Abstract
Stereotypy can be characterized as inflexible, repetitive behaviors that occur following repeated exposure to psychostimulants, such as cocaine (COC). Stereotypy may be related to preferential activation of the patch (striosome) compartment of striatum, as enhanced relative activation of the patch compartment has been shown to positively correlate with the emergence of stereotypy following repeated psychostimulant treatment. However, the specific contribution of the patch compartment to COC-induced stereotypy following repeated exposure is unknown. To elucidate the involvement of the patch compartment to the development of stereotypy following repeated COC exposure, we determined if destruction of this sub-region altered COC-induced behaviors. The neurons of the patch compartment were ablated by bilateral infusion of the neurotoxin dermorphin-saporin (DERM-SAP; 17 ng/μl) into the striatum. Animals were allowed to recover for eight days following the infusion, and then were given daily injections of COC (25 mg/kg) or saline for one week, followed by a weeklong drug-free period. Animals were then given a challenge dose of saline or COC, observed for 2h in activity chambers and sacrificed. The number of mu-labeled patches in the striatum were reduced by DERM-SAP pretreatment. In COC-treated animals DERM-SAP pretreatment significantly reduced the immobilization and intensity of stereotypy but increased locomotor activity. DERM-SAP pretreatment attenuated COC-induced c-Fos expression in the patch compartment, while enhancing COC-induced c-Fos expression in the matrix compartment. These data indicate that the patch compartment contributes to repetitive behavior and suggests that alterations in activity in the patch vs matrix compartments may underlie to this phenomenon.
Keywords: psychostimulant, immediate early gene, behavior, basal ganglia, sensitization, striosome
1. Introduction
The basal ganglia are critically important in the regulation of movement and alterations in the function of the striatum contributes to the development of movement disorders (Crittenden and Graybiel, 2011). The striatum can be divided into the patch (striosome) and matrix compartments based on differential connectivity and the expression of neuropeptides and receptors (Crittenden and Graybiel, 2011; Gerfen et al., 1985; Gerfen and Wilson, 1996; Gerfen and Young, 1988; Graybiel, 1990). The patch compartment is thought to be a limbic channel that runs through the striatum, as it receives inputs from prelimbic cortex and amygdala, while the matrix compartment is considered to be a motor channel traversing the striatum, since it receives inputs from sensorimotor and associative forebrain regions (Bolam et al., 1988; Gerfen, 1984; McDonald, 1992; Ragsdale and Graybiel, 1988). The specific functions of these compartments are not completely understood, but several lines of data suggest that the patch and matrix compartments may sub-serve different aspects of motor activity and behavior. For example, treatment with high or repeated doses of psychostimulants results in enhanced immediate early gene (Canales and Graybiel, 2000; Cole et al., 1995; Graybiel et al., 1990; Horner and Keefe, 2006; Moratalla et al., 1992; Tan et al., 2000; Wang et al., 1995) and neuropeptide expression (Adams et al., 2003; Cole et al., 1995; Fagergren et al., 2003; Horner and Keefe, 2006; Wang et al., 1995) in the patch compartment relative to the surrounding matrix.
Psychostimulant treatment can also result in stereotypy, which is defined as the development of abnormally repetitive motor actions that coincides with an inability to initiate normal adaptive responses (Canales and Graybiel, 2000; Cole et al., 1995; Graybiel et al., 1990; Graybiel and Rausch, 2000). Psychostimulant-induced stereotypic behaviors may be related to enhanced activation of the rostral aspects of the patch compartment relative to the surrounding rostral matrix compartment, as the relative degree of c-Fos expression in the patch compartment in the rostral region of striatum correlates positively with the development of stereotypic behavior following psychostimulant treatment (Canales and Graybiel, 2000). Interestingly, this relationship was specific only for the rostral aspects of striatum, as there was not a significant correlation between psychostimulant-induced patch-enhanced c-Fos expression and stereotypy in more middle or caudal regions of the striatum (Canales and Graybiel, 2000). Furthermore, a recent study from our laboratory found that ablation of the neurons of the rostral patch compartment resulted in reduced levels of stereotypy in response to a single, high dose of methamphetamine, suggesting that the rostral aspect of this striatal sub-region contributes to the expression of psychostimulant-induced repetitive behaviors (Murray et al., 2014).
While a single, high dose of methamphetamine is sufficient to induce significant stereotypy, as well as a patch-enhanced pattern of gene expression in the rostral striatum, a single high dose of COC induces locomotor activity without significant stereotypy and results in a more homogeneous pattern of striatal gene expression (Adams et al., 2003; Aliane et al., 2009; Canales and Graybiel, 2000; Graybiel et al., 1990; Horner and Keefe, 2006; Murray et al., 2014). However, when given repeatedly and followed by a challenge dose of the drug, COC can induce a patch-enhanced pattern of striatal activation in the rostral striatum and stereotypic behavior will develop (Aliane et al., 2012; Aliane et al., 2009; Canales, 2005a; Canales and Graybiel, 2000; Saka et al., 2004). It is thought that a shift towards patch-enhanced activity observed after repeated psychostimulant treatment reflects an accentuation of the limbic-based motivationally linked pathways through the striatum, relative to the sensorimotor striatal pathways, which may result in a narrowing of the behavioral focus, while promoting its repetition (Graybiel et al., 2000). Although a number of studies have examined the relative levels of activation of the patch vs. matrix compartments following psychostimulant exposure, the functional role of the patch compartment in the development of stereotypic behaviors when psychostimulants are repeatedly administered is heretofore unknown.
In order to address the role of the patch compartment of rostral striatum in the expression of stereotypy following repeated COC treatment, we utilized the neurotoxin dermorphin-saporin (DERM-SAP (Lawhorn et al., 2009; Murray et al., 2014; Tokuno et al., 2002); to specifically ablate the neurons that comprise the rostral patch compartment prior to exposure to COC. Mu opioid receptors are densely expressed by the neurons of the patch compartment, while the neurons of the matrix compartment contain relatively few mu opioid receptors (Herkenham and Pert, 1981; Pert et al., 1976; Tempel and Zukin, 1987). Dermorphin is a potent mu opioid receptor agonist that induces mu opioid receptor internalization (Giagnoni et al., 1984), while saporin is a cytotoxin that impairs ribosomal function (Wiley and Kline, 2000). Thus, internalization of the DERM-SAP complex will ultimately lead to the destruction of mu opioid receptor-containing neurons, such as those found in the patch compartment of striatum, while leaving non-mu opioid receptor expressing neurons, such as the majority of those found in the matrix compartment relatively intact.
The goal of this study was to determine whether preferential ablation of the patch compartment of the rostral striatum altered the development of stereotypic behavior after repeated treatment with COC, followed by a drug-free period and a subsequent exposure to a challenge dose of COC. We then examined c-Fos expression in the patch and matrix compartments of rostral striatum, as well as in the adjacent nucleus accumbens, in order to determine the impact of patch compartment lesions on the relative degree of compartmental activation following a challenge dose of COC.
2. Results
2.1. Effects of DERM-SAP infusion on mu opioid receptor immunoreactivity in the striatum
Infusion of DERM-SAP into striatum reduced mu opioid receptor immunoreactivity relative to infusion of unconjugated SAP (Fig. 2A). A student’s t-test revealed that DERM-SAP pretreatment significantly reduced the number of mu opioid receptor-labeled patches in the striatum (t = 7.66, p < 0.0001, Fig. 2B). An unpaired t-test also revealed that DERM-SAP pretreatment significantly reduced the total area of patches present in the striatum (t = 6.112, p < 0.0001, Fig. 2C).
Figure 2.
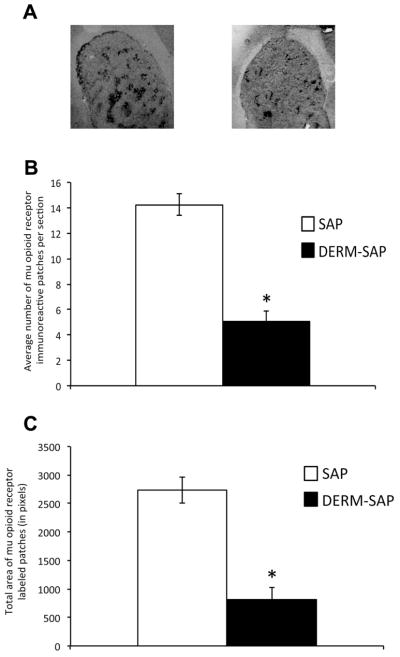
Effects of DERM-SAP infusion on mu opioid receptor immunoreactivity in the striatum. Infusion with the neurotoxin DERM-SAP (17 ng/μl) resulted in a decrease in mu opioid receptor immunoreactivity in the striatum (A). DERM-SAP treatment significantly reduced the overall number of mu-labeled patches in the striatum (B) and the total size of mu opioid receptor immunoreactive patches (C), as compared to animals treated with unconjugated SAP. *p<0.05 vs. SAP-treated control animals.
2.2. Effects of DERM-SAP pretreatment on COC-induced behaviors
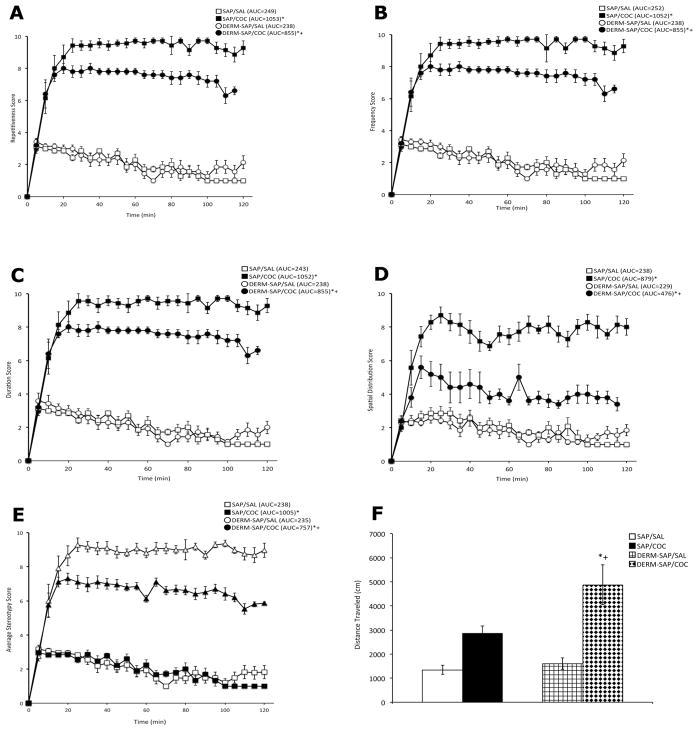
A two-way analysis of variance of the area under the curve (AUC) values for repetitiveness scores, which is an index of the degree to which an animal repeated a particular behavior, (Fig. 3A) during the two hour observation period revealed a significant main effect for DERM-SAP pretreatment (F1,22 = 26.7, p < 0.0001), COC treatment (F1,22 = 1228, p < 0.0001), and a significant interaction of pretreatment X treatment (F1,22 = 21.2, p < 0.0001). Post-hoc analysis revealed that COC treatment significantly increased the degree of repetitiveness in both SAP (p < 0.0001) and DERM-SAP (p < 0.0001) pretreated animals, but the increase in the degree of repetition induced by COC was significantly lower in DERM-SAP-pretreated animals than in SAP-pretreated animals (p=0.0002). DERM-SAP pretreatment alone did not significantly affect the repetitiveness scores of saline-treated animals (p > 0.05).
Figure 3.
Effects of intrastriatal infusion of DERM-SAP (17 ng/μl) and repeated SAL or COC treatment (25 mg/kg, twice daily for one week, followed by a weeklong drug-free period) with a subsequent SAL or COC challenge (25 mg/kg) on the degree of behavioral repetition (A), the frequency of the repeated behavior (B), the duration of the repetitive behavior (C), the spatial distribution of the behavior (D), the average stereotypy score, which is the average of the above behavioral dimensions (E), and locomotor activity (F). Values are expressed as the mean ±SEM. Locomotor activity is expressed as the total distance traveled in cm for the entire 2h observation period. For all other behavioral measures, the AUC values are in parentheses. *Significantly different from respective control group, p<0.005; +Significantly different from SAP-pretreated COC-treated group, p<0.005.
A two-way analysis of variance of the AUC values for frequency scores, which is an index of how often the animal engaged in the repetitive behavior, (Fig. 3B) during the two hour observation period revealed a significant main effect for DERM-SAP pretreatment (F1,22 = 26.6, p < 0.0001), COC treatment (F1,22 = 1199, p < 0.0001), and a significant interaction of pretreatment X treatment (F1,22 = 19.9, p=0.0002). Post-hoc analysis revealed that COC treatment significantly increased the frequency of the repetitive behavior in both SAP (p < 0.0001) and DERM-SAP (p < 0.0001) pretreated animals, but the increase in the frequency of the behavior induced by COC was significantly lower in DERM-SAP-pretreated animals than in SAP-pretreated animals (p=0.0002). DERM-SAP pretreatment alone did not significantly affect the frequency scores of saline-treated animals (p > 0.05).
A two-way analysis of variance of the AUC values for duration scores, which is an index of the length of time the animal spent engaged in the repetitive behavior, (Fig. 3C) during the two hour observation period revealed a significant main effect for DERM-SAP pretreatment (F1,22 = 27.4 p < 0.0001), COC treatment (F1,22 = 1370, p < 0.0001), and a significant interaction of pretreatment X treatment (F1,22 = 27.8, p < 0.0001). Post-hoc analysis revealed that COC treatment significantly increased the duration of the repetitive behavior in both SAP (p < 0.0001) and DERM-SAP (p < 0.0001) pretreated animals, but the increase in the duration of the behavior induced by COC was significantly lower in DERM-SAP-pretreated animals than in SAP-pretreated animals (p=0.002). DERM-SAP pretreatment alone did not significantly affect the duration scores of saline-treated animals (p > 0.05).
A two-way analysis of variance of the AUC values for spatial distribution scores, which is an index of the degree of confinement of the animal to a single location within the apparatus (Fig. 3D) during the two hour observation period revealed a significant main effect for DERM-SAP pretreatment (F1,22= 91.50 p < 0.0001), COC treatment (F1,22= 520.9, p < 0.0001), and a significant interaction of pretreatment X treatment (F1,22 = 110.4, p < 0.0001). Post-hoc analysis revealed that COC treatment significantly increased the degree of confinement in both SAP (p < 0.05) and DERM-SAP (p < 0.05) pretreated animals, but the increase in spatial confinement induced by COC was significantly lower in DERM-SAP-pretreated animals than in SAP-pretreated animals (p < 0.05). DERM-SAP pretreatment alone did not significantly affect the spatial distribution scores of saline-treated animals (p > 0.05).
A two-way analysis of variance of the AUC values for overall stereotypy behavior scores, which are the averages of the four behavioral dimensions described above (Fig. 3E) during the two hour observation period revealed a significant main effect for DERM-SAP pretreatment (F1,22 = 51.0, p <0.0001), COC treatment (F1,22 = 3150.0, p < 0.0001), and a significant interaction of pretreatment X treatment (F1,22 = 49.0 p < 0.0001). Post-hoc analysis revealed that stereotyped behavior was significantly increased following COC treatment in both SAP- (p < 0.05) and DERM-SAP- (p < 0.05) pretreated animals. However, the COC-induced increase in stereotyped behavior was significantly lower in DERM-SAP-pretreated animals than in SAP-pretreated animals (p < 0.05). DERM-SAP pretreatment alone did not significantly affect stereotyped behavior in saline-treated animals (p > 0.05).
A two-way analysis of variance of total distance traveled (Fig. 3F) during the two hour observation period revealed a significant main effect for DERM-SAP pretreatment (F1,14 = 7.8, p = 0.0143), COC treatment (F1,14 = 35.0 p < 0.0001), and a significant interaction of pretreatment X treatment (F1,14 = 4.6, p = 0.0491). Post-hoc analysis revealed that COC treatment significantly increased the total distance traveled in both SAP (p < 0.05) and DERM-SAP (p < 0.05) pretreated animals, but the COC-induced increase in horizontal activity was significantly greater in DERM-SAP pretreated animals than in SAP-pretreated animals (p < 0.05). DERM-SAP pretreatment alone did not significantly affect horizontal activity in saline-treated animals (p > 0.05).
2.3. Effects of DERM-SAP pretreatment on c-Fos immunoreactivity in the core and shell of the nucleus accumbens
Analysis of c-Fos immunoreactivity was performed on a 400 × 400 square pixel area in the core and shell of NAc. Analysis of c-Fos immunoreactivity in the NAc core revealed a significant main effect for COC treatment (F1,21 =17.5, p=0.0004) but not a significant main effect for DERM-SAP pretreatment (F1,21 =0.694, p=0.415) or pretreatment X treatment interaction (F1,21=1.4, p=0.250; data not shown). Post-hoc analysis revealed that COC treatment significantly increased NAc core c-Fos immunoreactivity (p=0.0005). Analysis of c-Fos immunoreactivity in the NAc shell revealed a significant main effect for COC treatment (F1,22=22.22, p=0.0001) but not a significant main effect for DERM-SAP pretreatment (F1,22=0.153, p=0.702) or a pretreatment X treatment interaction (F1,22=0.04, p=0.844; data not shown). Post-hoc analysis revealed that COC treatment significantly increased NAc shell c-Fos immunoreactivity (p<0.0001).
2.4 Effects of DERM-SAP pretreatment on c-Fos immunoreactivity in the patch and matrix compartments of striatum
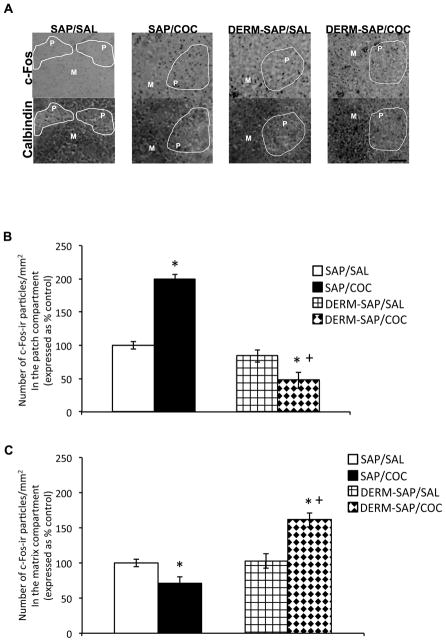
Analysis of c-Fos immunoreactivity in the patch and matrix compartments was performed on a 1024 × 768 square pixel area in the dorsal central region of rostral striatum. Prior ablation of the patch compartment with DERM-SAP appeared to prevent COC-induced increases in c-Fos immunoreactive particles in the patch compartment, while increasing c-Fos immunoreactive particles in the matrix compartment of the striatum (Fig. 4A). A two-way analysis of variance of c-Fos immunoreactivity in the patch compartment revealed a significant main effect of DERM-SAP pretreatment (F1,18=65.12, p<0.0001), a significant main effect of COC treatment (F1,18=9.03, p=0.0073) and a significant pretreatment × treatment interaction (F1,18=39.9, p<0.0001; Fig. 4B). Post-hoc analysis revealed that in DERM-SAP pretreated animals, COC treatment did not significantly increase the number of c-Fos immunoreactive particles/mm2 in the patch compartment, relative to DERM-SAP pretreated, saline-treated animals (p>0.05). In addition, there were significantly fewer c-Fos immunoreactive particles/mm2 in the patch compartment of DERM-SAP-pretreated, COC-treated animals, as compared to SAP-pretreated, COC-treated animals (p<0.05). COC treatment significantly increased the number c-Fos immunoreactive particles/mm2 in the patch compartment of SAP-pretreated animals (p<0.05), and DERM-SAP treatment alone did not significantly alter the number c-Fos immunoreactive particles/mm2 in the patch compartment (p>0.05). There was a significant correlation between the number of neutral red-labeled particles and c-Fos-labeled particles in several randomly selected striatal sections, which verified that we had positively identified Fos-labeled nuclei (p=0.0002; rs=0.89).
Figure 4.
Effects of DERM-SAP pretreatment on COC-induced immunoreactivity in the patch and matrix compartments of striatum. Representative photomicrographs showing calbindin and c-Fos immunoreactivity in adjacent sections of the striatum (A). Scale bar represents 100 μM. Semi-quantitative analysis of c-Fos immunoreactivity in the patch (B) and matrix (C) compartments of rats intrastriatally infused with SAP or DERM-SAP (17 ng/μl), 8 days prior to repeated treatment with SAL oe COC (25 mg/kg, twice daily for one week, followed by a weeklong drug-free period), and a subsequent challenge dose of SAL or COC (25 mg/kg). Data are presented as the percentage of the number of c-Fos immunoreactive particles/mm2 in SAP-treated control animals. *p<0.05 vs. SAP-pretreated control animals; +p<0.05 vs. SAP-pretreated COC-treated animals.
A two-way analysis of variance of c-Fos immunoreactivity in the matrix compartment revealed a significant main effect of DERM-SAP pretreatment (F1,16=48.62, p<0.0001), a significant main effect of COC treatment (F1,16=8.03, p=0.012) and a significant pretreatment × treatment interaction (F1,16=42.62, p<0.0001; Fig. 4C). Post-hoc analysis revealed that COC treatment significantly increased the number c-Fos immunoreactive particles/mm2 in the matrix of DERM-SAP pretreated animals, as compared to DERM-SAP pretreated saline animals (p<0.05). COC treatment also significantly increased the number of c-Fos immunoreactive particles/mm2 in the matrix of DERM-SAP pretreated animals, as compared to SAP-pretreated, COC-treated animals (p<0.05). In SAP-pretreated animals, COC treatment significantly reduced the number of c-Fos immunoreactive particles/mm2 in the matrix compartment, as compared to SAP-pretreated, saline-treated animals (p<0.05). DERM-SAP pretreatment alone did not significantly alter the number of c-Fos immunoreactive particles/mm2 in the matrix compartment (p>0.05).
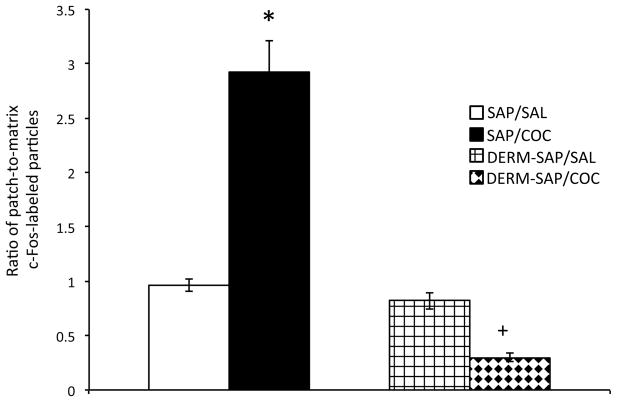
A two-way analysis of variance of the patch-to-matrix ratio of c-Fos immunoreactive particles was calculated by dividing the number of particles in patch by the number of particles in the matrix in the 1024 × 760 square pixel area for each animal, and revealed that there were significant main effects of DERM-SAP pretreatment (F1,19=55.24, p<0.0001; Fig. 5) and COC treatment (F1,19=14.87, p=0.001) and a significant pretreatment × treatment interaction (F1,19=44.4, p<0.0001). Post-hoc analysis of significant effects indicated that there was a significant difference in the ratio of patch-to-matrix c-Fos immunoreactivity in the striatum of SAP-pretreated, SAL-treated animals and SAP-pretreated, COC-treated animals (p<0.05), and that DERM-SAP pretreatment abolished this effect, as the patch-to-matrix ratio of c-Fos immunoreactivity was significantly reduced in DERM-SAP-pretreated, COC-treated animals, as compared to SAP-pretreated, COC-treated animals (p<0.05). However, there was not a significant difference in the patch-to-matrix ratio of c-Fos immunoreactivity in DERM-SAP-pretreated, SAL-treated and DERM-SAP-pretreated, COC-treated animals (p>0.05). DERM-SAP pretreatment alone did not significantly alter the patch-to-matrix ratio of c-Fos immunoreactivity (p>0.05).
Figure 5.
Effects of DERM-SAP pretreatment on the ratio of patch-to-matrix c-Fos immunoreactivity in the rostral striatum following repeated COC treatment and challenge. Repeated treatment with SAL or COC (25 mg/kg, twice daily for one week, followed by a weeklong drug-free period) and a subsequent SAL or COC challenge (25 mg/kg) significantly increased the ratio of patch-to-matrix c-Fos immunoreactivity in SAP-pretreated animals, an effect that was attenuated by prior treatment with DERM-SAP (17 ng/μl). *p<0.05 vs. SAP-pretreated control animals; +p<0.05 vs. SAP-pretreated COC-treated animals.
3. Discussion
The goal of the current study was to determine whether perturbation of the patch compartment of rostral striatum would have an impact on the behaviors induced by repeated COC treatment followed by a subsequent challenge dose and alter the relative levels of immediate early gene activation in the rostral patch vs. matrix compartments of striatum. DERM-SAP administered intrastriatally resulted in approximately a 60–70% reduction in the size and total area of mu opioid receptor-labeled patches in the rostral striatum. Lesions of the rostral patch compartment resulted in a significant decrease in the stereotypic behavior and spatial confinement typically observed with repeated COC treatment, while significantly increasing horizontal activity. Repeated COC treatment followed by a challenge dose of the drug resulted in an increase in c-Fos immunoreactivity in the rostral patch compartment, coupled with a decrease in c-Fos immunoreactivity in the rostral matrix compartment, while ablation of the rostral patch compartment prevented induction of c-Fos by COC within this region, and enhanced COC-induced c-Fos expression in the rostral matrix compartment. These data are among the first to specifically demonstrate a role for the patch compartment of rostral striatum in the development of stereotypy following repeated exposure to COC and a subsequent challenge dose of the drug. These data indicate that preferential involvement of the patch-based limbic circuits that traverse the rostral striatum are necessary for the development of intense and focused repetitive behaviors and that the patch compartment may provide an interface whereby limbic-mediated internally-driven states can modify and influence the motoric and adaptive behavioral functions mediated by the basal ganglia.
Previous studies indicate that treatments which induce a low level of stereotypy result in relatively similar levels of immediate early gene and neuropeptide induction in the rostral patch vs. matrix compartments, while treatments that induce a high level of stereotypy induce significant stereotypy and result in greater relative immediate early gene and neuropeptide induction of the rostral patch compartment versus the matrix compartment (Adams et al., 2003; Aliane et al., 2009; Canales and Graybiel, 2000; Graybiel et al., 1990; Horner and Keefe, 2006). Alternatively, free movement or sensorimotor stimulation is linked to increased activation of the matrix compartment, as measured by glucose utilization (Brown et al., 2002) and animals will repeatedly electrically self-stimulate when the electrode is placed in or near the patch compartment (White and Hiroi, 1998). Based on these findings, as well as the differential inputs that have been described for the patch (i.e., limbic inputs from prelimbic cortex and amygdala) and matrix compartments (i.e., sensorimotor inputs from sensorimotor cortex), it has been suggested that the patch compartment may mediate motivationally-based, internally-cued, repetitive behaviors, while the matrix compartment may be related to the processing of externally-based sensory cues and sensorimotor information (Bolam et al., 1988; Canales, 2005b; Canales and Graybiel, 2000; Crittenden and Graybiel, 2011; Gerfen, 1984; Gerfen, 1989; Graybiel et al., 2000; Ragsdale and Graybiel, 1988; Saka and Graybiel, 2003).
The expression of stereotypy is thought to compete with locomotor activity, and it has been suggested that differential circuitry may contribute to the expression of each (Aliane et al., 2009; Canales and Graybiel, 2000). Specifically, psychostimulant-induced hyperlocomotor activity is the result of increased activation of the direct pathway, and while both compartments contain neurons that project to direct pathway nuclei, the bulk of the direct pathway arises from the matrix compartment, which is due in part to the fact that the matrix compartment is larger than the patch compartment (Crittenden and Graybiel, 2011; Fujiyama et al., 2011; Gerfen and Young, 1988; Nestler, 2001; Tokuno et al., 2002; Xu et al., 2000; Xu et al., 1994). The current data show that animals that underwent patch compartment lesions prior to repeated exposure to COC and a COC challenge dose exhibited increased locomotor activity and diminished stereotypy and enhanced relative immediate gene activation in the matrix compartment. In terms of how increased c-Fos induction in the matrix compartment relative to the patch compartment might relate to changes in behavior following repeated COC treatment and a subsequent challenge, when the patch compartment is fully intact, a subsequent dose of COC may result in relatively greater c-Fos induction in patch-based circuits and thereby masks the effects of c-Fos induction in matrix-based pathways, leading to greater levels of stereotypy as compared to locomotor activity. On the other hand, when the patch compartment is not fully functional, a subsequent dose of COC may tip the balance of c-Fos induction in favor of the matrix compartment following repeated COC treatment, leading to a greater degree of c-Fos induction in the direct pathway, and increased locomotor activity, as compared to stereotypy. It is important to note that while stereotypic behaviors involve the basal ganglia circuits that traverse the dorsal striatum, locomotor activity is thought to be associated with alterations in basal ganglia circuits involving the nucleus accumbens (Aliane et al., 2009; Bradberry, 2008; Broderick et al., 1984; Canales and Graybiel, 2000; Delfs et al., 1990; Ito et al., 2002; Kelly et al., 1975). Thus, compromising the patch compartment of dorsal striatum may have resulted in an unmasking of COC-induced locomotor activity as mediated by circuits through the nucleus accumbens. However, our data indicate that while COC treatment alone increased c-Fos immunoreactivity in the core and shell of the nucleus accumbens, pretreatment with DERM-SAP did not significantly enhance c-Fos immunoreactivity in the nucleus accumbens of COC-treated animals, which would seem to suggest that the increased locomotor activity observed in these animals may not have been the result of increased activation of the nucleus accumbens, which is in agreement with previous data (Murray et al., 2014).
The mechanisms that allow for the enhanced activation of the matrix compartment in response to a COC challenge following repeated COC treatment in animals with patch compartment lesions are not clear. The shift in patch-enhanced activation that is observed following chronic intermittent exposure to COC reflects a down-regulation of c-Fos induction in the matrix compartment, coupled with an up-regulation of c-Fos induction in the patch compartment (Canales and Graybiel, 2000; Saka et al., 2004). It is thought that this shift in relative compartmental immediate early gene induction may be due in part, to the induction of long-term depression within the matrix compartment (Canales, 2005a; Graybiel et al., 2000; Saka et al., 2004). The sensorimotor cortex, which sends inputs to the matrix compartment, is strongly activated by psychostimulant treatment, and a gradual depression of the glutamatergic inputs to the striatum from this region may underlie the reduced induction of c-Fos within the matrix compartment upon subsequent drug exposure (Graybiel and Canales, 2000; Graybiel et al., 2000; O’Dell and Marshall, 2000). Cholingeric and/or nitric oxide synthase (NOS)-positive striatal interneurons may also contribute to the down-regulation of immediate early gene induction in the matrix following repeated psychostimulant treatment, as targeted ablation of these interneurons leads to greater c-Fos inducibility in the projection neurons of the matrix relative to the patch compartment (Saka et al., 2002). Enhanced activity of the prelimbic cortex, which sends projections to the patch compartment, may also underlie the shift in the responsiveness of neurons in the patch and matrix compartments that occur following repeated psychostimulant exposure, as recurrent stimulation of the prelimbic cortex prior to a single dose of cocaine results in patch-enhanced pattern of c-Fos induction in the striatum, similar to what is seen following chronic cocaine treatment (Canales, 2005b). On the other hand, in animals with patch compartment lesions, the loss of inhibitory striatal inputs from the patch compartment to the dopaminergic neurons of the substantia nigra pars compacta could translate into enhanced dopamine release in the nigrostriatal pathway, which in the absence of fully intact patch compartment, could have preferentially activated matrix-based circuits, resulting in a release of locomotor activity following subsequent COC exposure.
Alternatively, changes in cross-compartmental communication following patch compartment lesions may have resulted in enhanced c-Fos activation in the matrix compartment upon subsequent COC exposure. It has been suggested that the patch compartment, through modulation of cholinergic and GABAergic interneurons that lie near the borders of the patch and matrix compartments can inhibit the projection neurons of the matrix compartment (Aosaki and Kawaguchi, 1996; Miura et al., 2007). Thus, in the absence of a fully intact patch compartment, the matrix compartment may be lifted from patch-mediated inhibition, allowing for enhanced c-Fos activation to occur in response to COC treatment. However, it is also important to note that under circumstances which are poorly understood, the patch compartment through enkephalin signaling, may release the neurons of the matrix from inhibition, which would suggest that ablation of the patch compartment may result in decreased activity of the matrix compartment (Miura et al., 2007; Stanford and Cooper, 1999). Clearly, additional studies are needed to examine the role that patch-matrix compartmental cross-talk through striatal interneurons may play in the relative levels of activation of these compartments.
Although our lesions were targeted to mu opioid receptor-expressing neurons of the rostral patch compartment (Herkenham and Pert, 1981; Pert et al., 1976; Tempel and Zukin, 1987), a very small portion of mu opioid receptors exist on the medium spiny neurons of the matrix compartment (Guttenberg et al., 1996; Kaneko et al., 1995), as well as on a subset of cholinergic interneurons (Jabourian et al., 2005), raising the possibility that regions outside of the patch compartment may have been compromised by DERM-SAP treatment. However, our group and others have previously shown that both the matrix compartment and cholinergic interneurons were unaffected following DERM-SAP treatment (Lawhorn et al., 2009; Murray et al., 2014). In addition, following DERM-SAP treatment, roughly 30% of mu opioid receptor-labeled patches remained intact, which could explain why there was not a complete abolishment of stereotypy following repeated COC treatment and challenge. It is also possible that extrastriatal regions such as the pedunculopontine nucleus or subthalamic nucleus may also contribute to psychostimulant-induced stereotypic behavior (Aliane et al., 2012; Barwick et al., 2000; Inglis et al., 1994; Mathur et al., 1997; Pallanti et al., 2010). Nevertheless, despite these potential limitations, these are among the data first to specifically examine the role of the patch compartment in the development of stereotypic behaviors following repeated COC treatment and challenge.
In summary, our study is the first to examine the functional role of the patch compartment in the development of stereotyped behaviors following persistent psychostimulant exposure by using a targeted neurotoxin to specifically lesion the patch compartment prior to repeated COC treatment and challenge. The current data demonstrate that the patch compartment must be intact in order for maximal expression of stereotypy in response to a challenge dose of COC following repeated COC exposure, indicating that activation of limbic-associated, patch-based circuits contributes to psychostimulant-induced repetitive behaviors. Lesions of the patch compartment also increased COC-induced locomotor activity and increased COC-induced activation of the matrix compartment, indicating that in the absence of enhanced activation of the patch compartment, the matrix-based circuits that mediate locomotor activity may be exposed, leading to decreased spatial confinement and augmented locomotor activity. Together, these data indicate that the patch compartment is an important component of the basal ganglia circuitry that mediates repetitive behaviors, and suggest that when the activity of this region is enhanced as a result of repeated psychostimulant exposure, internally-driven motivational states may overrule ongoing adaptive behaviors, leading to focused stereotypy and perhaps maladaptive habitual behaviors.
4. Experimental Procedures
4.1. Animals
All experiments utilized male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN, USA) weighing between 250–350g. Animals were housed in groups of four in plastic cages in a temperature-controlled room on a 14:10h light/dark cycle with ad libitum access to food and water. Animal housing and experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, as well as the Mercer University School of Medicine Institutional Animal Care and Use Committee. Power analyses were used to select the minimum number of animals needed for the experiments and effort was made to minimize pain and suffering during the course of the procedures.
4.2. Drugs and Chemicals
Ketamine hydrochloride and xylazine hydrochloride were obtained from Sigma Aldrich (St. Louis, MO, USA). (−) Cocaine (COC) hydrochloride was obtained as a generous gift from the National Institute of Drug Abuse (Bethesda, MD, USA). Free base conversions were used to calculate the doses for COC, ketamine, and xylazine dissolved in saline, and all drugs were delivered in volumes of 1 ml/kg. Demorphin-saporin (DERM-SAP) and unconjugated saporin (SAP) were obtained from Advanced Targeting Systems (San Diego, CA, USA) and dissolved in buffered artificial cerebrospinal fluid (aCSF; 144 mM NaCl, 2.68 mM KCl, 1.6 mM CaCl2, 2.6 mM MgCl2, and 0.4 mM KH2PO4; pH, 7.2).
4.3. DERM-SAP Infusion
Animals were fixed on a stereotaxic frame (Stoelting Company, Wood Dale, IL, USA) after being anesthetized with ketamine (90 mg/kg, i.p.) and xylazine (9 mg/kg, i.p.). Bilateral infusions of DERM-SAP and SAP were made by inserting a 31-gauge needle into the rostral striatum (coordinates based on bregma: +1.5 mm anterior, 2.5 mm lateral, −5.0 mm ventral (Paxinos and Watson, 2005) through a burr hole drilled into the exposed skull. Total infusion volumes were 2 μl (Tokuno et al., 2002) at a rate of 0.5 μl/min. After infusions were complete the needles were left in place for five minutes before being slowly removed in an effort to minimize fluid backflow. Animals were allowed to recuperate before being moved back to their home cages. Experiments began eight days after infusions (Tokuno et al., 2002). Animals were only included in subsequent analyses if during sectioning, their infusions were found to be located in the rostral striatum.
4.4. Experimental Design and Behavior
Eight days after infusions, animals (n=5–7/group) were injected i.p. bi-daily with COC (25 mg/kg) or saline (SAL) at 10:00 am and 5 pm for one week, followed by one week of drug withdrawal (Canales and Graybiel, 2000). At the end of the withdrawal period, COC-treated animals were given a challenge dose of COC (25 mg/kg, i.p.), while SAL-treated animals were challenged with SAL and subsequently placed in plexiglass activity chambers (46 × 46 × 12 cm; (Frankel et al., 2007; Horner et al., 2012; Horner et al., 2010; Murray et al., 2014) for 2 hours, during which time behavior was recorded. Behavioral footage for each animal was analyzed post-hoc by a rater blind to experimental conditions. Behavior was sampled for one minute every five minutes for the entire 2h observation period after injection of the challenge dose. Stereotypy was rated on a scale of 1–10, with 1 representing the minimal degree of response and 10 representing the maximal degree of response (Canales and Graybiel, 2000; Creese and Iverson, 1973; Horner et al., 2012; Horner et al., 2010; Murray et al., 2014). Stereotypy scores were obtained by averaging the scores from four other behavioral dimensions: repetitiveness/flexibility (the number of alternative motor responses emitted), frequency (the number of responses per unit time), duration (the percentage of time spent performing the most dominant response(s)), and the spatial distribution of the motor response which is an index of the confinement of the animal to a single location within the apparatus, with a higher score indicating a greater degree of confinement (Canales and Graybiel, 2000). Horizontal activity was defined as the number of quadrants the animal crossed on a 4 × 4 grid projected onto the recorded video using AnyMaze software (Stoelting, Wood Dale, IL, USA) and converted into centimeters (Frankel et al., 2007; Horner et al., 2012; Horner et al., 2010; Murray et al., 2014).
4.5. Tissue processing for immunohistochemistry
Immediately after the completion of the behavioral monitoring phase (i.e. 2h after injection of the challenge dose), rats were sacrificed by CO2 exposure for one minute followed by decapitation. Brains were then quickly harvested, quick-frozen in isopentane on dry ice, and stored at −80°C until they were cut with a cryostat (Minotome Plus, Triangle Biomedical Sciences, Durham, N.C., USA) into 12-μm sections through the striatum (at approximately +1.5 mm from bregma; (Paxinos and Watson, 2005)).
4.6. Mu opioid receptor immunohistochemistry
Sections through striatum were post-fixed in 4% paraformaldehyde/0.9% NaCl and then rinsed three times in 0.1 M phosphate-buffered saline (PBS). Afterward, slides were blocked with 10% bovine serum albumin (BSA)/0.3% Triton X-100 (TX)/0.1 M PBS for 2 h. Slides were then incubated overnight at 4°C with a polyclonal antibody for the mu opioid receptor (Immunostar, Hudson, WI, USA), diluted in 1:1000 in 0.3% TX/0.1M PBS/5% BSA. The following morning, slides were washed several times in PBS and then incubated for 2 h at room temperature in biotinylated goat anti-rabbit IgG antiserum (Vector Laboratories, Burlingame, CA, USA) diluted 1:200 in 0.1M PBS/5% BSA. Once this step was complete, slides were again washed three times in PBS, incubated 1 h in ABC solution (Elite ABC Kit, Vector Laboratories) and washed three more times in PBS. Bound antibody was detected using a 3′,3-diaminobenzidine/Ni+ solution (Vector Laboratories). Finally, slides were washed with deionized H2O, dehydrated in a series of alcohols and coverslipped out of xylene.
4.7. c-Fos immunohistochemistry
Sections through striatum were post-fixed in 4% paraformaldehyde, pH 7.4, and rinsed three times in PBS before being blocked with 4% normal goat serum (NGS)/0.3% TX for 1h. Slides were then incubated overnight at 4°C with a polyclonal antibody for c-Fos (Abeam, Cambridge, MA, USA) diluted 1:1000 in 0.3% TX/0.1M PBS. The following morning slides were washed three times in PBS and incubated at room temperature for 2h with biotinylated goat anti-rabbit IgG antiserum (Vector Laboratories) diluted 1:200 in 0.1M PBS/1% NGS. Slides were then washed three times in PBS, incubated at room temperature for 1h with ABC solution (Elite ABC Kit, Vector Laboratories), and washed three more times in PBS. Bound antibody was detected using a 3′,3-diaminobenzidine/Ni+ solution (Vector Laboratories). Finally, slides were washed with deionized water, dehydrated in a series of increasing alcohol concentrations, and coverslipped out of xylene.
4.8. Calbindin immunohistochemistry
Striatal sections were post-fixed in 4% paraformaldehyde, pH 7.4, and rinsed three times in PBS before being blocked with 4% NGS/0.3% TX for 1h. Slides were then incubated overnight at 4°C with a polyclonal antibody for calbindin (Immunostar) diluted 1:1000 in 0.3% TX/0.1M PBS. The following morning slides were washed three times in PBS and incubated at room temperature for 2h in biotinylated goat anti-rabbit IgG antiserum (Vector Laboratories) diluted 1:200 in 0.1M PBS/1% NGS. Slides were then washed three times in PBS, incubated for 1h with ABC solution (Elite ABC Kit, Vector Laboratories), and washed three more times in PBS. Bound antibody was detected using a 3′,3-diaminobenzidine/Ni+ solution (Vector Laboratories). Finally, slides were washed with deionized water, dehydrated in a series of increasing alcohol concentrations, and coverslipped out of xylene.
4.9. Image analysis
For patch density counts and c-Fos particle counts, the left and right hemispheres of one striatal section at the level of the infusion (i.e., where cannulae tracks were present, at approximately +1.5 mm anterior to bregma) was analyzed and the values from the left and right hemispheres averaged for each animal. In order to confirm the loss of mu opioid receptor immunoreactive patches in the striatum, slides from mu opioid receptor immunohistochemistry were captured with a video camera (CCD IEEE-1394, Scion Corporation, Frederick, MD, USA). The ImageJ software package (National Institutes of Health; http://rsb.info.nih.gov/ij) was used to outline patches of mu opioid receptor immunoreactivity in the whole rostral striatum (5–7 animals per treatment group), designated as the area below the corpus callosum and above the anterior commissure, ending approximately at the ventral tip of the lateral ventricle at approximately +1.5 mm anterior to bregma. The particle analysis option in ImageJ was used to determine the number of mu opioid receptor labeled patches that exceeded the threshold density which was adjusted to eliminate background staining, as described previously (Murray et al., 2014). Prior to analysis, the pixel range for patch size was determined by outlining approximately 5–15 positively labeled patches from 10–15 randomly selected sections and taking the average size of these labeled patches in respect to pixel area. The lower limit for a “labeled patch” on the particle analysis setting was set to the smallest number of pixels measured for any patch, whereas the upper limit was set at the maximal particle size on the particle analysis option of ImageJ. In order to determine whether DERM-SAP treatment altered the overall size of the patch compartment, the total area, in pixels, of the mu-immunoreactive patches highlighted by thresholding for each animal.
In order to determine the relative level of activation in the patch and matrix compartments of striatum, sections immediately adjacent (12 μm away) to those used for c-Fos immunohistochemistry were labeled for calbindin (Murray et al., 2014). We opted to use calbindin immunohistochemistry rather than mu opioid receptor immunohistochemistry to delineate the patch and matrix compartments of striatum, since the destruction of mu opioid-receptor containing neurons in the patch compartment might make such delineation difficult in DERM-SAP treated animals. On the other hand, calbindin is preferentially expressed in the matrix compartment (Gerfen et al., 1985), and calbindin staining is unaltered following DERM-SAP treatment (Murray et al., 2014). Pixel range determination and particle analysis of c-Fos immunoreactivity was performed as previously described (Murray et al., 2014). Prior to analysis, the pixel range for c-Fos particle size was determined by outlining approximately 15–20 positively labeled cells from 10–15 randomly selected sections and taking the average size of the labeled cells in respect to pixel area. The lower limit for a “labeled cell” on the particle analysis setting was then set to the smallest number of pixels measured for any cell, whereas the upper limit was set at the maximal particle size on the particle analysis option of ImageJ. The threshold density was adjusted to eliminate background staining and the number of immunoreactive pixels per selected area in each region of interest was measured above this threshold. Both the c-Fos-labeled sections and the adjacent calbindin-labeled sections were captured using a VistaVision (VWR) microscope with a video camera (CCD Moticam 2300) using a 2.5X objective. ImageJ was used to outline regions of either dense calbindin immunoreactivity (matrix) or absent calbindin immunoreactivity (patch). These areas were then superimposed over corresponding areas of the adjacent c-Fos-labeled striatal section, which was a 1024 × 768 pixel square area in the dorsal central region of rostral striatum, and the number of c-Fos-labeled particles were then counted in the region of interest. Counts of c-Fos-labeled particles were expressed as the number of c-Fos-ir particles per mm2. A ratio of patch-to-matrix c-Fos labeled particles was calculated by dividing the number of c-Fos-ir particles/mm2 for the patch by the number of c-Fos-ir particles/mm2 for the matrix, in the 1024 × 768 square pixel area for each animal in the study. Particle analysis of c-Fos immunoreactivity was also measured in a 400 × 400 square pixel area in the core and shell of the nucleus accumbens. In addition, randomly chosen sections from all groups labeled with the nuclear stain neutral red, and a correlation performed between the number neutral red-labeled particles and c-Fos labeled particles in order to verify that c-Fos-labeled particles corresponded to nuclear labeling.
4.10. Statistical analysis
The effects of DERM-SAP pretreatment on the number of and total area of mu opioid receptor-labeled patches was analyzed using a student’s t-test. Stereotypy and spatial confinement rating data was represented as the area under the curve (AUC) for the two-hour observation period, while horizontal activity was represented as the total distance traveled over the two-hour observation period. All measures were analyzed using a two-way (pretreatment X treatment) analysis of variance (ANOVA). The effects of DERM-SAP pretreatment on COC-induced c-Fos immunoreactivity were analyzed using a two-way (pretreatment X treatment) ANOVA, with a separate analysis for the patch and matrix compartments. Correlations between neutral red-labeled nuclei and c-Fos labeled nuclei were performed using a Spearman correlation. Tukey multi-comparison tests were used for post-hoc analysis of significant effects. The alpha level for all analyses was set at 0.05.
Figure 1.
Infusion sites in the dorsal striatum. Schematic images showing lines that indicate the placement of microinjection cannulae tips observed during sectioning. Measurements are given in mm relative to bregma.
Highlights.
Dermorphin---saporin was used to ablate the neurons of the patch compartment of rostral striatum.
Animals Were treated repeatedly with cocaine.
Dermorphin---saporin Reduced cocaine---induced stereotypy.
Dermorphin---saporin attenuated patch---enhanced c---Fos expression in The rostral striatum by cocaine.
Acknowledgments
This work was supported by a grant from the National Institute on Drug Abuse (DA025303) and a Mercer University Seed Grant to KAH.
Abbreviations
- DERM-SAP
Dermorphin-saporin
- SAP
unconjugated saporin
- COC
cocaine
- SAL
saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Adams DH, Hanson GR, Keefe KA. Distinct effects of methamphetamine and cocaine on preprodynorphin messenger RNA in rat striatal patch and matrix. J Neurochem. 2003;84:87–93. doi: 10.1046/j.1471-4159.2003.01507.x. [DOI] [PubMed] [Google Scholar]
- Aliane V, Perez S, Deniau JM, Kernel ML. Raclopride or high-frequency stimulation of the subthalamic nucleus stops cocaine-induced motor stereotypy and restores related alterations in prefrontal basal ganglia circuits. The European journal of neuroscience. 2012;36:3235–3245. doi: 10.1111/j.1460-9568.2012.08245.x. [DOI] [PubMed] [Google Scholar]
- Aliane V, Perez S, Nieoullon A, Deniau JM, Kemel ML. Cocaine-induced stereotypy is linked to an imbalance between the medial prefrontal and sensorimotor circuits of the basal ganglia. Eur J Neurosci. 2009;30:1269–1279. doi: 10.1111/j.1460-9568.2009.06907.x. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Kawaguchi Y. Actions of substance P on rat neostriatal neurons in vitro. J Neurosci. 1996;16:5141–5153. doi: 10.1523/JNEUROSCI.16-16-05141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barwick VS, Jones DH, Richter JT, Hicks PB, Young KA. Subthalamic nucleus microinjections of 5-HT2 receptor antagonists suppress stereotypy in rats. Neuroreport. 2000;11:267–270. doi: 10.1097/00001756-200002070-00009. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Izzo PN, Graybiel AM. Cellular substrate of the histochemically defined striosome and matrix system of the caudate nucleus: a combined golgi and immunohistochemical study. Neuroscience. 1988;24:853–875. doi: 10.1016/0306-4522(88)90073-5. [DOI] [PubMed] [Google Scholar]
- Bradberry CW. Comparison of acute and chronic neurochemical effects of cocaine and cocaine cues in rhesus monkeys and rodents: focus on striatal and cortical dopamine systems. Rev Neurosci. 2008;19:113–128. doi: 10.1515/revneuro.2008.19.2-3.113. [DOI] [PubMed] [Google Scholar]
- Broderick PA, Gardner EL, van Praag HM. In vivo electrochemical and behavioral evidence for specific neural substrates modulated differentially by enkephalin in rat stimulant stereotypy and locomotion. Biol Psychiatry. 1984;19:45–54. [PubMed] [Google Scholar]
- Brown LL, Feldman SM, Smith DM, Cavanaugh JR, Ackermann RF, Graybiel AM. Differential metabolic activity in the striosome and matrix compartments of the rat striatum during natural behaviors. J Neurosci. 2002;22:305–314. doi: 10.1523/JNEUROSCI.22-01-00305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales JJ. Intermittent cortical stimulation evokes sensitization to cocaine and enduring changes in matrix and striosome neuron responsiveness. Synapse. 2005a;57:56–60. doi: 10.1002/syn.20149. [DOI] [PubMed] [Google Scholar]
- Canales JJ. Stimulant-induced adaptations in neostriatal matrix and striosome systems: transiting from instrumental responding to habitual behavior in drug addiction. Neurobiol Learn Mem. 2005b;83:93–103. doi: 10.1016/j.nlm.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy. Nat Neurosci. 2000;3:377–383. doi: 10.1038/73949. [DOI] [PubMed] [Google Scholar]
- Cole RL, Konradi C, Douglass J, Hyman SE. Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron. 1995;14:813–823. doi: 10.1016/0896-6273(95)90225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I, Iverson SD. Blockage of amphetamine-induced motor stimulation and stereotypy in the adult rat following neonatal treatment with 6-hydroxydopamine. Brain Res. 1973;55:369–382. doi: 10.1016/0006-8993(73)90302-8. [DOI] [PubMed] [Google Scholar]
- Crittenden JR, Graybiel AM. Basal Ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front Neuroanat. 2011;5:59. doi: 10.3389/fnana.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfs JM, Schreiber L, Kelley AE. Microinjection of cocaine into the nucleus accumbens elicits locomotor activation in the rat. J Neurosci. 1990;10:303–310. doi: 10.1523/JNEUROSCI.10-01-00303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagergren P, Smith HR, Daunais JB, Nader MA, Porrino LJ, Hurd YL. Temporal upregulation of prodynorphin mRNA in the primate striatum after cocaine self-administration. The European journal of neuroscience. 2003;17:2212–2218. doi: 10.1046/j.1460-9568.2003.02636.x. [DOI] [PubMed] [Google Scholar]
- Frankel PS, Hoonakker AJ, Danaceau JP, Hanson GR. Mechanism of an exaggerated locomotor response to a low-dose challenge of methamphetamine. Pharmacol Biochem Behav. 2007;86:511–515. doi: 10.1016/j.pbb.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama F, Sohn J, Nakano T, Furuta T, Nakamura KC, Matsuda W, Kaneko T. Exclusive and common targets of neostriatofugal projections of rat striosome neurons: a single neuron-tracing study using a viral vector. Eur J Neurosci. 2011;33:668–677. doi: 10.1111/j.1460-9568.2010.07564.x. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984;311:461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: striatal patch-matrix organization is related to cortical lamination. Science. 1989;246:385–388. doi: 10.1126/science.2799392. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Baimbridge KG, Miller JJ. The neostriatal mosaic: compartmental distribution of calcium-binding protein and parvalbumin in the basal ganglia of the rat and monkey. Proc Natl Acad Sci U S A. 1985;82:8780–8784. doi: 10.1073/pnas.82.24.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Wilson CJ. The Basal Ganglia. In: Swanson LW, Bjorklund A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy. Elsevier Sciences; Amsterdam: 1996. [Google Scholar]
- Gerfen CR, Young WS., 3rd Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 1988;460:161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- Giagnoni G, Parolaro D, Casiraghi L, Crema G, Sala M, Andreis C, Gori E. Dermorphin interaction with peripheral opioid receptors. Neuropeptides. 1984;5:157–160. doi: 10.1016/0143-4179(84)90051-9. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Canales JJ. The neurobiology of repetitive behaviors: clues to the neurobiology of Tourette syndrome. Adv Neurol. 2000;85:123–131. [PubMed] [Google Scholar]
- Graybiel AM, Canales JJ, Capper-Loup C. Levodopa-induced dyskinesias and dopamine-dependent stereotypies: a new hypothesis. Trends Neurosci. 2000;23:S71–S77. doi: 10.1016/s1471-1931(00)00027-6. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci U S A. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Rausch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- Guttenberg ND, Klop H, Minami M, Satoh M, Voorn P. Co-localization of mu opioid receptor is greater with dynorphin than enkephalin in rat striatum. Neuroreport. 1996;7:2119–2124. doi: 10.1097/00001756-199609020-00011. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Pert CB. Mosaic distibution of opiate receptors, parafascilular projections and acetylcholinesterase in rat striatum. Nature. 1981;291:415–418. doi: 10.1038/291415a0. [DOI] [PubMed] [Google Scholar]
- Horner KA, Hebbard JC, Logan AS, Vanchipurakel GA, Gilbert YE. Activation of mu opioid receptors in the striatum differentially augments methamphetamine-induced gene expression and enhances stereotypic behavior. J Neurochem. 2012;120:779–794. doi: 10.1111/j.1471-4159.2011.07620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner KA, Keefe KA. Regulation of psychostimulant-induced preprodynorphin, c-fos and zif/268 messenger RNA expression in the rat dorsal striatum by mu opioid receptor blockade. Eur J Pharmacol. 2006;532:61–73. doi: 10.1016/j.ejphar.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Horner KA, Noble ES, Gilbert YE. Methamphetamine-induced stereotypy correlates negatively with patch-enhanced prodynorphin and arc mRNA expression in the rat caudate putamen: the role of mu opioid receptor activation. Pharmacol Biochem Behav. 2010;95:410–421. doi: 10.1016/j.pbb.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis WL, Allen LF, Whitelaw RB, Latimer MP, Brace HM, Winn P. An investigation into the role of the pedunculopontine tegmental nucleus in the mediation of locomotion and orofacial stereotypy induced by d-amphetamine and apomorphine in the rat. Neuroscience. 1994;58:817–833. doi: 10.1016/0306-4522(94)90459-6. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabourian M, Venance L, Bourgoin S, Ozon S, Perez S, Godeheu G, Glowinski J, Kemel ML. Functional mu opioid receptors are expressed in cholinergic interneurons of the rat dorsal striatum: territorial specificity and diurnal variation. Eur J Neurosci. 2005;21:3301–3309. doi: 10.1111/j.1460-9568.2005.04154.x. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Minami M, Satoh M, Mizuno N. Immunocytochemical localization of mu-opioid receptor in the rat caudate-putamen. Neurosci Lett. 1995;184:149–152. doi: 10.1016/0304-3940(94)11192-l. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Lawhorn C, Smith DM, Brown LL. Partial ablation of mu-opioid receptor rich striosomes produces deficits on a motor-skill learning task. Neuroscience. 2009;163:109–119. doi: 10.1016/j.neuroscience.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur A, Shandarin A, LaViolette SR, Parker J, Yeomans JS. Locomotion and stereotypy induced by scopolamine: contributions of muscarinic receptors near the pedunculopontine tegmental nucleus. Brain Res. 1997;775:144–155. doi: 10.1016/s0006-8993(97)00928-1. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Projection neurons of basolateral amygdala: a correlative golgi and retrograde tract tracing study. Brain Res Bull. 1992;28:179–185. doi: 10.1016/0361-9230(92)90177-y. [DOI] [PubMed] [Google Scholar]
- Miura M, Saino-Saito S, Masuda M, Kobayashi K, Aosaki T. Compartment-specific modulation of GABAergic synaptic transmission by mu-opioid receptor in the mouse striatum with green fluorescent protein-expressing dopamine islands. J Neurosci. 2007;27:9721–9728. doi: 10.1523/JNEUROSCI.2993-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratalla R, Robertson HA, Graybiel AM. Dynamic regulation of NGFI-A [zif268, egr1) gene expression in the striatum. J Neurosci. 1992;12:2609–2622. doi: 10.1523/JNEUROSCI.12-07-02609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RC, Gilbert YE, Logan AS, Hebbard JC, Horner KA. Striatal patch compartment lesions alter methamphetamine-induced behavior and immediate early gene expression in the striatum, substantia nigra and frontal cortex. Brain Struct Funct. 2014;219:1213–1229. doi: 10.1007/s00429-013-0559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- O’Dell SJ, Marshall JF. Repeated administration of methamphetamine damages cells in the somatosensory cortex: overlap with cytochrome oxidase-rich barrels. Synapse. 2000;37:32–37. doi: 10.1002/(SICI)1098-2396(200007)37:1<32::AID-SYN4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Pallanti S, Bernardi S, Raglione LM, Marini P, Ammannati F, Sorbi S, Ramat S. Complex repetitive behavior: punding after bilateral subthalamic nucleus stimulation in Parkinson’s disease. Parkinsonism Relat Disord. 2010;16:376–380. doi: 10.1016/j.parkreldis.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier Academic Press; San Diego, CA: 2005. [Google Scholar]
- Pert CB, Kuhar M, Snyder SH. Opiate receptor: autoradiographic localization in the rat brain. Proc Natl Acad Sci USA. 1976;73:3729–3733. doi: 10.1073/pnas.73.10.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale CW, Jr, Graybiel AM. Fibers from the basolateral amygdala selectively innervate the striosomes in the caudate nucleus of the cat. J Comp Neurol. 1988;269:506–522. doi: 10.1002/cne.902690404. [DOI] [PubMed] [Google Scholar]
- Saka E, Goodrich C, Harlan P, Madras BK, Graybiel AM. Repetitive behaviors in monkeys are linked to specific striatal activation patterns. J Neurosci. 2004;24:7557–7565. doi: 10.1523/JNEUROSCI.1072-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka E, Graybiel AM. Pathophysiology of Tourette’s syndrome: striatal pathways revisited. Brain Dev. 2003;25(Suppl 1):S15–19. doi: 10.1016/s0387-7604(03)90002-7. [DOI] [PubMed] [Google Scholar]
- Saka E, Iadarola M, Fitzgerald DJ, Graybiel AM. Local circuit neurons in the striatum regulate neural and behavioral responses to dopaminergic stimulation. Proc Natl Acad Sci U S A. 2002;99:9004–9009. doi: 10.1073/pnas.132212499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford IM, Cooper AJ. Presynaptic mu and delta opioid receptor modulation of GABAA IPSCs in the rat globus pallidus in vitro. J Neurosci. 1999;19:4796–4803. doi: 10.1523/JNEUROSCI.19-12-04796.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A, Moratalla R, Lyford G, Worley PF, Graybiel AM. The activity-regulated cytoskeletal-associated protein Arc is expressed in different striosome-matrix patterns following exposure to amphetamine and cocaine. J Neurochem. 2000;74:2074–2078. doi: 10.1046/j.1471-4159.2000.0742074.x. [DOI] [PubMed] [Google Scholar]
- Tempel A, Zukin RS. Neuroanatomical patterns of the mu, delta and kappa opioid receptors of the rat brain as determined by quantitative in vitro autoradiography. Proc Natl Acad Sci USA. 1987;43:4308–4312. doi: 10.1073/pnas.84.12.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuno H, Chiken S, Kametani K, Moriizumi T. Efferent projections from the striatal patch compartment: anterograde degeneration after selective ablation of neurons expressing mu-opioid receptor in rats. Neurosci Lett. 2002;332:5–8. doi: 10.1016/s0304-3940(02)00837-6. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Smith AJ, McGinty JF. A single injection of amphetamine or methamphetamine induces dynamic alterations in c-fos, zif/268 and preprodynorphin messenger RNA expression in the rat forebrain. Neuroscience. 1995;68:83–95. doi: 10.1016/0306-4522(95)00100-w. [DOI] [PubMed] [Google Scholar]
- White NM, Hiroi N. Preferential localization of self-stimulation sites in striosomes/patches in the rat striatum. Proc Natl Acad Sci U S A. 1998;95:6486–6491. doi: 10.1073/pnas.95.11.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley RG, Kline IR. Neuronal lesioning with axonally transported toxins. J Neurosci Methods. 2000;103:73–82. doi: 10.1016/s0165-0270(00)00297-1. [DOI] [PubMed] [Google Scholar]
- Xu M, Guo Y, Vorhees CV, Zhang J. Behavioral responses to cocaine and amphetamine administration in mice lacking the dopamine D1 receptor. Brain Res. 2000;852:198–207. doi: 10.1016/s0006-8993(99)02258-1. [DOI] [PubMed] [Google Scholar]
- Xu M, Hu XT, Cooper DC, Moratalla R, Graybiel AM, White FJ, Tonegawa S. Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell. 1994;79:945–955. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]