excess resulting in differences in longevity and specific causes of death in subsequent generations expressed through the paternal line.17,21 Although these observations were in concordance with expected differences between X versus Y chromosome transmission, mammalian experiments having shown sex-specific effects of paternal exposures before breeding in the offspring generation,23,24 work in progress in the Överkalix study might further clarify matrilineal transmission.
These initial observations, and the results of studies such as the Avon Longitudinal Study of Parents and Children (ALSPAC) and others,17 indicate the importance of expanding current and future efforts to include, whenever possible, a transgenerational perspective.17
Panel discussion: John G. Kral (SUNY Downstate Medical Center), Lawrence B. Finer, Stephen A. Krawetz, and Nico S. Rizzo
John Kral: I ask Dr. Finer and Dr. Krawetz to join us here while the audience thinks of some questions. I’m going to use my prerogative to ask a very quick and small question of each of you. I want you to say, in 60 seconds, what your vision is from your perspective for the future: what you would like to see next in your field? Dr. Finer?
Lawrence Finer: The stubborn unintended pregnancy problem in the United States is tied to inconsistent or even non-existent contraceptive use. One of the goals should be to try to improve use overall in the population of long-acting methods such as the IUD implant.
I don’t mean to imply that there is a magic bullet or one method that is best. There is certainly no best contraceptive method; and the particular method that you use really should depend on your own personal child-bearing goals, your relationship situation, etc. We are under-utilizing current technology to protect the population against unplanned pregnancy. This needs to change: some professional societies are making efforts in that direction. That’s where I think we need to go.
Kral: Thank you. We’re setting an agenda here. Dr. Krawetz?
Stephen Krawetz: My primary concern—the primary reason for my studies—is to ensure the birth and long life of a healthy child. Precision medicine, the ability to have a substantial amount of information on the genetic background of each individual, will allow us to assess the potential risks for individuals having children. Hopefully, we will ultimately be able to develop strategies to repair genetic damage in order to minimize risk. There are methods on the horizon demonstrating that there are various “resetting” mechanisms that are very simple, that can be implemented. We are very close to being able to recognize risks, to then develop strategies to reverse or minimize them.
Kral: You’re really asking for planned parenthood…
Krawetz: More for people to take responsibility.
Kral: Dr. Rizzo?
Nico Rizzo: In the future, by having learned what our grandparents did that might have had adverse effects on our lives, we might be able to develop positive interventions affecting the lives of our children and grandchildren.
We want to recognize mechanisms to reverse adverse transgenerational nutritional effects on individual and population levels. We need to identify the most effective respective critical periods for intervention. Are there specific periods in life when countermeasures are especially effective during early childhood, or during puberty? Are there other periods when specific interventions, programs, or a public health agenda could be most effective? What can we do today so that in future generations, our children might have a better life and be prepared to a better life in an always-changing environment?
Kral: Questions from the audience?
Question: Dr. Finer, I am curious to know if you’re planning to collect information on the relationship between unintended pregnancy and BMI. Because while yours was certainly a very interesting talk, I am just curious to know about rates of unintended pregnancy based on BMI. Are obese individuals more likely to experience unintended pregnancies?
Finer: That’s a very interesting question. I believe that there are data that could answer the question. I’m not sure if the data set I have been using has BMI, but there’s a second source of information on pregnancy intendedness collected by the CDC: the Pregnancy Risk Assessment Monitoring System surveys women about 6 months after they’ve given birth asking them about many aspects of their pregnancy, including intendedness and also measures like BMI. Possibly those measures are made at the time the mother is interviewed. This would require a little exploration on my part, but it’s a certainly provocative question.
Question: I have a question for Dr. Krawetz following up on Dr. Rizzo’s talk. We heard about these very interesting observations about trans-generational influences of grandpaternal nutrition. I’m just wondering if you could expound a little bit on the topic. What types of developmental changes are occurring in the sperm around 10–12 years of age, the “slow-growth period”?
Krawetz: From 10–12 years of age, reaching puberty, is the first differentiation of the primordial germ cells (PGCs), forming active spermatozoa. However, at that point the PGCs have already been laid out, so it’s hard for me to envisage how they could actually be modified. To me, that’s the million dollar unanswered question. With respect to timing, I envisage it happening later, when extra RNA is being delivered and taken up through pathways, as is the case with soluble RNAs, while the sperm is actually resting or transiting through the epididymis. We haven’t really considered this yet—the techniques are just being developed. That’s where I think there is a possibility to actually see the effectors.
Question: This is for you, Dr. Rizzo. When we look at the direct effect of a paternal grandfather on a grandson, it’s a really dramatic narrative. And yet we don’t see the same narrative for the granddaughter, from either the maternal grandmother or grandfather? What’s your interpretation?
Rizzo: That’s a very, very good question that I wish I could answer definitively. There might be some differences in the Y-line, in the sperm line. However, the mechanisms are not clear yet. But first, we need to replicate these results to validate them. We also must study animal models. In mice grandfathers have effects on the granddaughter, but there might be species differences.
Question: I’ll put Dr. Krawetz on the stand here. There are recent studies showing heritability of histone expression patterns, but are there individual differences in patterns of heritability of the microRNA being expressed within the sperm?
Krawetz: Sure. In terms of the consistency of the RNAs: they’re identified in sperm among individuals and among populations of cells, not individual cells. It might become possible to sequence the 50 femtograms of RNA in an individual cell, but the technology is not there yet. I shouldn’t say it’s possible. It’s very doubtful right now.
We have to do it on a population basis. We’ve looked at over 400 individuals and can classify these individuals in terms of their fertility and fecundity potential. In doing so, we notice that there are consistencies in the patterns. We know that it’s reproducible among different individuals. Data suggest that we will be able to identify those key players: we have some pretty good evidence for them, for example microRNA 34C181C and other microRNAs.
We have some really good evidence that they are important and are actually paternally derived, paternally inherited. The genome in the sperm is actually more compacted in mice, where it’s almost like a crystalline structure, whereas in humans it’s floppy. There’s a whole series of regions of human chromatin, about 15%, which is in a type of poised, some of which is bivalently marked, chromatin state.
Question: I’m a retired primary care physician in New York and have been very frustrated for many years that the scientific advances have failed to curb the rise in obesity and diabetes. I have started a community-based activity group to try to change this.
A question for all of the panelists— is our science too narrow and reductionist? How can the community affect the controversies over important findings in nutrition and contraception that have such a profound influence? Also, how can we spread the knowledge that well-being and stress and physical activity are associated with better health?
Kral: That’s an important agenda question where we hope to get more information during the day that will help us to formulate practical approaches.
Question: Dr. Rizzo, can you give us a sense of the prevalence of obesity in the Överkalix population? Is obesity part of the story at all?
Rizzo: Even in today’s society in Sweden, the obesity rate among youth is very, very low compared with the European Youth Study and other studies. Our study looked at grandparents born in 1820, when the obesity problem did not really exist. However, the interesting part is that fluctuations in food availability, though not related directly to obesity, had an impact in the form of metabolic, obesity-type disturbances, even with no obvious signs of obesity.
Kral: Thank you panelists and audience.
The microbiome and global health
Maria Gloria Dominguez-Bello (New York University School of Medicine) discussed global health implications of the human microbiome, the microbial genes that complement the human genome in body niches, and how these microbial communities exert important endocrine, immune, and digestive functions.
The microbiota have been linked to obesity risk and immune-related diseases, among others. Over the past four decades, epidemics of immune-related and metabolic disorders, such as asthma, type 1 diabetes, celiac disease, and obesity, have emerged globally and are associated with industrialization.25,26 Many of these diseases are associated with excessive or aberrant immune responsiveness mediated by T helper (TH1, TH2, and TH17) and/or T regulatory (Treg) immune cells and are believed to originate in early life.
Dominguez-Bello described how the maternal microbiome naturally colonizes various body sites of the newborn and the compelling epidemiological evidence that C-section and/or early exposure to antibiotics is associated with increased risk of immune-related diseases.7 In addition to this epidemiological evidence, Dominguez-Bello continued, results in experimental animals support a causal link between specific states of intestinal microbes and obesity38,39 and asthma.40–42 Research has shown, for example, that the microbiome of Westernized people has a lower diversity of microorganisms than microbiomes of tribal people43–45 indicating that Westernized lifestyle greatly affects the microbiota of humans directly compounded through maternal transgenerational transmission.
Offspring effects of maternal exercise before and during gestation
Linda M. Szymanski (Johns Hopkins University School of Medicine) discussed investigations into the benefits of exercise before, during, and after pregnancy. There is little debate over the health benefits of physical activity for the general population. However, questions remain when it comes to the effects of physical activity during pregnancy. Many benefits have been suggested and supported, including enhanced maternal health, both short and long term, and improved pregnancy outcome. Equally important, the fetus and neonate may derive benefits, perhaps even as adults. Szymanski focused on three antepartum complications that impact short- and long-term maternal and offspring health owing to reduction or absence of physical activity, primarily related to obesity.
Excessive gestational gain (GWG), gestational diabetes mellitus (GDM), and hypertensive disorders are frequently encountered complications of pregnancy that may be modified by physical activity (Table 1). Data are the most robust for GDM, with the greatest risk reduction (more than 50%) seen in highly active women who exercise before pregnancy. Much less information is available on the impact of physical activity on hypertension in pregnancy; thus, there is little mention of exercise in the majority of obstetric reviews on management of women with hypertension during pregnancy.
Table 1.
| Gestational weight gain | Gestational diabetes | Hypertensive disorders | |
|---|---|---|---|
| Maternal impact |
|
|
|
| Pregnancy outcome |
|
|
|
| Offspring impact |
|
|
|
| Effect of exercise |
|
|
|
Similar to the general population, one of the major problems surrounding physical activity and pregnancy is not the lack of data supporting exercise in pregnancy, rather it is the lack of participation in physical activity. The data are not encouraging. Only a minority of pregnant women are physically active enough to meet current recommendations. Moreover, many previously active women either reduce or stop exercise during pregnancy. To further complicate the issue, many obstetric providers either do not discuss exercise with their patients or are hesitant to encourage exercise. The apparent lack of counseling on exercise during pregnancy may deprive women of overall health benefits, pregnancy-specific benefits, and consequent offspring health benefits.
Research on fetal responses to exercise, Szymanski continued, should focus on a broad goal of providing additional evidence to encourage exercise during pregnancy and enable the development of evidence-based guidelines for both women and their providers. When healthy pregnant women exercise according to available guidelines,49 the activity is well tolerated by both mother and fetus, as indicated by a variety of commonly used tests of fetal well-being.50 The hope is that data such as these will reassure healthcare providers that women can exercise during pregnancy when following existing recommendations.
Concluding, Szymanski emphasized that while more data are needed to develop evidence-based guidelines, particularly in women with comorbidities, such as obesity, hypertension, and diabetes, physical activity during pregnancy is a low-cost, potentially high-yield intervention that may significantly improve pregnancy outcome and enhance both maternal and offspring health in the short and long term.
Intrauterine environment and programming
Risk of childhood obesity
Shari Barkin (Vanderbilt University Medical Center) discussed how obesity and chronic disease are the result of complex interactions between genetic, behavioral, and environmental factors that occur during sensitive periods of development, including pregnancy and early childhood. Susceptibility to obesity within an obesogenic environment appears to vary greatly among individuals, suggesting interactions between genetic variation and the environment. Exposure to an obesogenic environment and its effect on genetic expression can begin in utero and extend through early childhood development.51 Periods of genetic plasticity occurring in the prenatal and postnatal environments are critical because exposure of the developing fetus, infant, or young child to certain environmental factors has been linked to phenotypic expression of patterns of growth and adiposity that can result in lifelong increased risk of overweight, obesity, type II diabetes,51 and breast cancer. During fetal development, for example, adipocyte differentiation—a predictor of future adiposity—may be directly affected by maternal genetic, hormonal, and behavioral factors.51,52
Evidence exists, continued Barkin, for specific vulnerability genes—owing to single nucleotide polymorphisms (SNPs)—which place the individual at risk for obesity when the affected genes are expressed or silenced. As one example, in the United Kingdom several longitudinal cohorts of children, including the ALSPAC, assessed genetic contributions to childhood obesity and confirmed a strong association with a polymorphism in the FTO gene (fat mass and obesity associated). Expression of the SNP-related allele is correlated with increased body mass index, adiposity, circulating leptin, energy intake, impaired control of energy balance, and satiety. Similar associations (of SNP and phenotype) have also shown age-dependent gene expression that changes as a child develops.
Epigenetics presents a far more dynamic potential than SNPs when considering a mechanism to explain the complexities of individual variation in susceptibility to developing obesity. For example, whereas genomic studies detect specific gene variants (SNPs) that serve as indicators of an expected response to nutrients or other environmental factors, epigenetic studies consider how nutrients that interact with genes to modify their expression, for example, through posttranslational modifications of DNA such as methylation and histone acetylation, become heritable changes passed on to several generations.53 The epigenome appears to be highly dynamic in response to nutrition, physical activity, and chronic stress, Barkin emphasized.
The evidence for epigenetic modification as a significant modulator of obesity and related diseases compels a deeper examination of exposure to environmental factors that may affect gene expression, particularly during periods of genetic plasticity, that is, from conception through early childhood. By exploring the strength of the biologic evidence surrounding the contributions of early-life experiences in conjunction with genetic processes to childhood obesity, unique opportunities to identify potentially plastic periods ripe for prevention strategies could be uncovered.
Nutritional influences on human developmental epigenetics
Robert A. Waterland (Baylor College of Medicine/USDA/ARS Children’s Nutrition Research Center) discussed the developmental origins hypothesis, which proposes that, during critical periods of development, environmental stimuli guide ontogenic processes, leading to persistent alterations in metabolism and risk of disease. Of various potential mechanisms that could mediate such developmental plasticity,54 induced alterations in epigenetic gene regulation have attracted the most attention in recent years. DNA methylation occurs predominantly at palindromic CpG dinucleotides in mammals. Genomic patterns of CpG methylation, once established during development, are highly stable, affording an outstanding candidate mechanism to explain the life-scale persistence that is the hallmark of the developmental origins paradigm.
However, significant obstacles have impeded progress toward testing the hypothesis that epigenetic mechanisms mediate developmental programming, said Waterland, including the inherent cell-type specificity of most epigenetic marks, the influence of genetics on inter-individual epigenetic variation, and the potential for reverse causality (i.e., a disease causes epigenetic changes and vice versa).55 Importantly, a select class of genomic loci, metastable epialleles (MEs), circumvents these obstacles. At Mes, establishment of epigenotype occurs stochastically in the early embryo, but then is maintained and propagated stably to all tissue lineages during subsequent differentiation. This leads to systemic inter-individual variation in DNA methylation that is not genetically mediated. Waterland described his group’s earlier studies in murine ME models, including agouti viable yellow (Avy)56 and AxinFused,57 which found that stochastic establishment of epigenotype at Mes is particularly sensitive to maternal nutrition before and during pregnancy.
To identify genomic regions in the human genome with systemic inter-individual variation in epigenetic regulation, Waterland discussed a reduced-representation method for genome-scale DNA-methylation profiling. Comparing methylation profiles in both peripheral blood lymphocytes (PBLs) and hair follicle (HF) DNA from healthy Caucasian adults identified a small set of candidate MEs.58 Systemic inter-individual variation in DNA methylation was confirmed by studying liver, kidney, and brain tissues of Vietnamese cadavers, and studies in monozygotic twins demonstrated that such variation occurred even in the absence of genetic variation.58,59
To test for effects of maternal periconceptional nutritional status, Waterland teamed up with Andrew Prentice and his colleagues, who have for decades been studying the effects of seasonal variation on maternal nutritional status and reproductive outcomes in rural Gambia.60 In this population, the single annual rainy season, combined with a reliance on own-grown foods, induces a dramatic and repetitive annual variation in energy balance and micronutrient status. Waterland and Prentice initially employed a retrospective design, examining peripheral blood lymphocyte DNA from children (average age 7) who had been conceived during the peak of either the rainy or dry season. At all five human MEs examined, average methylation was higher in children conceived during the rainy season.58
Although these findings were generally consistent with the hypothesis that maternal nutritional status affects establishment of DNA methylation at MEs, the retrospective study design made it impossible to rule out the possibility that—rather than maternal nutrition—some other seasonally variable environmental factor had induced the epigenetic changes. Moreover, since only one tissue (PBL) was studied, it was not clear that the environmental effect occurred in the early embryo. For these reasons, again in collaboration, Prentice and Waterland conducted a prospective study of maternal nutritional status and offspring DNA methylation. Across 34 villages in West Kiang, Gambia, over 2000 women of childbearing age were recruited and visited monthly. At the first report of a missed menses, a peripheral blood sample was collected; once pregnancy was confirmed, each woman was enrolled into the main group, which ensured that maternal nutritional status biomarkers were measured early in pregnancy in the women, who incidentally also had conceived during the peak of either the rainy or the dry season (~70 per season). PBLs and HFs were collected from the infants (average age 6 months) for measurement of DNA methylation. An indicator group of women was studied concurrently. These 30 women of childbearing age from the same villages as the main group provided blood samples monthly for 1 year. Their seasonal variations in biomarker status were used to back-extrapolate those in the main group to estimate nutritional status at the time of conception.59
As in the earlier study, infants conceived during the rainy season had higher DNA methylation at all six MEs studied. This increment was observed in both PBL and HF DNA, indicating an environmental effect that occurred in the early embryo and was maintained during subsequent differentiation of cellular lineages.59 Of 13 maternal nutritional-status biomarkers examined, two predicted systemic offspring DNA methylation at MEs. Elevated levels of maternal homocysteine and cysteine, downstream by-products of transmethylation, around the time of conception predicted lower DNA methylation in both PBLs and HFs in her infant. These data, therefore, suggest that, rather than indicators of methyl donor supply (such as folate and methionine), the best indicators of methylation capacity in the early embryo may be circulating biomarkers related to product inhibition.
Waterland concluded that the data indicate that Mes are not merely an epigenetic oddity in mouse models but they also exist in the human genome. And similar to mouse Mes, human Mes are demonstrated to have stochastic establishment of systemic inter-individual epigenetic variation that is influenced by maternal nutrition in early pregnancy. Using genome-wide bisulfite sequencing coupled with their multiple-tissue inter-individual screen, future work will aim to identify many more human MEs, which may help advance our understanding of how early nutrition determines epigenetic regulation and risk of diseases—including obesity.
Ontogeny of taste preferences: basic biology and health implications
Early life exposures—both biological and social—explain trajectories of health decades later in adulthood.61 Julie A. Mennella (Monell Chemical Senses Center) offered that, because many illnesses of modern society are in part the consequence of poor food choices dictated by our taste preferences, research on the chemical senses that contribute to the flavor of foods becomes paramount to both understanding (children’s) proclivity for some foods while rejecting others and developing evidence-based strategies to foster healthy food habits.
Basic research has shown that children live in different sensory worlds than adults. Evolution, moreover, has helped define the types of foods initially preferred and rejected by infants and children. For example, they naturally prefer foods with higher levels of sweet (the signal for calories) and salty (the signal for needed minerals)62 and reject those with lower levels of bitter (the signal for poison) than do adults.63 The adult-like pattern does not emerge until mid-adolescence. Further, tasting something sweet reduces the stress from pain in children. Children’s basic biology, a consequence of a long evolutionary history, does not predispose them to favor the recommended low-sugar, low-sodium, vegetable-rich diet, and makes them especially vulnerable to our current food environment, which contains foods high in salt and refined sugars.
If this is the bad news, Mennella continued, the good news is that sensory experiences— beginning as early as fetal life —can shape food and flavor preferences.64 While children do not have to learn to like sweet and salty foods, they do have to learn the context in which these taste experiences occur. Through familiarization, children develop a sense of what should, or should not, taste sweet or salty. The mechanisms for sensing foods operate before birth and throughout childhood. However, since vegetable acceptance, in particular, is low during childhood through adulthood, many infants are not given the opportunity to taste these foods, and thus to learn to like them. Mothers eating diets rich in healthy foods, including a range of vegetables, can get children off to a good start, since flavors are transmitted from the maternal diet to the amniotic fluid and mother’s milk, and experience with such flavors leads to greater acceptance of those foods at weaning. In contrast, infants fed formula learn to prefer its unique flavor profile and may have more difficulty initially accepting flavors of fruits and vegetables found normally in breast milk of mothers eating a rich diet.
Once weaned, regardless of early feeding mode, infants can learn through repeated exposure and dietary variety, and some evidence suggests that there may be sensitive periods for learning about tastes and flavors early in life.64 Infants will consume more of foods that have a familiar flavor, and are more accepting of novel flavors if they have experience with flavor variety. Although infants often appear to reject vegetables that have a bitter taste, caregivers can enhance acceptance by focusing on infants’ willingness to actually eat a food instead of their facial expressions, and by providing repeated opportunities to taste a food. Introducing children repeatedly to individual as well as a variety of fruits and vegetables, both within and between meals, may help them be more accepting of fruits and vegetables, which is difficult to achieve beyond toddlerhood.65
Mennella summarized by saying that early-life experiences with healthy tastes and flavors may go a long way toward promoting healthy eating and growth, which could have a significant positive influence on many chronic illnesses associated with poor food choices. Mothers feed their children the foods the mothers like and enjoy. Research is needed to improve dietary habits of women during pregnancy and the postpartum period, as well as understanding of how and when infants learn to like foods; and whether, as for other senses, there are sensitive periods for learning about flavors and foods.64
Obesogenic effects of developmental programming
Mina Desai (University of California, Los Angeles Medical Center) discussed how the developing fetus is dependent upon maternal nutritional, hormonal, and metabolic environments, and as such any perturbations may program organ structure, cellular composition, gene expression, and/or the epigenome, ultimately altering metabolism and function.66 Maternal gestational over- and undernutrition can each result in obese adult offspring. To understand putative underlying mechanism(s) contributing to offspring obesity, Desai and Michael Ross’s laboratory have established rat models of divergent maternal nutritional exposures: (1) maternal undernutrition (food restriction (FR)) and (2) maternal overnutrition (high-fat diet (HF)). Their studies have shown that both offspring exhibit early onset of hyperphagia and increased adiposity, suggesting common mechanisms of programmed hypothalamic appetite pathways and adipogenic signals.67,68
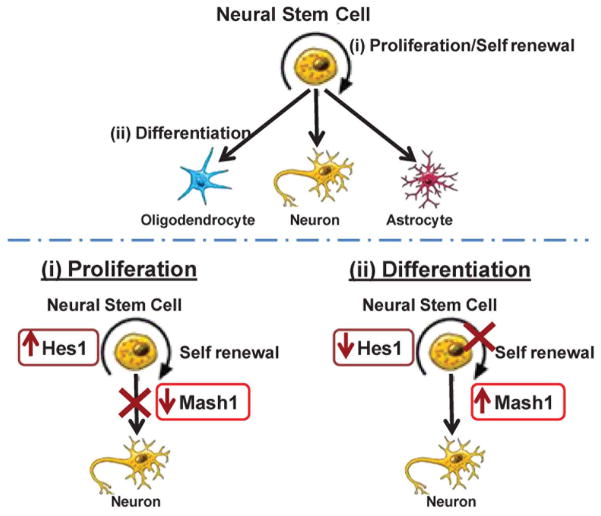
Appetite regulation develops first in utero, with continued neural development and maturation during the neonatal period. The predominant appetite-regulatory site, the hypothalamic arcuate nucleus (ARC), contains two populations of neurons derived from neural stem cell (NSC) progenitors that have opposing actions on food intake: orexigenic (agouti-related protein, AgRP and neuropeptide Y (NPY)) and anorexigenic (pro-opiomelanocortin, POMC). The development of these neurons is regulated by the basic helix–loop–helix (bHLH) neuroproliferative factor Hes1, which promotes NSC proliferation and inhibits downstream bHLH neurodifferentiation factors (e.g., Mash1 and Ngn3). Once activated, Ngn3 further promotes the development of anorexigenic POMC neurons, while inhibiting AgRP expression (Fig. 3A).
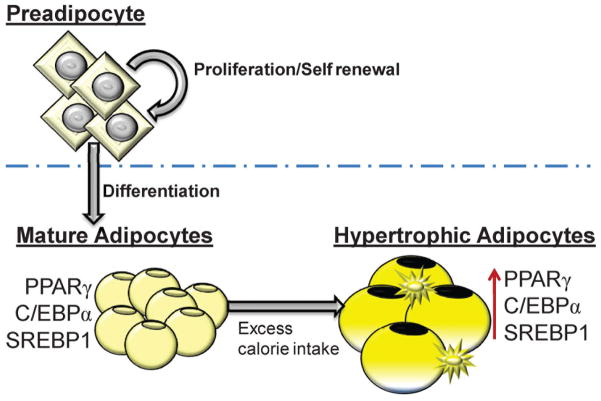
Figure 3.
(A) Neural stem cells (NSCs) can proliferate and self-renew or NSCs can differentiate to neuronal or glial cells. Hes1 promotes NSC proliferation and inhibits Mash1 and NSC differentiation. Reduced Hes1 suppresses NPC proliferation and alleviates Mash1 inhibition allowing NSC differentiation. (B) Preadipocytes (adipocyte stem cell) can proliferate and self-renew or can differentiate to adipocytes. Mature adipocytes: adipogenic (PPARγ, C/EBPα) and lipogenic (SREBP1) transcription factors are induced. Hypertrophic adipocytes: availability of excess calories facilitates increased lipid storage and promotes increase cell size with greater expression of PPARγ, C/EBPα and SREBP1. Increased macrophage presence is also evident.
Desai and Ross described studies demonstrating that FR and HF offspring exhibit dysfunction at several points in the appetite/satiety pathway. In both groups of offspring, appetite regulation is biased toward orexigenic (AgRP/NPY) neurons and away from anorexigenic (POMC) neurons, resulting in an increased ratio of hypothalamic ARC appetite/satiety gene expression. The underlying mechanism involves reduced Hes1, with subsequent reduced Mash1/Ngn3 expression. They have further explored this pathway in FR offspring using hypothalamic NSCs. FR fetuses exhibit reduced NSC migration (from the periventricular region to the ARC) in vivo, with reduced proliferation and neuronal differentiation in vitro. Importantly, even in culture media outside the fetal environment, FR NSCs are programmed to preferentially differentiate to appetite as compared to satiety neurons.69,70
In addition to programmed appetite/satiety, these studies indicate that adipogenesis is also a contributory factor to the development of programmed obesity. Increases in adipogenesis and fat mass occur pre- and postnatally, though some adipogenesis continues during adulthood. Adipose tissue grows through both cell hyperplasia and hypertrophy. The process of adipogenesis involves differentiation of fibroblasts and pre-adipocytes to mature fat-storing adipocytes. The differentiation pathway is highly organized, with precisely controlled expression of a cascade of transcription factors. Of these, the principal adipogenic transcription factors PPARγ (peroxisome proliferator-activated receptor) and C/EBPα (CCAAT enhancer–binding protein) promote adipogenesis. The downstream target of PPARγ is the lipogenic transcription factor SREBP1 (sterol regulatory element–binding protein), which facilitates lipogenesis (Fig. 3B).
In these studies, both FR and HF offspring exhibit enhanced adipogenesis. In both offspring, there is early induction of the adipogenic signaling cascade promoting fat storage, as evidenced by increased gene expression of PPARγ, C/EBPα, and SREBP1, as well as increased de novo fatty acid synthesis. Utilizing primary preadipocyte and adipocyte cultures, Desai and Ross have further explored whether the increased adipogenic potential of FR adipocytes is due to intrinsic cellular changes. FR preadipocytes have comparatively increased proliferative potential and, when allowed to differentiate to mature adipocytes, exhibit higher PPARγ expression with greater lipid content than those of controls.71,72 Importantly, FR preadipocytes and adipocytes are more sensitive to insulin, a potent growth factor during fetal life. Overall, FR adipocytes in culture retain the phenotype of enhanced adipogenesis and lipogenesis, evidenced by growth in culture, as well as enzyme/signaling expression.
Programmed hyperphagia and adiposity are secondary to enhanced appetite and impaired satiety responses, intrinsic traits of increased adipocyte differentiation, and enhanced propensity for fat storage. These phenotypes result from alterations in stem cell precursors of both appetite/satiety neurons and adipocytes, changes that promote offspring obesity. Early modifications of the maternal nutrient environment may prevent altered development and regulation of appetite and adipogenesis.
Breast feeding: molecules, nutrients, and context
Nancy F. Krebs (University of Colorado School of Medicine) discussed the relationship between bioactive components in breast milk and infant growth, with implications for lifelong adipogenesis. Rapid weight gain during the first weeks to months of post-natal life is associated with later obesity.73 The growth acceleration hypothesis proposes that rapid growth programs infant metabolism to be susceptible to obesity and comorbidities later in life.74 Abundant epidemiologic data support the importance of a critical post-natal window, but the underlying mechanisms of programming remain unknown.75,76 While breastfeeding protects against later obesity in humans,77 animal data suggest distinct effects of maternal obesity on milk composition that program offspring for increased risk of obesity and metabolic disease.78,79
Krebs suggested that maternal phenotype (normal weight, NW versus overweight/obese, OW/Ob) may influence the nutrient profile (bioactive components) of human milk, thus influencing patterns of infant weight gain and fat deposition. Krebs’ group has compared the macronutrient content, cytokines, adipokines, and pro-oxidant mediators in human milk between two groups (NW and QW/Ob), and have related the findings to infant growth (n = 46) and body composition (n = 50) in the first 4 months of life in two separate studies.80,81
Their results identified hormonal differences in maternal circulation, but the general composition of human milk was similar between groups. Leptin and insulin were consistently higher in milk of OW/Ob women across time points (P < 0.05). However, macronutrients, adiponectin, ghrelin, and markers of oxidative stress in milk did not differ. The concentration of inflammatory cytokines in human milk also did not differ between the two groups, though the range and variability in cytokine concentrations may obscure meaningful differences.82
In a study assessing infant body composition, infants of OW/Ob mothers were heavier (P < 0.01) and fatter (P < 0.02) at birth. However, weight (adjusted for sex), percent body fat, weight gain, and fat deposition did not differ from NW comparisons thereafter. Another difference, the measured rate of infant lean mass gain was higher in infants of OW/Ob mothers. A multivariate model of infant lean mass deposition was constructed using backwards stepwise regression; in the final model, only three variables remained and explained 31% of the variability in lean mass deposition: infant sex and milk IL-10 concentrations were positively associated, while milk glucose concentrations were negatively associated, with the outcome. None of the biochemical compounds measured in milk was predictive of infant fat deposition over time.83 Similar results were detected in a second study of similar design that lacked measures of body composition: no differences in rates of infant weight gain were detected between groups. The most robust predictor of rate of infant weight gain over the first 4 months of life was caloric density of human milk at 4 months (P < 0.001).84
In addition to the biochemical composition of human milk, Krebs’ group assessed, in the larger cohort, maternal beliefs and behaviors surrounding infant feeding, as these have been hypothesized to play a role in development of later obesity.85 No differences in maternal beliefs between groups were detected, though OW/Ob women were more likely to feed their infants on a schedule by 4 months than were NW women (P < 0.03). Independent of maternal group, maternal concern about infant milk intake and/or weight gain was associated with a variety of poor outcomes, including less exclusive breastfeeding (P < 0.02), a “pressuring” feeding style (P < 0.01), and more feeding for infant fussiness (P < 0.01).86
According to Krebs, the data suggest that bioactive components in human milk and maternal feeding practices may be synergistic drivers of early infant growth. Of particular note, maternal obesity did not affect the general composition of human milk or the body-fat trajectory of infants. The findings support the conclusion that there are potential drivers of lean body mass deposition in infants and highlight the need for research that will identify the drivers of infant adipogenesis. Breastfeeding may attenuate the adverse impact of maternal obesity, and therefore may be especially important to limit the mother-to-child transmission of obesity.
Resetting the program: neonatal to adolescence
Appetite as a susceptibility factor for obesity
Jane Wardle (University College London) argued that the tripling of obesity rates over the past 30 years is undoubtedly due to environmental changes, most likely a combination of increased availability and use of energy-dense, accessible, intensively-marketed food and diminished physical activity. However, Wardle also mentioned that within-population variation in adiposity is largely caused by genetic differences, with heritability estimates exceeding 70% (even since the beginning of the current obesity epidemic). A resolution to the seeming paradox of obesity being both highly environmental and simultaneously highly genetic is the idea that genes do not act directly on adipogenesis but rather determine intermediate phenotypes whose expression are sensitive to the environment. In the context of a behavioral susceptibility model, Wardle proposed that two appetitive traits, food responsiveness (which upregulates the urge to eat in the presence of food cues) and satiety responsiveness (which downregulates the urge to eat following food intake), are candidates for the intermediate phenotype.87 These traits were implicated decades ago in seminal work by pioneers in the field Jean Meyer, Mickey Stunkard, and Stanley Schachter, who demonstrated striking differences between obese patients and healthy controls, although they did not specifically emphasize genetic or environmental processes.
Wardle’s group has carried out a series of studies designed to elaborate the behavioral susceptibility model, focusing specifically on satiety responsiveness (SR), partly because it has been neglected in the recent canon of obesity research, and partly because it has particular salience in early life. The research has aimed to (1) test for quantitative, prospective associations between SR and weight, (2) assess the heritability of SR and determine its association with obesity genes, and (3) examine associations between SR and food intake in daily life.
To obtain large enough sample sizes to have the power to address these questions, Wardle’s group used validated, psychometric measures of SR from the Child Eating Behaviour Questionnaire or its infant version (the Baby Eating Behaviour Questionnaire). In several community-based samples with objectively measured weights, they confirmed lower SR in obese than in normal-weight children, and a quantitative association between SR and weight across the distribution. To test the direction of causation more stringently (e.g., with a prospective design and holding environmental factors constant), they examined within-pair differences in growth trajectories up to 15 months in DZ twins differing in SR at 3 months.87 Weight data were predominantly from medical records of infant growth, and otherwise from parent-reported weights using scales sent to the home. As predicted, the twin with lower SR gained significantly more weight up to 15 months.
Wardle’s group has previously demonstrated high heritability (> 70%) of SR in two twin samples, and used the twin design to show a bivariate genetic association between SR and weight, implicating shared genetic etiology. More recent work has taken this forward by showing that a 32-SNP polygenic risk score (PRS) is linearly related to SR, and that SR mediated the PRS–adiposity association.88 The effort now is to look for associations between SR, meal patterns (from 3-day diet diaries), and growth. Their preliminary data show an association between SR and meal size at age 21 months, with no association between SR and either meal frequency or the energy density of meals. Meal size also appears to have a much stronger association with weight gain up to age 5 than does meal frequency or dietary energy density. Wardle’s group is planning to examine whether home-environment characteristics modify associations between appetite and weight.
The data support the conclusion that SR is an intermediate phenotype associated with obesity genes, and they help to explain how genetic and environmental influences are linked. The findings may stimulate interest in the role of satiety in the etiology of obesity.
Exercise and neurodegeneration; a potential therapeutic role for FNDC5/irisin
According to Christiane D. Wrann (Dana-Farber Cancer Institute and Harvard Medical School), studies in humans and several rodent models have shown that exercise can improve cognitive function; this has been linked to increased expression of brain-derived neurotrophic factor (BDNF).90 However, the underlying mechanisms driving the elevation of this neurotrophin remain unknown.
Cognitive impairment is a major socioeconomic health burden that is caused by a variety of conditions, including aging and neurodegenerative disorders such as Alzheimer’s disease. The disability associated with cognitive impairment is devastating, and the economic and social costs of caring for the affected individuals are staggering; yet effective treatment options are woefully inadequate, primarily due to a lack of therapeutic targets. For example, it is estimated that 5.1 million Americans may have Alzheimer’s disease and prevalence doubles every 5 years beyond age 65. The national costs of caring for individuals with Alzheimer’s disease are estimated at more than $100 billion annually.91 On the other side starkly stands the lack of effective therapeutic options.
Exercise—especially endurance exercise—is known to have beneficial effects on brain health and cognitive function.90,92 The improvement in cognitive function with exercise, namely in learning and memory, has been most prominently observed in the aging population.93 Exercise has also been reported to ameliorate outcomes in neurological diseases like depression, epilepsy, stroke, and Alzheimer’s and Parkinson’s diseases. The effects of exercise on the brain are most apparent in the hippocampus and the dentate gyrus, parts of the brain involved in learning and memory. Specific beneficial effects of exercise in the brain include increases in blood flow to the hippocampus and increased hippocampal size in humans. Exercise also causes morphological changes in dendrites and dendritic spines, increased synaptic plasticity, and de novo neurogenesis in the dentate gyrus in various rodent models of exercise.
Wrann, Spiegelman et al. previously reported that the protein FNDC5 and its secreted form, irisin, are important mediators of the beneficial effects of exercise on metabolism. They showed that FNDC5 is also elevated by endurance exercise in the hippocampus and reported a novel pathway regulating BDNF expression in the brain after exercise. Mice were exercised for 30 days using voluntary free-wheel running and compared to sedentary control mice, which were housed without a running wheel. Subsequent gene expression analysis revealed increased Fndc5 in the hippocampus.
Further mechanistic studies in primary neuronal cell cultures and in mice lacking PGC-1a (Pgc1a−/− mice) identified the metabolic transcriptional co-activator PGC-1a as a major inducer of FNDC5, which supports the notion of cross talk between metabolism and the brain in exercise. In turn, FNDC5 can drive increased expression of the aforementioned important neurotrophin Bdnf and other neuroprotective genes. In contrast, decreasing FNDC5 through RNA interference (RNAi)-mediated knockdown reduces Bdnf.
Having shown that FNDC5 is a molecular link between exercise and increased BDNF in the brain, Wrann and colleagues asked whether genetically increasing FNDC5 in the absence of exercise would have a similar effect to exercise itself. Using adenoviral vectors, the FNDC5 levels in the liver and, consequently, the amount of circulating irisin in the blood, were raised. Seven days later, the brains of the mice were examined, and a significant increase in BDNF and other neuroprotective proteins was observed in the hippocampus.94
Taken together, their findings link endurance exercise and the important metabolic mediators, PGC-1a and FNDC5, with BDNF expression in the brain. They are currently investigating the identity of the irisin receptor. While more research will be required to determine whether the FNDC5/irisin protein actually improves cognitive function in animals, this study suggest that a natural substance given in the bloodstream might mimic some of the effects of endurance exercise on the brain.
Description of a study modifying young couples’ lifestyle choices to prevent diabetes in the next generation
Susanne Stormer of Novo Nordisk described the company’s pilot initiative to improve women’s health and lifestyle choices before and during pregnancy to reduce maternal and offspring risk of developing diabetes later in life, that can be replicated, scaled, and sustainably run in different settings. The project is underway in partnership with the Ministry of Health in Malaysia, where rapid socioeconomic, dietary, and lifestyle changes have let to a threefold increase in diabetes prevalence in women of reproductive age. The program targets young couples responsibly planning pregnancies before conception, seen as the best time to prevent GDM and other forms of diabetes. After studying lifestyles habits of this young population, the Malaysian pilot initiative will explore ways to encourage women to adopt healty lifestyles, including changing their diets and exercise habits, to demonstrate that prepregnancy interventions can improve health during pregnancy, reduce GDM, and show that these interventions improve long-term health outcomes for both mothers and children.
Urban adolescent fitness for the next generation
Fitness goes beyond appearance or athletic ability. It is adaptive and expresses the physiological status of an individual and, in distinction to “health” is measurable. Examples of measures of physical fitness are cardiorespiratory endurance, muscular endurance and strength, body composition, and flexibility.95 Among these cardiorespiratory fitness is an important indicator of health and cardiometabolic risk in children and adolescents.96
In his second talk, Nico Rizzo discussed the latest brief on fitness among U.S. youth aged 12–15 years released by the National Center for Health Statistics,97 which reported that adequate fitness levels of cardiorespiratory fitness decreased from 52.4% in 1999–2000 to 42.2% in 2012, a 20% decrease in one decade. Overweight and obese children had substantially lower levels of cardiorespiratory fitness compared to normal-weight youth,97 related to the fact that approximately 30% of children age 6–11 are overweight and 15% are obese, with similar prevalences among adolescents age 12–19: 30% overweight, 16% obese.98 These developments demand integrative interventions that can reverse these negative trends and equip a new generation of urban adolescents with the necessary conditions for improving their fitness and health.
Longer duration and higher intensity of physical activity result in improved cardio-respiratory fitness.96 Rizzo argued that interventions and programs should address the need for safe local places and sufficient time for urban adolescents to pursue physical activities that will develop and improve their fitness. Such programs should include school curricula with regular physical activity classes adapted to the needs of all students, focusing on activities that improve cardiovascular fitness more than once per week. Urban planning and community initiatives must include “green zones” and parks that provide spaces for play and exercise, safe pedestrian and bicycle paths that allow for physical activity on the go, with events that ignite and foster the fun of physical activity on a community scale. Sweden and other countries have implemented policies that facilitate safe physical activity and mobility in urban environments. Cities such as Curitiba, Brazil and Austin, Texas and many others including the EPODE program7 mentioned in Dr. Wahlqvist’s presentation, are focusing on livability in modern urban centers. However, to be successful in improving the fitness of a new generation of urban adolescents, any initiative should make the healthy choice the easier or natural choice to make, even for those that are disadvantaged.
Summary and review: diabesity in 2015—healthy habits and possible policies
Problems
Maladaptive mechanisms
Human evolution has qualitatively and quantitatively favored physiologic homeostatic mechanisms for detecting, assimilating, and storing nutrients, which, in the preindustrial era, was offset by the exertion of obtaining, producing, processing, and preserving nutrients. The transition from foraging to agriculture increased sedentism, decreasing lower-body bone density and muscle mass. Mechanization and the built environment of developed nations also effectively diminished exertion without providing sufficient necessary alternatives for modern humans to prevent the dysmetabolic diathesis of diabesity caused by overnutrition and underactivity leading to molecular stress, inflammation, dysbiosis, insulin resistance, and, ultimately, sarcopenic organ failure.
Seemingly paradoxically, undernutrition and privation in less developed settings have similar consequences, particularly during (rapid) re-alimentation exceeding the anabolic capacity of individuals, presenting a “dilemma of philanthropy.” Increased exports to undeveloped nations are causing similar problems of mechanization locally, although at more rapid rates and with more severe consequences. In developed nations, pharmacological and surgical methods are available to remedy diabesity but are rarely used to prevent it, owing to the prevailing disease-oriented model of medical care neglecting prevention and/or emanating from the conviction that drugs and operations are intrinsically dangerous or simply too costly.
Poverty and ignorance
The two greatest obstacles to achieving energy balance, the ultimate objective of human behavior, are poverty and ignorance. Neither were obstacles to the coexistence of species competing for energy, mates, and habitat until Homo sapiens evolved the drive to overcome shortages of these commodities (Latin: commoditas = fitness, adaptation). The question is whether Homo sapiens are wise enough to allocate enough resources to provide necessary and sufficient education to overcome poverty.
Poverty underlies many of the subjects of this conference, and was aptly identified but not addressed directly, although Wahlqvist elegantly explained the importance of loss of sustainable ecosystems and the role of agricultural productivity in remedying micro- and macronutrient deficiencies. Problematic in this setting is the trade-off between low-cost, energy-rich, and palatable calories (junk food) and the low quality of the nutrients—the contrast between “tasty” and “healthy” and the effects of dietary choices driving environmental sustainability.99
Diet and exercise
Natural growth is slow and consistent (although predictably circadian and seasonal), whereas accelerated or sudden methods of decreasing intake (dieting), and/or increasing exertion (exercising), termed lifestyle modification, are maladaptive in the developed nations where they are volitionally practiced. In the presence of numerous redundant counter-regulatory physiologic mechanisms preserving lean body mass (LBM) or set point, these methods may cause illness. Ratchet effects of cycling, as in dieting or repeated pregnancies, characteristic of working life and modern aging, create new allostatic plateaus, difficult to reverse, impairing quality and length of life. Intake is a greater determinant of energy balance than expenditure in the mechanized world, where overnutrition and sedentism are driving the dysmetabolic diathesis of diabesity.
In commenting on the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study addressing the current 50% prevalence of the disease in the United States in the New England Journal of Medicine in 2012, David B. Allen, pointed out: “Fifty years ago [when prevalences were 3%] children did not avoid obesity by making healthy choices: they simply lived in an environment that provided fewer calories and included more physical activity for all.”100 The huge business of dangling tempting palatable choices before the eyes of children participating in parental food shopping exerts real pressure on the parent in industrialized nations. Although this specifically refers to affluent overnutrition, there are substantial similarities with underresourced populations everywhere.
Physical activity
In a recent systematic review, Hall et al. created a mathematical model of childhood growth at ages 5–18, predicting the energy equivalents of healthy and obese children of both sexes and proposing the magnitude and timing of treatment that might inform policy: “At the population level, the excess weight of US children in 2003–06 was associated with a mean increase in energy intake of roughly 200 kcal per day per child compared with similar children in 1976–80.”101 Clearly, there is a need for reasonably simple methods to increase daily expenditure by 200 kcal/day in school children.
Just as there are few studies of mechanisms maintaining leanness, remarkably little attention is paid to maintaining wellness or fitness. Prevention and quality-of-life aspects of medical care and patient satisfaction have only recently emerged as significant goals of the medical–industrial complex, yet are the ultimate determinants of sustainability of proposed remedies. Conflict between econometric public health models and care of individual patients (i.e., between policy and medical practice) is based on the dilemma of choosing between investing in prevention versus paying to reduce extant suffering. Effective global policies, if achievable, cannot mitigate the differential distribution of natural resources and impoverishing, unpredictable catastrophes, thus arguing for local policies except to address truly global threats (e.g., anthropogenic climate change, pollution, and depletion of unrenewable resources).
The goal of reversing maladaptive behavior requires durable adherence to health-sustaining behaviors (habits) that can realistically be sustained on the individual level. Such habits are easiest to establish during the period of greatest neuroplasticity starting in the womb and continuing through pubarche, where the intrauterine environment is the most sensitive and responsive.102
Solutions?
The magnitude and scope of the problems are well-known and authoritatively documented with impressive supporting information (Wahlqvist). New knowledge about contributions to the problem have surfaced: remote patrilineal inheritance of famine-induced genomic changes (Rizzo), outcomes of unintended pregnancies (Finer), RNA damage in sperm (Krawetz), effects of urbanization on the microbiome (Dominguez-Bello), food choices during gestation (Menella), and disturbances of satiety responsiveness in children engendering behavioral susceptibility (Wardle). New tools for assessing population-level nutritional exposure have been proposed, paving the way for epigenetic epidemiology (Waterland).
In the domain of potentially malleable mechanisms, we have learned that RNA damage in sperm might be reversible (Krawetz), that metastable epialleles are sensitive to periconceptional maternal diet (Waterland), and that novel molecules affecting metabolic fitness and related neurocognitive function have been identified (Wrann), as well as new data on the effects of dietary phenol exposure on appetitive behavior and adiposity (Desai, Ross).
The following provides constructive suggestions related to measuring and predicting the dysmetabolic diathesis and methods for prevention and treatment (Szymanski, Krebs), while identifying major systemic obstacles, including limitations to the sustainability of methods.
Measurement
Obesity is easy and inexpensive to detect and predict. It is recognized by preschool children expressing preferences for classmates according to size and shape, reflecting corpulence or large body size. Adiposity, considered synonymous with obesity, more narrowly refers to increased body fat, requiring body composition measurement for accurate assessment. It is unfortunate that obesity (derived from the Latin ob = over and edere = to eat), a size phenotype, has become a catch-all for a multifaceted collection of metabolic disorders, related but with very different manifestations. Diabesity defines the dysmetabolic or diseased state.103 The preferred metric of obesity is the body mass index (BMI), adjusting weight for height (stature) squared. It requires a scale and a reasonably precise measure of height.
Metabolic phenotype is better reflected in measures of fat distribution validated across races and over the life cycle, where central or abdominal accumulation, measured as waist (defined as maximum circumference between bottom of rib cage to top of hip bone), is a robust marker of cardiometabolic and mortality risk. The necessary adjustment for stature is achieved by the weight:height ratio (WHtR)—not to be confused with the waist:hip ratio (WHR). Margaret Ashwell has provided a most practical primary screening tool: the use of a stretched string to measure height (or recumbent length), folding the string in the middle (halving it) and then using the resultant length to measure waist circumference. If the length is too short to encircle the waist, WHtR is greater than the optimal 0.5, thus indicating increased metabolic risk,104 requiring action.
I propose a minimal metabolic exam that includes (1) subjective strength of handshake (surrogate for hand dynamometry), (2) standard medical history, and (3) height and waist circumference (waist:height ratio by “string test”), augmented by resource requiring (4) body weight (scales), (5) urine microalbumin, and (6) finger-stick HbA1c, HDL, and C-reactive protein, these chemical analyses requiring transfer to expensive analytical equipment in high-capacity centralized reference laboratories in a functioning public health system.
Education
Education is primal. Although obesity is easy to detect, there is an important awareness gap, not only for recognizing obesity but also for understanding its health implications. The barriers are not exclusively related to education and poverty, but also to perpetuated ethnic or cultural biases that affect access to care. If parents and caregivers in the strata with the highest prevalences of obesity are ignorant, in denial, or confused, it will be very difficult to prevent diabesity in children. If benevolent leaders of populations do not understand or believe the severity of threats to the health of their people, it is not likely that they will enact possible policies or allocate resources to implement and sustain them. On the other hand, if the people have access to credible information (enabled in this age of communication) they might be able to adopt healthy habits aimed at reversing maladaptive behavior, regardless of their ability to hold their leaders accountable. These processes require effective, accessible public education with well-defined reformulated goals incorporating the neglected topic of human fitness.
Predictors
There is ample evidence from epidemiologic studies to predict risk of offspring diabesity without the use of scarce resources. The majority of items in Table 2 only require physical presence and obtaining a competent history, which are, unfortunately, not universally possible in vulnerable populations susceptible to diabesity. Each item alone is robustly associated with diabesity and should trigger preventive measures, without a need for sophisticated technology.
Table 2.
Predictors of diabesity in girls and boys
| Genetic | Environmental |
|---|---|
| Gene polymorphisms/mutations | Socioeconomic |
| Ethnicity | Low income |
| Hispanic | Food uncertainty |
| Black | High-fat, low-protein diet |
| Pacific islander | Low education |
| Native American | “Traditional beliefs” |
| Female family history | Sedentism |
| Obesity | Television |
| Impaired glucose tolerance | Computers |
| Type 2 diabetes mellitus | Labor-saving devices |
| Automobile | |
| Elevator | |
| Gestational | |
| Maternal disease | Fetal factors |
| Obesity | Macrosomia |
| Diabetes | Undernutrition |
| Gestational | Hyperinsulinemia |
| Eclampsia, pre-eclampsia | Stress |
| Toxemia | Maternal |
| Substance abuse | diet |
| Dysbiosis | medication |
| Gestational stress | bacteria (dysbiosis) |
| Cesarean section | |
| Antibiotics | |
| Postnatal factors | |
| High birth weight | Lack of exercise |
| Low birth weight/catch-up growth | Television watching |
| Rapid neonatal weight gain (>100 g/month for 4 months) | Web surfing |
| “Eating style” | Lack of sleep |
| Maternal stress | Evening chronotype |
| Formula feeding | Excess consumption of added sugars (e.g., soft drinks (SSB)) |
| Distracted mothering | Restaurant eating |
| Chaotic environment | Stress |
Prevention
Prevention and treatment are inseparable: healthy habits, when adopted, have palliative as well as curative potential and provide primary and secondary prevention. Correction of intrauterine energy balance can override genetic susceptibility to obesity and obesogenic lifestyle during childhood, leveling the trajectory of the dysmetabolic syndrome and pre-empting adult disease,105,106 providing pre-emptive or primordial prevention, which has been demonstrated using metabolic surgery. Regardless, since operations cannot realistically be offered to the increasing numbers of metabolically unfit fertile girls and women, large-scale accessible methods must be given priority.
Health by a thousand cures
In the final analysis, changing maladaptive behavior has the capacity to mitigate effects of maladapted genes by “fighting fire with fire” through mechanisms of epigenetics, cellular plasticity, and pluripotency, some of which have contributed to the maladaptation in the first place. Contrary to “death by a thousand cuts,” the present paradigm suggests “health by a thousand cures.” Table 3 is an inventory ranging from small, simple, cheap “cures” (e.g., healthy habits) to ambitious policy-requiring initiatives, altogether resulting in creeping normalcy based on the premise that major positive change, which occurs slowly, in many unnoticed increments, is not perceived as burdensome or objectionable, thus more likely to succeed (possible policy).
Table 3.
“Health by a thousand cures”
| Home and neighborhood (healthy habits) |
Anthropometry
|
Energy balance
|
Sleep
|
| Ecosystem targets (possible policies) |
Food providers
|
Schools
|
Departments of Health
|
Medical practice
|
Summary
Achieving energy balance for sustainable metabolic fitness requires cutting food intake: quantity, rate, and choices, as well as increasing exertion, sleep, and socializing, adapting both ingestion and exertion to human chronobiology. Changes must start before conception, extending beyond the years of reproductive capacity throughout the entire life cycle, keeping in mind “grandparent effects” benefiting the next generation but also the substantial costs of caring for unhealthy aging populations threatening gross national products worldwide. With an evolutionary developmental perspective, a plan of action would start by prioritizing the fitness of the founder generation. Maybe extension of the concept of fetal rights to include provision of an optimal intrauterine environment preventing known contributors to postnatal disease carried into adulthood could move politicians to support educational programs, including preconception counseling, effective long-term anticontraceptives, and expanded gestational services. Table 4 summarizes potential items for action, chronologically, without any rank order, and without ruling out concomitant implementation of several items.
Table 4.
Educational targets for reversing the dysmetabolic environment.
| 1. Fitness and developmental origins of disease | 6. Breast feeding |
| 2. Protection of girls and women | 7. Eating rate (behavior) |
| 3. Contraception | 8. Chronobiology, Sleep |
| 4. Physical activity, sedentism, gestation | 9. Environmental temperature |
| 5. Endocrine disruptors | 10. Early education (preschool) |
We cannot and will not reverse evolved advances in technology and medical care that benefit and improve the quality and duration of lives of billions of people, although leaving many more behind. The true quandary, after identifying immutable obstacles imposed by physical determinants of life on Earth, is to balance the benefits against the risks inherent in these benefits, as is done in many settings, especially the responsible practice of medicine. Achieving consensus on this balance consonant with equitable distribution of humane values transcends human understanding and decisional capacity. Homo sapiens are maybe not so sapient after all.
Acknowledgments
Dr. Mennella is supported by grant DC011287 from the National Institute of Deafness and Other Communication Disorders and by grants HD37119 and HD072307 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. Dr. Wrann’s work was supported by the JPB Foundation and NIH grants (DK31405 and DK90861) and a K99/R00 Pathway to Independence Award (NS087096).
Appendix: supplementary reading
Biology of metabolism
- Arruda AP, Pers BM, Parlakgül G, Güney E, Inouye K, Hotamisligil GS. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med. 2014;20:1427–35. doi: 10.1038/nm.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Yuk F, Puri R, Janssen WG, Rapp PR, Morrison JH. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc Natl Acad Sci U S A. 2014;111:486–91. doi: 10.1073/pnas.1311310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, McEwen BS. Mitochondria impact brain function and cognition. Proc Natl Acad Sci U S A. 2014;111:7–8. doi: 10.1073/pnas.1321881111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Juster RP, McEwen BS. Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids. Nat Rev Endocrinol. 2014;10:303–10. doi: 10.1038/nrendo.2014.22. [DOI] [PubMed] [Google Scholar]
- Dalmas E, Donath MY. A role for interleukin-22 in the alleviation of metabolic syndrome. Nat Med. 2014;20:1379–81. doi: 10.1038/nm.3748. [DOI] [PubMed] [Google Scholar]
- Reig-Viader R, Capilla L, Vila-Cejudo M, et al. Telomere homeostasis is compromised in spermatocytes from patients with idiopathic infertility. Fertil Steril. 2014;102:728–738. doi: 10.1016/j.fertnstert.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Meyer SN, Amoyel M, Bergantiños C, et al. An ancient defense system eliminates unfit cells from developing tissues during cell competition. Science. 2014 Dec 5;346(6214):1258236. doi: 10.1126/science.1258236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ascertainment/monitoring
- Levitsky DA, Garay J, Nausbaum M, Neighbors L, Dellavalle DM. Monitoring weight daily blocks the freshman weight gain: a model for combating the epidemic of obesity. Int J Obes (Lond) 2006;30:1003–10. doi: 10.1038/sj.ijo.0803221. [DOI] [PubMed] [Google Scholar]
- Helander EE, Vuorinen AL, Wansink B, Korhonen IK. Are breaks in daily self-weighing associated with weight gain? PLoS One. 2014 Nov 14;9(11):e113164. doi: 10.1371/journal.pone.0113164. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison CL, Lombard CB, Teede HJ. Limiting postpartum weight retention through early antenatal intervention: the HeLP-her randomised controlled trial. Int J Behav Nutr Phys Act. 2014;11:134. doi: 10.1186/s12966-014-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Saunders DH, Shenkin SD, Sproule J. Lifestyle intervention for improving school achievement in overweight or obese children and adolescents. Cochrane Database Syst Rev. 2014 Mar 14;3:CD009728. doi: 10.1002/14651858.CD009728.pub2. [DOI] [PubMed] [Google Scholar]
- Luley C, Blaik A, Aronica S, Dierkes J, Kropf S, Westphal S. Evaluation of three new strategies to fight obesity in families. J Nutr Metab. 2010 doi: 10.1155/2010/751905. pii: 751905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley P, Levine E, Askew S, Puleo E, et al. Weight gain prevention among black women in the rural community health center setting: the Shape Program. BMC Public Health. 2012 Jun 15;12:305. doi: 10.1186/1471-2458-12-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg DM, Levine EL, Lane I, et al. Adherence to self-monitoring via interactive voice response technology in an eHealth intervention targeting weight gain prevention among Black women: randomized controlled trial. J Med Internet Res. 2014 Apr 29;16(4):e114. doi: 10.2196/jmir.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radesky J, Miller AL, Rosenblum KL, Appugliese D, Kaciroti N, Lumeng JC. Maternal Mobile Device Use During a Structured Parent-Child Interaction Task. Acad Pediatr. 2014 Nov 22; doi: 10.1016/j.acap.2014.10.001. pii: S1876–2859(14)00338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Predictors
- Kral JG. Preventing and treating obesity in girls and young women to curb the epidemic. Obes Res. 2004;12:1539–46. doi: 10.1038/oby.2004.193. [DOI] [PubMed] [Google Scholar]
- McAllister EJ, Dhurandhar NV, Keith SW, et al. Ten putative contributors to the obesity epidemic. Crit Rev Food Sci Nutr. 2009;49:868–913. doi: 10.1080/10408390903372599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng SF, Redsell SA, Swift JA, Yang M, Glazebrook CP. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97:1019–26. doi: 10.1136/archdischild-2012-302263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Contraception
- Bailey MJ, Malkova O, Norling J. Do family planning programs decrease poverty? Evidence from public census data. CESifo Econ Stud. 2014;60:312–337. doi: 10.1093/cesifo/ifu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Physical activity, sedentism, gestational exercise
- Kibbe DL, Hackett J, Hurley M, et al. Ten Years of TAKE 10!(®): Integrating physical activity with academic concepts in elementary school classrooms. Prev Med. 2011 Jun;52(Suppl 1):S43–50. doi: 10.1016/j.ypmed.2011.01.025. http://www.nea.org/tools/in-classroom-exercise.html. [DOI] [PubMed] [Google Scholar]
- Vara L, Agras S. Caloric intake and activity levels are related in young children. Int J Obes. 1989;13:613–7. [PubMed] [Google Scholar]
- Francois ME, Baldi JC, Manning PJ, et al. ‘Exercise snacks’ before meals: a novel strategy to improve glycaemic control in individuals with insulin resistance. Diabetologia. 2014;57:1437–45. doi: 10.1007/s00125-014-3244-6. [DOI] [PubMed] [Google Scholar]
- Lee DC, Pate RR, Lavie CJ, et al. Leisure-time running reduces all-cause and cardiovascular mortality risk. J Am Coll Cardiol. 2014;64:472–81. doi: 10.1016/j.jacc.2014.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen CP, Tsai MK, Wai JP, Wu X. Promoting increased physical activity and reduced inactivity - Authors’ reply. Lancet. 2013;381:114–5. doi: 10.1016/S0140-6736(13)60046-X. [DOI] [PubMed] [Google Scholar]
- May LE, Scholtz SA, Suminski R, Gustafson KM. Aerobic exercise during pregnancy influences infant heart rate variability at one month of age. Early Hum Dev. 2014;90:33–8. doi: 10.1016/j.earlhumdev.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Jukic AM, Lawlor DA, Juhl M, et al. Physical activity during pregnancy and language development in the offspring. Paediatr Perinat Epidemiol. 2013;27:283–93. doi: 10.1111/ppe.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson AM, Bucci DJ. Physical exercise during pregnancy improves object recognition memory in adult offspring. Neuroscience. 2014;256:53–60. doi: 10.1016/j.neuroscience.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Endocrine disruptors
- de Cock M, de Boer MR, Lamoree M, Legler J, van de Bor M. First year growth in relation to prenatal exposure to endocrine disruptors – a Dutch prospective cohort study. Int J Environ Res Public Health. 2014;11:7001–21. doi: 10.3390/ijerph110707001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Breastfeeding, diet
- Reyes M, Hoyos V, Martinez SM, et al. Satiety responsiveness and eating behavior among Chilean adolescents and the role of breastfeeding. Int J Obes (Lond) 2014;38:552–7. doi: 10.1038/ijo.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehler HL, Chaput KH, Tough SC. Risk factors for cessation of breastfeeding prior to six months postpartum among a community sample of women in Calgary, Alberta. Can J Public Health. 2009;100:376–80. doi: 10.1007/BF03405274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok E, Multon C, Piguel L, et al. Decreased full breastfeeding, altered practices, perceptions, and infant weight change of prepregnant obese women: a need for extra support. Pediatrics. 2008;121(5):e1319–24. doi: 10.1542/peds.2007-2747. [DOI] [PubMed] [Google Scholar]
- Odom EC, Li R, Scanlon KS, Perrine CG, Grummer-Strawn L. Reasons for earlier than desired cessation of breastfeeding. Pediatrics. 2013;131(3):e726–32. doi: 10.1542/peds.2012-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauff LE, Leonard SA, Rasmussen KM. Associations of maternal obesity and psychosocial factors with breastfeeding intention, initiation, and duration. Am J Clin Nutr. 2014;99:524–34. doi: 10.3945/ajcn.113.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JO, Wright G, Herman AN, et al. “Snacks are not food”. Low-income, urban mothers’ perceptions of feeding snacks to their preschool-aged children. Appetite. 2015;84:61–7. doi: 10.1016/j.appet.2014.09.007. [DOI] [PubMed] [Google Scholar]
Eating rate (eating style or behavior)
- Stunkard A, Kaplan D. Eating in public places: a review of reports of the direct observation of eating behavior. Int J Obes. 1977;1:89–101. [PubMed] [Google Scholar]
- Agras WS, Kraemer HC, Berkowitz RI, Korner AF, Hammer LD. Does a vigorous feeding style influence early development of adiposity? J Pediatr. 1987;110:799–804. doi: 10.1016/s0022-3476(87)80029-x. [DOI] [PubMed] [Google Scholar]
- Kral JG, Buckley MC, Kissileff HR, Schaffner F. Metabolic correlates of eating behavior in severe obesity. Int J Obes Relat Metab Disord. 2001;25:258–64. doi: 10.1038/sj.ijo.0801469. [DOI] [PubMed] [Google Scholar]
- Otsuka R, Tamakoshi K, Yatsuya H, et al. Eating fast leads to insulin resistance: findings in middle-aged Japanese men and women. Prev Med. 2008;46:154–9. doi: 10.1016/j.ypmed.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Galhardo J, Hunt LP, Lightman SL, et al. Normalizing eating behavior reduces body weight and improves gastrointestinal hormonal secretion in obese adolescents. J Clin Endocrinol Metab. 2012;97:E193–201. doi: 10.1210/jc.2011-1999. [DOI] [PubMed] [Google Scholar]
- Zandian M, Ioakimidis I, Bergström J, et al. Children eat their school lunch too quickly: an exploratory study of the effect on food intake. BMC Public Health. 2012 May 14;12:351. doi: 10.1186/1471-2458-12-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Ranawana DV, Tan WJ, Quek YC, Henry CJ. The impact of eating methods on eating rate and glycemic response in healthy adults. Physiol Behav. 2015;139:505–10. doi: 10.1016/j.physbeh.2014.12.014. [DOI] [PubMed] [Google Scholar]
Sleep duration, chronotype: “morningness”
- Morselli LL, Guyon A, Spiegel K. Sleep and metabolic function. Pflugers Arch. 2012;463:139–60. doi: 10.1007/s00424-011-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen EA, Zhao X, Rother KI, et al. Evening chronotype is associated with changes in eating behavior, more sleep apnea and increased stress hormones in short sleeping obese individuals. PLoS One. 2013;8(3):e56519. doi: 10.1371/journal.pone.0056519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Environmental temp
- Celi FS, Brychta RJ, Linderman JD, et al. Minimal changes in environmental temperature result in a significant increase in energy expenditure and changes in the hormonal homeostasis in healthy adults. Eur J Endocrinol. 2010;163:863–72. doi: 10.1530/EJE-10-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Preschool programs
- Campbell F, Conti G, Heckman JJ. Early childhood investments substantially boost adult health. Science. 2014;343:1478–85. doi: 10.1126/science.1248429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Microbiome
- Jasti C, Hug B, Waters JL, Whitaker RJ. How do small things make a big difference? Activities to teach about human-microbe interactions. Am Biol Teach. 2014;76:601–608. doi: 10.1525/abt.2014.76.9.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Brooks D. In praise of small miracles. http://www.nytimes.com/2014/12/12/opinion/david-brooks-in-praise-of-small-miracles.
- 2. [Accessed September 26, 2014];FAO Report The State of Food and Agriculture. 2013 http://www.fao.org/publications/sofa/2013/en/
- 3.Wahlqvist ML, Specht RL. Food variety and biodiversity: Econutrition. Asia Pac J Clin Nutr. 1998 Dec;7(3/4):314–9. [PubMed] [Google Scholar]
- 4.Healthy Americans. [Accessed September 26, 2014];F as in Fat: How Obesity Threatens America’s Future. 2012 http://www.healthyamericans.org/assets/files/TFAH2012FasInFat18.pdf.
- 5.Organisation for Economic Co-operation and Development. [Accessed September 26, 2014];2014 Report on Obesity. http://www.oecd.org/els/health-systems/Obesity-Update-2014.pdf.
- 6.Siahpush M, Huang TT, Sikora A, Tibbits M, Shaikh RA, Singh GK. Prolonged financial stress predicts subsequent obesity: results from a prospective study of an Australian national sample. Obesity. 2013;22:616–621. doi: 10.1002/oby.20572. [DOI] [PubMed] [Google Scholar]
- 7.Borys JM1, Le Bodo Y, Jebb SA, Seidell JC, Summerbell C, Richard D, De Henauw S, Moreno LA, Romon M, Visscher TL, Raffin S, Swinburn B EEN Study Group. EPODE approach for childhood obesity prevention: methods, progress and international development. Obes Rev. 2012 Apr;13(4):299–315. doi: 10.1111/j.1467-789X.2011.00950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health. 2014;104:S43–48. doi: 10.2105/AJPH.2013.301416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American College of Obstetricians and Gynecologists. Adolescents and Long-Acting Reversible Contraception: Implants and Intrauterine Devices. Committee Opinion No. 539. Obstet Gynecol. 2012;120:983–988. doi: 10.1097/AOG.0b013e3182723b7d. [DOI] [PubMed] [Google Scholar]
- 10.Jodar M, Selvaraju S, Sendler E, Diamond MP, Krawetz SA for the Reproductive Medicine Network. The presence, roles and clinical use of spermatozoal RNAs Human Reproduction Update. 2013;19:604–24. doi: 10.1093/humupd/dmt031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krawetz SA, Kruger A, Lalancette C, Tagett R, Anton E, Draghici S, Diamond M. A survey of small RNAs in human sperm. Human Reproduction. 2011;26:3401–3412. doi: 10.1093/humrep/der329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sendler E, Johnson GD, Mao S, Goodrich RJ, Diamond MP, Hauser R, Krawetz SA. Stability, Delivery and Functions of Human Sperm RNAs at Fertilization. Nucleic Acids Research. 2013;41:4104–4117. doi: 10.1093/nar/gkt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fullston T, Ohlsson Teague EM, Palmer NO, DeBlasio MJ, Mitchell M, Corbett M, Print CG, Owens JA, Lane M. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J. 2013;27:4226–43. doi: 10.1096/fj.12-224048. [DOI] [PubMed] [Google Scholar]
- 14.Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, Farinelli L, Miska E, Mansuy IM. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci. 2014;17:667–9. doi: 10.1038/nn.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moskovtsev SI, Jodar M, Mackie P, Kenigsberg S, Krawetz SA, Librach CL. RNA sequencing analysis of extracellular RNAs in human seminal fluid. CFAS 2014 Annual Meeting; Quebec City, Quebec, Canada. [Google Scholar]
- 16.Rizzo NS. Health Determinants - Epigenetics and an Integrative Model for Public Health. 141st APHA Annual Meeting and Exposition; November 2-November 6, 2013; Boston, Massachusetts: APHA; 2013. [Google Scholar]
- 17.Pembrey M, Saffery R, Bygren LO. Human transgenerational responses to early-life experience: potential impact on development, health and biomedical research. Journal of medical genetics. 2014;51:563–572. doi: 10.1136/jmedgenet-2014-102577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michels KB. The promises and challenges of epigenetic epidemiology. Experimental gerontology. 2010;45:297–301. doi: 10.1016/j.exger.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Rizzo NS. Epigenetics - A Public Health Perspective. 140th APHA Annual Meeting and Exposition; October 27-October 31, 2012; San Francisco, California: APHA; 2012. [Google Scholar]
- 21.Kaati G, Bygren LO, Pembrey M, Sjostrom M. Transgenerational response to nutrition, early life circumstances and longevity. European journal of human genetics: EJHG. 2007;15:784–790. doi: 10.1038/sj.ejhg.5201832. [DOI] [PubMed] [Google Scholar]
- 22.Bygren LO, Tinghog P, Carstensen J, et al. Change in paternal grandmothers’ early food supply influenced cardiovascular mortality of the female grandchildren. BMC genetics. 2014;15:12. doi: 10.1186/1471-2156-15-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zerres K, Eggermann T. Genetics and epigenetics. Explanatory approaches for (gender-specific) mechanisms of disease development. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz. 2014;57:1047–1053. doi: 10.1007/s00103-014-2013-5. [DOI] [PubMed] [Google Scholar]
- 24.Soubry A, Hoyo C, Jirtle RL, Murphy SK. A paternal environmental legacy: evidence for epigenetic inheritance through the male germ line. BioEssays: news and reviews in molecular, cellular and developmental biology. 2014;36:359–371. doi: 10.1002/bies.201300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. The New England journal of medicine. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham SA, Kramer MR, Narayan KMV. Incidence of childhood obesity in the United States. The New England journal of medicine. 2014;370:403–411. doi: 10.1056/NEJMoa1309753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huh SY, et al. Delivery by caesarean section and risk of obesity in preschool age children: a prospective cohort study. Archives of disease in childhood. 2012;97:610–616. doi: 10.1136/archdischild-2011-301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ege MJ, et al. Exposure to environmental microorganisms and childhood asthma. The New England journal of medicine. 2011;364:701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 29.Couzin-Frankel J. Bacteria and asthma: untangling the links. Science. 2010;330:1168–1169. doi: 10.1126/science.330.6008.1168. 330/6008/1168 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. The Journal of infectious diseases. 2008;198:553–560. doi: 10.1086/590158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167:821–827. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]
- 32.Kero J, et al. Mode of delivery and asthma -- is there a connection? Pediatric research. 2002;52:6–11. doi: 10.1203/00006450-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Roduit C, et al. Asthma at 8 years of age in children born by caesarean section. Thorax. 2009;64:107–113. doi: 10.1136/thx.2008.100875. [DOI] [PubMed] [Google Scholar]
- 34.Decker E, et al. Cesarean delivery is associated with celiac disease but not inflammatory bowel disease in children. Pediatrics. 2010;125:e1433–1440. doi: 10.1542/peds.2009-2260. [DOI] [PubMed] [Google Scholar]
- 35.Marild K, Stephansson O, Montgomery S, Murray JA, Ludvigsson JF. Pregnancy outcome and risk of celiac disease in offspring: a nationwide case-control study. Gastroenterology. 2012;142:39–45. e33. doi: 10.1053/j.gastro.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Algert CS, McElduff A, Morris JM, Roberts CL. Perinatal risk factors for early onset of Type 1 diabetes in a 2000–2005 birth cohort. Diabetic medicine: a journal of the British Diabetic Association. 2009;26:1193–1197. doi: 10.1111/j.1464-5491.2009.02878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonifacio E, Warncke K, Winkler C, Wallner M, Ziegler AG. Cesarean section and interferon-induced helicase gene polymorphisms combine to increase childhood type 1 diabetes risk. Diabetes. 2011;60:3300–3306. doi: 10.2337/db11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho I, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henao-Mejia J, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell SL, et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO reports. 2012;13:440–447. doi: 10.1038/embor.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aumeunier A, et al. Systemic Toll-like receptor stimulation suppresses experimental allergic asthma and autoimmune diabetes in NOD mice. Plos One. 2010;5:e11484. doi: 10.1371/journal.pone.0011484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Codolo G, et al. The neutrophil-activating protein of Helicobacter pylori down-modulates Th2 inflammation in ovalbumin-induced allergic asthma. Cellular microbiology. 2008;10:2355–2363. doi: 10.1111/j.1462-5822.2008.01217.x. CMI1217 [pii] [DOI] [PubMed] [Google Scholar]
- 43.Yatsunenko T, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schnorr SL, et al. Gut microbiome of the Hadza hunter-gatherers. Nature communications. 2014;5:3654. doi: 10.1038/ncomms4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Filippo C, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. PNAS. 2010 doi: 10.1073/pnas.1005963107. Early edition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Streuling I, Beyerlein A, Rosenfeld E, Hofmann H, Schulz T, von Kries R. Physical activity and gestational weight gain: a meta-analysis of intervention trials. BJOG. 2011;118:278–84. doi: 10.1111/j.1471-0528.2010.02801.x. [DOI] [PubMed] [Google Scholar]
- 47.Tobias DK, Zhang C, van Dam RM, Bowers K, Hu FB. Physical activity before and during pregnancy and risk of gestational diabetes mellitus: A meta-analysis. Diabetes Care. 2011;34:223–9. doi: 10.2337/dc10-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bellamy L, Casas J-P, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systemic review and meta-analysis. BMJ. 2007;335:974–86. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. Department of Health and Human Services; Washington, DC: 2008. [Google Scholar]
- 50.Szymanski LM, Satin AJ. Exercise during pregnancy: Fetal responses to current public health guidelines. Obstet Gynecol. 2012;119:603–10. doi: 10.1097/AOG.0b013e31824760b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hochberg Z, Feil R, Constancia M, et al. Child health, developmental plasticity, and epigenetic programming. Endocrine Reviews. 2011;32(2):159–224. doi: 10.1210/er.2009-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Symonds ME, Sebert SP, Hyatt MA, Budge H. Nutritional programming of the metabolic syndrome. Nature Reviews Endocrinology. 2009;5(11):604–610. doi: 10.1038/nrendo.2009.195. [DOI] [PubMed] [Google Scholar]
- 53.Salsberry PJ, Reagan PB. Effects of heritability, shared environment, and nonshared intrauterine conditions on child and adolescent BMI. Obesity. 2010;18(9):1775–1780. doi: 10.1038/oby.2009.485. [DOI] [PubMed] [Google Scholar]
- 54.Waterland RA, Garza C. Potential mechanisms of metabolic imprinting that lead to chronic disease. Am J Clin Nutr. 1999;69:179–197. doi: 10.1093/ajcn/69.2.179. [DOI] [PubMed] [Google Scholar]
- 55.Waterland RA, Michels KB. Epigenetic Epidemiology of the Developmental Origins Hypothesis. Annu Rev Nutr. 2007;27:363–388. doi: 10.1146/annurev.nutr.27.061406.093705. [DOI] [PubMed] [Google Scholar]
- 56.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waterland RA, et al. Maternal methyl supplements increase offspring DNA methylation at Axin fused. Genesis. 2006;44:401–406. doi: 10.1002/dvg.20230. [DOI] [PubMed] [Google Scholar]
- 58.Waterland RA, et al. Season of conception in rural gambia affects DNA methylation at putative human metastable epialleles. PLoS Genet. 2010;6:e1001252. doi: 10.1371/journal.pgen.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dominguez-Salas P, et al. Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nature communications. 2014;5:3746. doi: 10.1038/ncomms4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore SE, et al. Season of birth predicts mortality in rural Gambia. Nature. 1997;388:434. doi: 10.1038/41245. [DOI] [PubMed] [Google Scholar]
- 61.Glass TA, McAtee MJ. Behavioral science at the crossroads in public health: extending horizons, envisioning the future. Soc Sci Med. 2006;62(7):1650–71. doi: 10.1016/j.socscimed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 62.Mennella JA, Finkbeiner S, Lipchock SV, Hwang LD, Reed DR. Preferences for salty and sweet tastes are elevated and related to each other during childhood. PLoS One. 2014;9:e92201. doi: 10.1371/journal.pone.0092201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mennella JA, Pepino MY, Duke FF, Reed DR. Age modifies the genotype-phenotype relationship for the bitter receptor TAS2R38. BMC Genet. 2010;11:60. doi: 10.1186/1471-2156-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trabulsi JC, Mennella JA. Diet, sensitive periods in flavour learning, and growth. Int Rev Psychiatry. 2012;24(3):219–30. doi: 10.3109/09540261.2012.675573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mennella JA, Trabulsi JC. Complementary foods and flavor experiences: setting the foundation. Ann Nutr Metab. 2012;60 (Suppl 2):40–50. doi: 10.1159/000335337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 67.Desai M, Gayle DA, Babu J, Ross MG. Programmed obesity in intrauterine growth restricted newborns: Modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol. 2005;288:R91–R96. doi: 10.1152/ajpregu.00340.2004. [DOI] [PubMed] [Google Scholar]
- 68.Desai M, Jellyman KJ, Han G, Ross MG. Rat Maternal Obesity and High Fat Diet Program Offspring Metabolic Syndrome. Am J Obstet Gynecol. 2014;237:e1–e13. doi: 10.1016/j.ajog.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Desai M, Li T, Ross MG. Hypothalamic neurosphere progenitor cells in low birth-weight rat newborns: neurotrophic effects of leptin and insulin. Brain Res. 2011;1378:29–42. doi: 10.1016/j.brainres.2010.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Desai M, Li T, Han G, Ross MG. Programmed hyperphagia secondary to increased hypothalamic SIRT1. Brain Res. 2014;14:252–259. doi: 10.1016/j.brainres.2014.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Desai M, Han G, Ferelli M, Kallichanda N, Lane RH. Programmed Upregulation of Adipogenic Transcription Factors in Intrauterine Growth Restricted Offspring. Reprod Sci. 2008;15:785–796. doi: 10.1177/1933719108318597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yee JK, Lee WN, Ross MG, Lane RH, Han G, Vega J, Desai M. Peroxisome proliferator-activated receptor gamma modulation and lipogenic response in adipocytes of small-for-gestational age offspring. Nutr Metab (Lond) 2012;22:62–73. doi: 10.1186/1743-7075-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Young BE, Johnson SL, Krebs NF. Biological determinants linking infant weight gain and child obesity: current knowledge and future directions. Adv Nutr. 2012;3(5):675–686. doi: 10.3945/an.112.002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singhal A. Does breastfeeding protect from growth acceleration and later obesity? Nestle Nutr Workshop Ser Pediatr Program. 2007;60:15–25. doi: 10.1159/000106357. [DOI] [PubMed] [Google Scholar]
- 75.Taveras EM, Rifas-Shiman SL, Sherry B, et al. Crossing growth percentiles in infancy and risk of obesity in childhood. Arch Pediatr Adolesc Med. 2011;165(11):993–998. doi: 10.1001/archpediatrics.2011.167. [DOI] [PubMed] [Google Scholar]
- 76.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331(7522):929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weng SF, Redsell SA, Swift JA, Yang M, Glazebrook CP. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97(12):1019–1026. doi: 10.1136/archdischild-2012-302263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Du Y, Yang M, Lee S, et al. Maternal western diet causes inflammatory milk and TLR2/4-dependent neonatal toxicity. Genes Dev. 2012;26(12):1306–1311. doi: 10.1101/gad.191031.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oben JA, Mouralidarane A, Samuelsson AM, et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol. 2010;52(6):913–920. doi: 10.1016/j.jhep.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 80.Young BE, Morrow A, Davidson B, Geraghty S, Patinkin Z, Krebs NF. 4-Hydroxynonenol (HNE) is present in human milk and related to gestational age at delivery and excessive infant weight gain. FASEB J. 2014;28:2476. (Oral Presentation) [Google Scholar]
- 81.Young BE, Patinkin Z, Reynolds R, DeLaHoussaye R, Barbour L, Hernandez T, Friedman J, Krebs N. Differences in Comprehensive Characterization of Human Milk between Lean and Overweight Women do not Affect Infant Fat or Fat-Free Mass Deposition; Presented at the International Society of Research in Human Milk and Lactation (ISRHML) Bi-Annual Conference; October, 2014; pp. 56–7. Available at: http://isrhml.net/download/proceedings-from-17th-isrhml-conference/Abstract T3 006. (Oral Presentation) [Google Scholar]
- 82.Young BE, Morrow A, Davidson B, Geraghty S, Patinkin Z, Krebs NF. 4-Hydroxynonenol (HNE) is present in human milk and related to gestational age at delivery and excessive infant weight gain. FASEB J. 2014;28:2476. (Oral Presentation) [Google Scholar]
- 83.Young BE, Patinkin Z, Reynolds R, DeLaHoussaye R, Barbour L, Hernandez T, Friedman J, Krebs N. Differences in Comprehensive Characterization of Human Milk between Lean and Overweight Women do not Affect Infant Fat or Fat-Free Mass Deposition; Presented at the International Society of Research in Human Milk and Lactation (ISRHML) Bi-Annual Conference; October, 2014; pp. 56–7. Available at: http://isrhml.net/download/proceedings-from-17th-isrhml-conference/Abstract T3 006. (Oral Presentation) [Google Scholar]
- 84.Young BE, Patinkin Z, Reynolds R, DeLaHoussaye R, Barbour L, Hernandez T, Friedman J, Krebs N. Differences in Comprehensive Characterization of Human Milk between Lean and Overweight Women do not Affect Infant Fat or Fat-Free Mass Deposition; Presented at the International Society of Research in Human Milk and Lactation (ISRHML) Bi-Annual Conference; October, 2014; pp. 56–7. Available at: http://isrhml.net/download/proceedings-from-17th-isrhml-conference/Abstract T3 006. (Oral Presentation) [Google Scholar]
- 85.Baughcum AE, Powers SW, Johnson SB, et al. Maternal feeding practices and beliefs and their relationships to overweight in early childhood. J Dev Behav Pediatr. 2001;22(6):391–408. doi: 10.1097/00004703-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 86.Young BE, Farazandeh S, Westra K, Krebs NF. Maternal Beliefs Surrounding Infant Feeding, but not Maternal BMI or Hospital Experience, Predict Breastfeeding Exclusivity and Behavior; Presented at the International Society of Research in Human Milk and Lactation (ISRHML) Bi-Annual Conference; October, 2014; p. 161. Available at: http://isrhml.net/download/proceedings-from-17th-isrhml-conference/Abstract CS 017. Poster Presentation. [Google Scholar]
- 87.Carnell S, Wardle J. Appetite and adiposity in children – evidence for a behavioral susceptibility theory of obesity. American Journal of Clinical Nutrition. 2008;88:22–29. doi: 10.1093/ajcn/88.1.22. [DOI] [PubMed] [Google Scholar]
- 88.van Jaarsveld CH, Boniface D, Llewellyn CH, Wardle J. Appetite and growth: a longitudinal sibling analysis. JAMA Pediatrics. 2014;168:345–350. doi: 10.1001/jamapediatrics.2013.4951. [DOI] [PubMed] [Google Scholar]
- 89.Llewellyn CH, Trzaskowski M, van Jaarsveld CH, Plomin R, Wardle J. Satiety mechanisms in genetic risk of obesity. JAMA Pediatrics. 2014;168:338–344. doi: 10.1001/jamapediatrics.2013.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends in cognitive sciences. 2013;17(10):525–44. doi: 10.1016/j.tics.2013.08.001. Epub 2013/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.http://www.alz.org/downloads/Facts_Figures_2014.pdf
- 92.Prakash RS, Voss MW, Erickson KI, Kramer AF. Physical Activity and Cognitive Vitality. Annual review of psychology. 2014 doi: 10.1146/annurev-psych-010814-015249. Epub 2014/09/25. [DOI] [PubMed] [Google Scholar]
- 93.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–30. doi: 10.1111/1467-9280.t01-1-01430. Epub 2003/03/29. [DOI] [PubMed] [Google Scholar]
- 94.Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18:649–59. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public health reports (Washington, DC: 1974) 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 96.Rizzo NS, Ruiz JR, Hurtig-Wennlof A, Ortega FB, Sjostrom M. Relationship of physical activity, fitness, and fatness with clustered metabolic risk in children and adolescents: the European youth heart study. The Journal of pediatrics. 2007;150:388–394. doi: 10.1016/j.jpeds.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 97.Gahche J, Fakhouri T, Carroll DD, Burt VL, Wang CY, Fulton JE. NCHS data brief: 1–8. 2014. Cardiorespiratory fitness levels among U.S. youth aged 12–15 years: United States, 1999–2004 and 2012. [PubMed] [Google Scholar]
- 98.CDC NCHS Health E-Stat. Prevalence of overweight, obesity and extreme obesity among adults: United States, trends 1960–62 through 2005–2006. National Center for Health Statistics; [Accessed December 1, 2014]. http://www.cdc.gov/nchs/data/hestat/overweight/overweight_adult.pdf. [Google Scholar]
- 99.Tilman D, Clark M. Global diets link environmental sustainability and human health. Nature. 2014;515:518–22. doi: 10.1038/nature13959. [DOI] [PubMed] [Google Scholar]
- 100.Allen DB. TODAY--a stark glimpse of tomorrow. N Engl J Med. 2012;366:2315–6. doi: 10.1056/NEJMe1204710. [DOI] [PubMed] [Google Scholar]
- 101.Hall KD, Butte NF, Swinburn BA, Chow CC. Dynamics of childhood growth and obesity: development and validation of a quantitative mathematical model. Lancet Diabetes Endocrinol. 2013;1(2):97–105. doi: 10.1016/s2213-8587(13)70051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roth TL, Sweatt JD. Annual research review: epigenetic mechanisms and environmental shaping of the brain during sensitive periods of development. J Child Psychol Psychiat. 2011;52:398–408. doi: 10.1111/j.1469-7610.2010.02282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kral JG. Diabesity: palliating, curing or preventing the dysmetabolic diathesis. Maturitas. 2014;77:243–8. doi: 10.1016/j.maturitas.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 104.Ashwell M, Gibson S. A proposal for a primary screening tool: ‘Keep your waist circumference to less than half your height’. BMC Med. 2014;12:207. doi: 10.1186/s12916-014-0207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kral JG. ABC of obesity. Management: Part III--surgery. BMJ. 2006;333:900–3. doi: 10.1136/bmj.333.7574.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guénard F, Deshaies Y, Cianflone K, Kral JG, Marceau P, Vohl MC. Differential methylation in glucoregulatory genes of offspring born before vs. after maternal gastrointestinal bypass surgery. Proc Natl Acad Sci U S A. 2013;110:11439–44. doi: 10.1073/pnas.1216959110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378(9793):804–14. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]