Abstract
The concentration and composition of cardiolipin (CL) in mitochondria are altered in age-related heart disease, Barth Syndrome, and other rare genetic disorders, resulting in mitochondrial dysfunction. To explore whether exogenous CL can be delivered to cells, CL was combined with apolipoprotein A-I to generate water-soluble, nanoscale complexes termed nanodisks (ND). Mass spectrometry HL60 myeloid progenitor cell extracts revealed a 30-fold increase in cellular CL content following incubation with CL-ND. When CL-ND containing a fluorescent CL analogue was employed, confocal microscopy revealed CL localization to mitochondria. The ability of CL-ND to elicit a physiological response was examined in an HL60 cell culture model of Barth Syndrome neutropenia. siRNA knockdown of the phospholipid transacylase, tafazzin (TAZ), induced apoptosis in these cells. When TAZ knockdown cells were incubated with CL-ND, the apoptotic response was attenuated. Thus, CL-ND represent a potential intervention strategy for replenishment of CL in Barth Syndrome, age-related heart disease, and other disorders characterized by depletion of this key mitochondrial phospholipid.
Keywords: cardiolipin nanodisk, neutropenia, HL60 myeloid progenitor cells, Barth Syndrome, mitochondrial dysfunction, heart disease
Introduction
Cardiolipin (CL) is a unique, highly specialized phospholipid that differs from other glycerophospholipids in that it contains four fatty acyl chains and three glycerol moieties, giving rise to a negatively charged, cone-shaped structure. In animals, CL is found almost exclusively in mitochondria, mostly on the matrix side of the inner membrane [1] where it interacts with, and stabilizes, electron transport chain (ETC) proteins. CL binds tightly to proteins that participate in oxidative phosphorylation including complex IV [2], ATP/ADP exchange protein [3], F0F1 ATP synthase [4], the orthophosphate transporter [5] and the cytochrome bc1 complex [6]. Moreover, CL binding is also necessary for optimal activity of complex IV [2], complex I and complex III [7]. CL is also required for ADP/ATP carrier function and formation of ETC supercomplexes [8–11].
Reduced levels of CL are associated with a number of common conditions, including age-related heart failure [12–14] and diabetes [15]. Profound defects in CL content and composition are a feature of Barth Syndrome (BTHS), a rare, life threatening X-linked disorder caused by loss of function mutations in the tafazzin gene (TAZ; OMIM entry *300394) [16]. TAZ encodes a phospholipid transacylase that localizes to mitochondria and functions in CL acyl chain remodeling [17]. BTHS patients are characterized by decreased amounts of CL, particularly in cardiac and skeletal muscle, altered CL molecular species composition and an increase in the ratio of monolyso CL / CL [18]. These alterations in CL lead to severe muscle weakness and cardiomyopathy, which can lead to heart failure, one of the leading causes of death among BTHS patients [19].
In addition to cardiomyopathy and skeletal muscle weakness, BTHS is characterized by neutropenia [19]. As neutrophils are the primary phagocytic cells of the human immune system, their depletion predisposes BTHS patients to infection. Based on their short half-life (~24 h) and relatively high abundance in circulation, the turnover rate of neutrophils is high. Thus, defective maturation can lead to a sharp decline in the concentration of circulating neutrophils. Makaryan et al [20] showed that HL60 myeloid progenitor cells transfected with a TAZ-specific shRNA undergo apoptosis. Based on this, it was proposed that neutropenia in BTHS arises from increased apoptosis of neutrophil precursor cells. In the present study we employed HL60 cells to evaluate the ability of exogenous CL to prevent the apoptotic phenotype induced by TAZ knockdown. The data indicate that CL, solubilized in nanodisk (ND) complexes, is taken up, localizes to mitochondria, and prevents TAZ knockdown-induced apoptosis.
Materials and Methods
Formulation of CL-ND
Tetralinoleoylcardiolipin [(18:2/18:2)2-cardiolipin] and tetra-myristoylcardiolipin [(14:0/14:0)2-cardiolipin] were purchased from Avanti Polar Lipids. Five mg of a given CL (stock solution in chloroform) was transferred to a glass tube and the solvent evaporated under a stream of N2 gas. Residual solvent was removed under vacuum. The prepared lipid was dispersed in phosphate buffered saline (PBS; 20 mM sodium phosphate, 150 mM sodium chloride, pH 7.0) followed by the addition of 2 mg recombinant human apoA-I [21] in a final volume of 1mL. The sample was subjected to bath sonication under a N2 atmosphere, with the temperature maintained between 22°C and 25 °C. During sonication, the turbid lipid dispersion became clear indicating apolipoprotein/phospholipid complexes (i.e. CL-ND) had formed. No pellet formed upon centrifugation. Control ND, containing dimyristoyl-phosphatidylcholine (Avanti Polar Lipids), were prepared in a similar manner. Where indicated, CL-ND were formulated in the presence (1 % w/w) of a flourescent CL (TopFlour-Cardiolipin; Avanti Polar Lipids).
Electron Microscopy
A drop of freshly prepared CL-ND was deposited on a carbon-coated grid and, after 15 sec, excess fluid was wicked away and a solution of 2% potassium phosphotungstate (pH 6.5) added. Excess stain was removed and the grid air-dried. Grids were examined at 80 kV in a JEM-1230 electron microscope (JEOL, Peabody, MA), and imaged with an UltraScan™ USC1000 charge-coupled device camera (Gatan, Warrendale, PA). Particle diameters were measured according to Forte and Nordhausen [22].
HL60 cell culture
Human HL60 promyelocytic leukemia cells were obtained from ATCC and cultured in RPMI 1640 media supplemented with 10% fetal bovine serum, 100 µg/mL penicillin, and 100 µg/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2. The cells were passaged every 3–4 days.
Cardiolipin uptake studies
HL60 cells (2 × 106) were incubated with PBS or tetralinoleoyl CL-ND (750 µg CL) in serum free medium for 24 h at 37°C. Following incubation, cells were washed and 100 µg of butylated hydroxytoluene and 5 µg tetramyristoyl CL (internal standard) were added. Cells were extracted according to Bligh and Dyer [23] with modifications of Garrett et al [24].
Liquid chromatography – mass spectrometry (LC-MS)
Negative ion electrospray ionization (ESI) LC-MS analysis of extracted CL was conducted on a Thermo Scientific (San Jose, CA) Vantage TSQ mass spectrometer with Thermo Accela UPLC operated by Xcalibur software. Separation of lipid was achieved by a Restek 150 × 2.1 mm (5 µm particle size) Viva C4 column at a flow rate of 260 µL/min. The mobile phase contained 10 mM ammonium formate in solvent A: acetonitrile:water (60:40, v:v); solvent B: 2-propanol:acetonitrile (90:10, v:v); and a gradient elution in the following manner was applied: 68% A, 0–1.5 min; 68−55% A, 1.5–4 min; 55−48% A, 4–5 min; 48−42% A, 5–8 min; 42−34% A, 8–11 min; 34−30% A, 11–14 min; 30−25% A, 14–18 min; 25−3% A, 18–23 min; 3−0% A, 25–30 min and kept at 0% A for 5 min. The tetramyristoyl CL internal standard (m/z 1240, [M – H]−) was eluted at 13.6 min, and the tetralinoleoyl CL (m/z 1448, [M – H]−) eluted at 14.4 min. Calculation of tetralinoleoyl CL content was based on the ratio of peak area of (18:2/18:2)2-CL and (14:0/14:0)2-CL from lipid extracts of cells incubated with PBS and CL-ND.
Confocal microscopy
HL60 cells (2 × 106) were incubated (24 h at 37°C) in 6 well plates containing poly-L-lysine-treated coverslips (BD Biosciences) in the presence of CL-ND (100 µg tetralinoleoyl CL + 1 µg TopFluor-CL). Following incubation, cells were washed with PBS and incubated with Mitotracker Orange (Life Technologies) according to the manufacturer’s protocol, and fixed with 4% paraformaldehyde (prepared in PBS containing 0.03 M sucrose) for 15 min at 22 °C. Hoechst 33342 was employed as a nuclear stai n. Cells were mounted on microscope slides, sealed with nail polish, and visualized at 63× with the Zeiss LSM710 confocal microscope.
TAZ knockdown experiments
HL60 cells (2 × 105) were seeded 2 days before the planned experiment. Cells were pelleted and re-suspended in nucleofection buffer containing 60 pmoles TAZ specific siRNA or a scrambled siRNA (Santa Cruz Biotechnology). The samples were electroporated with an Amaxa Cell Line Nucleofector Kit V, transferred to a 12 well plate at 1 × 106 cells/well and cultured in complete medium for 24 h. Where indicated, the media was supplemented with CL-ND (100 µg CL). Although nucleofection causes significant background apoptosis in this cell type, it remains the most effective method for HL60 cell transfection [25].
Reverse Transcriptase (RT)-PCR
Cells were processed with an Aurum Total RNA isolation kit (Bio-Rad) according to the manufacturer’s protocol. RT-PCR was performed using TaqMan PCR Reagent Kit (Applied Biosystems) and SYBR Green PCR Master Mix (Applied Biosystems). Primers specific for TAZ or GAPDH were employed, as described by Makaryan et al [20]. qPCR was performed using an Applied Biosystems 7900HT Fast Real-Time PCR System. Cycle threshold values derived from qPCR analysis were normalized to GAPDH mRNA levels.
Apoptosis studies
Following incubation in the presence or absence of CL-ND, TAZ knockdown and control HL60 cells were incubated with a) Alexa Fluor 488-labeled annexin V (Invitrogen) or b) propidium iodide (PI), as reported by Riccardi and Nicoletti [26]. In both assays, the cells were subject to flow cytometry analysis on a BD LSRFortessa and the data processed using FlowJo software.
Results
Effect of apoA-I on CL solubility
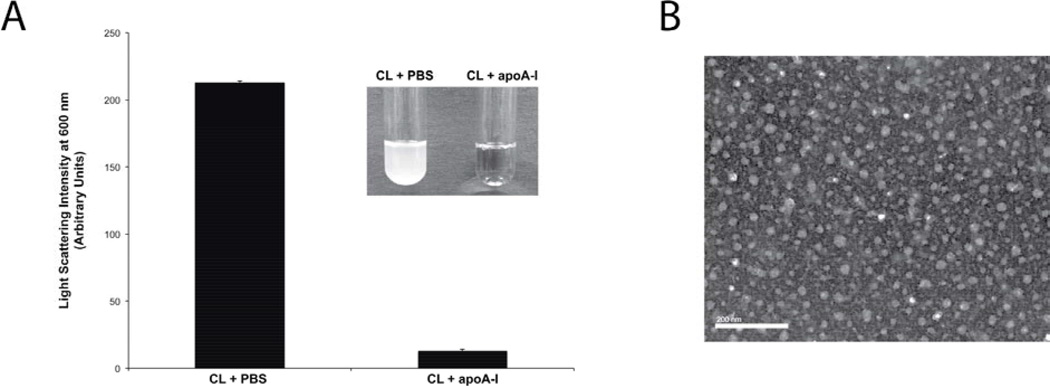
When 5 mg tetralinoleoyl CL was dispersed in PBS and bath sonicated, the sample remained turbid (Figure 1A). However, when CL was dispersed in PBS containing 2 mg recombinant human apoA-I, sample light scattering intensity was dramatically decreased. The extent of sample clarification was similar to that previously observed for phosphatidylcholine (PC) [27], indicating that CL behaves in a similar manner. The morphology of the complexes generated was examined by negative stain electron microscopy (Figure 1B), revealing a population of ND particles (seen en face) with diameters ranging from 18 to 31 nm.
Figure 1. Effect of apoA-I on CL solubility.
Panel A) Five mg tetralinoleoyl CL was dispersed in PBS or PBS containing 2 mg/ml apoA-I. Following bath sonication, sample light scattering intensity was measured at 600 nm on a Perkin Elmer Lamda 20 UV/Vis spectrophotometer. Inset: photographic images of the samples. Panel B) Negative stain electron microscopy of complexes generated upon incubation of CL with apoA-I in PBS. The bar represents 200 nm.
Uptake of CL-ND by HL60 cells
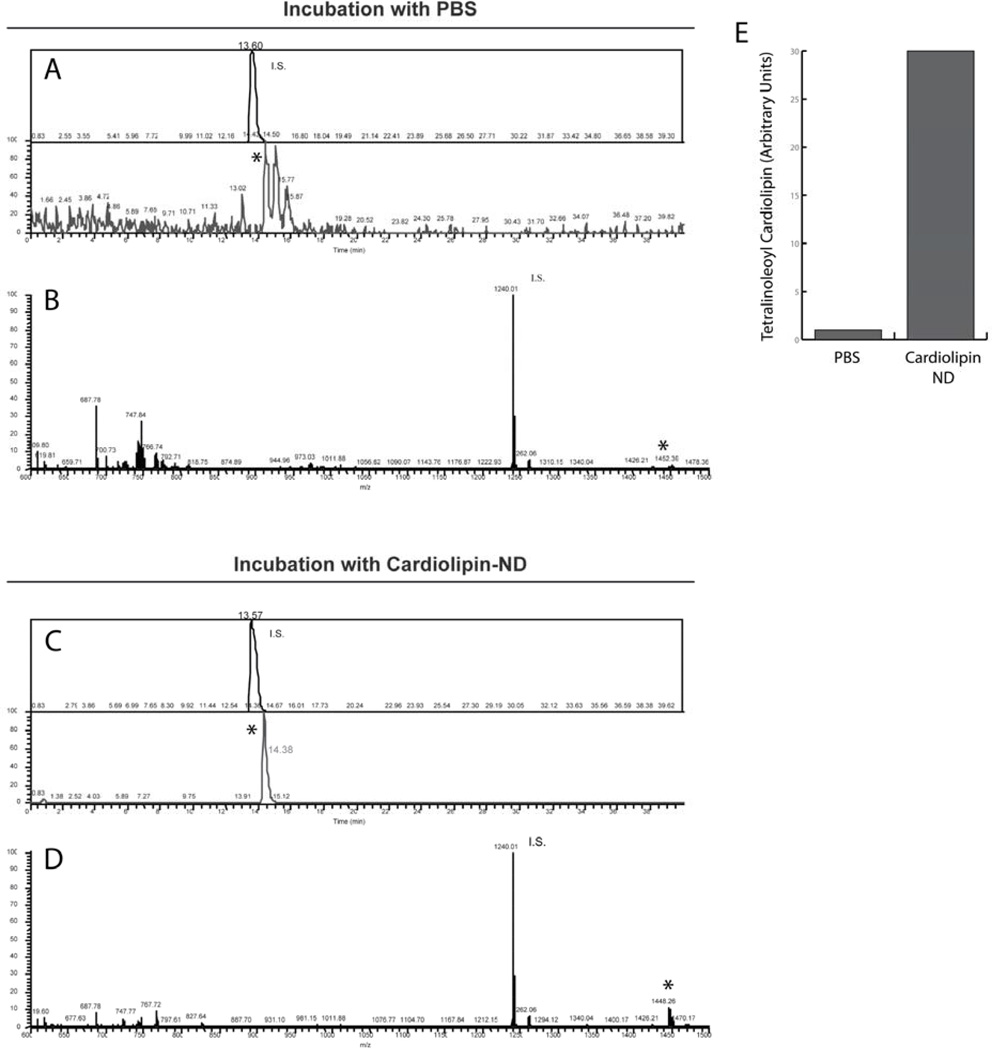
To assess if CL-ND can serve as a vehicle for delivery of CL to cells in culture, HL60 cells were incubated with tetralinoleoyl CL-ND. After 24 h, the cells were extracted and CL analyzed by LC-MS. In control cell extracts, the peak corresponding to the internal standard (tetramyristoyl CL) was prominent, while the peak corresponding to native tetralinoleoyl CL was just above background (Figure 2A). Upon treatment with CL-ND, however, the peak corresponding to tetralinoleoyl CL increased (Figure 2C). Similarly, an averaged spectrum view of control cell extracts shows a tetralinoleoyl CL peak that is low relative to the internal standard (Figure 2B), while in CL-ND treated cell extracts, this peak increased (Figure 2D). Peak quantification, relative to the internal standard, shows that, following incubation with CL-ND, the cellular content of tetralinoeoyl CL increased 30 fold (Figure 2E).
Figure 2. LC / MS of HL60 cell extracts.
HL60 cells were incubated with PBS or PBS containing tetralinoleoyl CL-ND. Panel A) LC/MS ion chromatograms of the internal standard, tetramyristoyl CL ("I.S."; top) and of tetralinoleoyl CL (" * "; bottom) from extracts of cells incubated with PBS. Panel B) The averaged ESI/MS spectrum of material eluted between 13.36–16.36 min from extracts of cells incubated with PBS. Panel C and D) Corresponding ion chromatograms and averaged mass spectrum, respectively, of lipid extracts from cells incubated with tetralinoleoyl CL-ND. Panel E) Histogram depicting the effect of CL-ND incubation on the tetralinoleoyl CL content of HL-60 cells, relative to I.S.
Exogenous CL homes to mitochondria
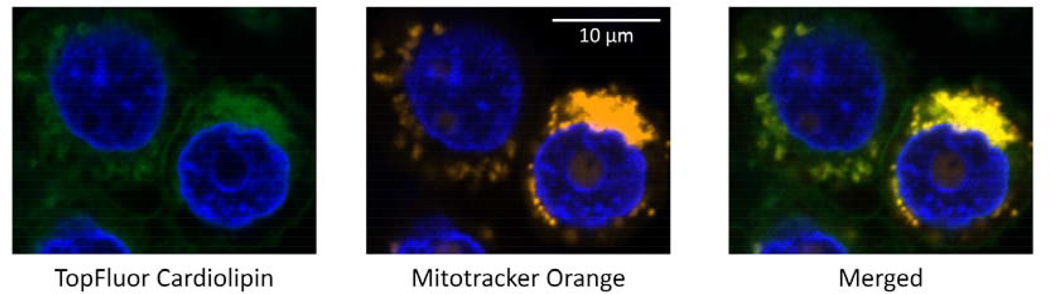
While the results presented above show CL uptake by HL60 cells, we sought to determine its intracellular fate following uptake. Cells were incubated with CL-ND containing small amounts of a fluorescent CL analogue and subjected to confocal fluorescence microscopy. Micrographs depicted in Figure 3 reveal a strong, punctate, perinuclear pattern of CL fluorescence within the cell and minor fluorescence associated with the plasma membrane. The punctate CL fluorescence signal co-localized with fluorescence derived from the mitochondria-specific reagent, MitoTracker, indicating exogenous CL localization to mitochondria.
Figure 3. Confocal microscopy of HL60 cells.
HL60 cells were incubated with CL-ND (100 µg tetralinoleoyl CL + 1 µg TopFluor-CL; left panel). Mitochondria were stained with MitoTracker Orange (middle panel). Nuclei were stained with Hoechst 33342. The right panel depicts a merged image with co-localization of mitochondria and TopFluor CL giving rise to a yellow fluorescence signal. Results depicted are representative images from three independent experiments.
Effect of CL-ND on TAZ knockdown-induced annexin V binding to HL60 cells
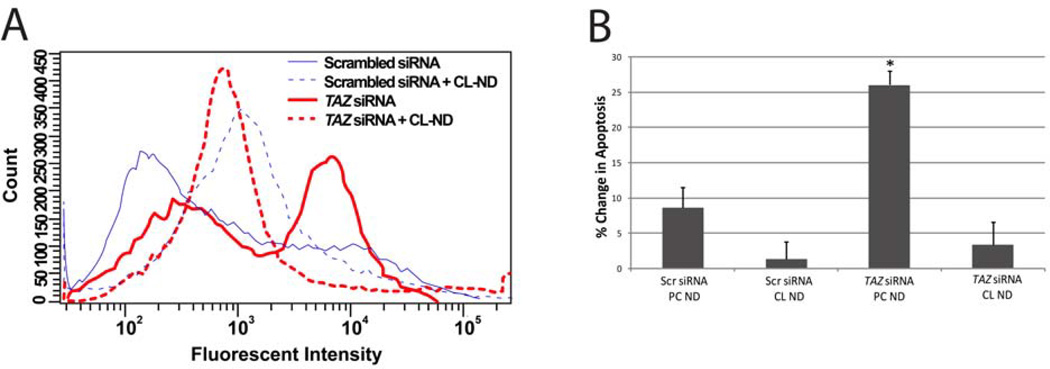
Compared to cells treated with a scrambled siRNA, TAZ specific siRNA induced a ~50% decline in TAZ mRNA levels (data not shown). To evaluate the effect of TAZ knockdown on HL60 cell apoptosis, cell surface exposure of phosphatidylserine was measured by flow cytometry following incubation with AlexaFluor 488-labeled annexin V. Consistent with results reported earlier [20], TAZ knockdown induced an increase in annexin V binding relative to cells treated with a scrambled siRNA (Figure 4A). Incubation of TAZ knockdown cells with CL-ND decreased annexin V binding to levels similar to that observed for cells treated with scrambled siRNA plus CL-ND, suggesting that CL-ND treatment prevents TAZ knockdown-induced apoptosis.
Figure 4. Effect of CL-ND on TAZ knockdown-induced apoptosis in HL60 cells.
HL60 cells were electroporated in the presence of a scrambled or TAZ-specific siRNA. Panel A) Following electroporation, the media was supplemented with PBS alone or PBS containing tetralinoleoyl CL-ND. After 24 h incubation, the cells were probed with AlexaFluor 488-labeled annexin V and analyzed by flow cytometry. Results depicted are representative of three independent experiments. Panel B) Following electroporation, the media was supplemented with PBS containing tetralinoleoyl CL-ND or PC-ND. After 24 h the cells treated with a permeabilizing PI solution and analyzed by flow cytometry. Values are the mean ± S.E. (n = 3); *P< 0.05 based on Student’s T test.
Effect of TAZ knockdown on PI staining of HL60 cells
To confirm the effect of CL-ND treatment on TAZ knockdown HL60 cells, an independent apoptosis assay, based on DNA degradation, was employed. Compared to cells treated with scrambled siRNA, TAZ siRNA-treated cells showed increased apoptosis (Figure 4B). Incubation of TAZ siRNA-treated cells with CL-ND eliminated this increase, while incubation of scrambled siRNA-treated cells with CL-ND had no significant effect, confirming that CL-ND treatment prevents TAZ knockdown-induced apoptosis.
Discussion
Reconstituted high-density lipoprotein (rHDL) are readily formed by combining glycerophospholipids, such as PC, with an apolipoprotein. Many different apolipoproteins, fragments thereof or synthetic peptides, possess the ability to form rHDL. In general, the particles created exist as nanoscale, disk-shaped phospholipid bilayers whose periphery is circumscribed by two or more apolipoprotein molecules. The protein/peptide “scaffold” of rHDL functions to stabilize the otherwise unstable edge of the phospholipid bilayer. Because different combinations of lipid and apolipoprotein can be used to formulate unique rHDL, this technology has been exploited for applications well beyond lipoprotein metabolism [27,28]. To distinguish rHDL engineered to possess additional features (e.g. inclusion of a hydrophobic drug), the term nanodisk (ND) is used.
In the present study, ND were formulated using CL. CL is different from other glycerophospholipids in that two acylated phosphoglycerol backbones share a third glycerol moiety as head group, giving rise to a distinctly cone-shaped anionic phospholipid possessing four esterified fatty acyl chains. In eukaryotes, CL is mainly confined to the inner membrane of mitochondria where it plays a key role in energy metabolism by establishing an optimal membrane environment for ETC proteins [29]. As such, defects in CL content and composition compromise mitochondrial function. In tissues with high oxidative metabolic capacity, including cardiac and skeletal muscle, CL fatty acyl chains are remodeled by the transacylase tafazzin to generate tetralinoleoyl CL as the major molecular species (> 90 %). It is conceivable that attainment of this fatty acyl chain composition enhances lipid packing, thereby establishing a membrane environment that facilitates optimal conditions for electron flux, generation / maintenance of a proton gradient, and ATP production. Indeed, individuals with BTHS harbor mutations in TAZ and display alterations in CL molecular species composition, decreased CL levels and increased amounts of monolyso-CL [19]. Similarly, CL-deficient mitochondria, isolated from hypothyroid patients, manifest impaired oxidative function [30]. Interestingly, treatment of isolated mitochondria from these subjects with exogenous CL restored normal activity. In cultured BTHS fibroblasts, linoleic acid supplementation of growth medium led to a time and dose dependent restoration of total CL levels and a significant increase in tetralinoleoyl CL [31]. Thus, it is conceivable that tafazzin-dependent CL remodeling can be bypassed by increasing substrate availability for direct de novo synthesis of tetralinoleoyl CL or by provision of exogenous tetralinoleoyl CL directly to the mitochondria.
Herein, it was hypothesized that the requirement for a functional tafazzin protein could be lessened or circumvented if exogenous tetralinoleoyl CL was provided to cultured cells. The work of Makaryan et al [20] established a cell culture model of BTHS neutropenia in which the functional effects of this hypothesis can be tested. The current study confirms that TAZ knockdown induces apoptosis. Moreover, we show that incubation of TAZ knockdown cells with CL-ND attenuates their apoptotic response. The lack of complete inhibition observed in annexin V binding assays could be due to annexin V recognition of CL [32] retained in the plasma membrane following incubation with CL-ND. To control for this, an independent, DNA quantification-based, PI binding apoptosis assay was performed [26]. The results of this assay confirm that incubation of TAZ knockdown HL60 cells with CL-ND attenuates their apoptotic response.
Although the present HL60 cell culture model of BTHS fails to manifest detectable changes in the monolyso CL / CL ratio typically associated with BTHS [20], this may be attributed to a high sensitivity of this cell type to mitochondrial dysfunction, resulting in apoptosis in response to minor changes in CL composition. Unlike muscle tissue, neutrophils possess few mitochondria, and thus may be more dependent on optimal mitochondrial function [33]. As such, although skeletal muscles of BTHS patients show modestly reduced function, neutrophil progenitors are susceptible to apoptosis.
In summary, we show that exogenously provided CL is taken up by HL60 cells, migrates to mitochondria and compensate for the effects of TAZ knockdown. The results suggest that CL-ND have the potential to bypass TAZ mutations in vivo. Because current therapies are largely palliative, this work represents the first direct intervention for BTHS. Future studies may also reveal a potential benefit of CL-ND for age-related heart disease and other CL-associated disorders.
Supplementary Material
Highlights.
Apolipoprotein A-I – cardiolipin complexes, termed nanodisks, were generated
Incubation of cardiolipin nanodisks with HL60 cells led to cellular cardiolipin uptake
Exogenous cardiolipin taken up by HL60 cells localized to mitochondria
Incubation of TAZ knockdown HL60 cells with cardiolipin nanodisks attenuated their apoptotic response
Acknowledgements
The authors thank Jennifer Beckstead for assistance with figure preparation and Dr. James Olzmann for advice. This work was funded by grants from the Barth Syndrome Foundation and the National Institutes of Health (R37 HL64159).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Horvath SE, Daum G. Lipids of mitochondria. Prog. Lipid Res. 2013;52:590–614. doi: 10.1016/j.plipres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Robinson NC, Zborowski J, Talbert LH. Cardiolipin-depleted bovine heart cytochrome c oxidase: binding stoichiometry and affinity for cardiolipin derivatives. Biochemistry. 1990;29:8962–8969. doi: 10.1021/bi00490a012. [DOI] [PubMed] [Google Scholar]
- 3.Horváth LI, Drees M, Beyer K, Klingenberg M, Marsh D. Lipid-protein interactions in ADP-ATP carrier/egg phosphatidylcholine recombinants studied by spin-label ESR spectroscopy. Biochemistry. 1990;29:10664–10669. doi: 10.1021/bi00499a013. [DOI] [PubMed] [Google Scholar]
- 4.Eble KS, Coleman WB, Hantgan RR, Cunningham CC. Tightly associated cardiolipin in the bovine heart mitochondrial ATP synthase as analyzed by 31P nuclear magnetic resonance spectroscopy. J. Biol. Chem. 1990;265:19434–19440. [PubMed] [Google Scholar]
- 5.Kaplan RS, Pratt RD, Pedersen PL. Purification and reconstitution of the phosphate transporter from rat liver mitochondria. Methods Enzymol. 1989;173:732–745. doi: 10.1016/s0076-6879(89)73047-0. [DOI] [PubMed] [Google Scholar]
- 6.Yu L, Yu C, King TE. The indispensability of phospholipid and ubiquinone in mitochondrial electron transfer from succinate to cytochrome c. J. Biol. Chem. 1978;253:2657–2663. [PubMed] [Google Scholar]
- 7.Fry M, Green DE. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J. Biol. Chem. 1981;256:1874–1880. [PubMed] [Google Scholar]
- 8.Claypool SM, Oktay Y, Boontheung P, Loo JA, Koehler CM. Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J. Cell Biol. 2008;182:937–950. doi: 10.1083/jcb.200801152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann B, Stöckl A, Schlame M, Beyer K, Klingenberg M. The reconstituted ADP/ATP carrier activity has an absolute requirement for cardiolipin as shown in cysteine mutants. J. Biol. Chem. 1994;269:1940–1944. [PubMed] [Google Scholar]
- 10.Pfeiffer K, Gohil V, Stuart RA, Hunte C, Brandt U, Greenberg ML, et al. Cardiolipin Stabilizes Respiratory Chain Supercomplexes. J. Biol. Chem. 2003;278:52873–52880. doi: 10.1074/jbc.M308366200. [DOI] [PubMed] [Google Scholar]
- 11.Zhang M, Mileykovskaya E, Dowhan W. Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria. J. Biol. Chem. 2005;280:29403–29408. doi: 10.1074/jbc.M504955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paradies G, Petrosillo G, Paradies V, Ruggiero FM. Oxidative stress, mitochondrial bioenergetics, and cardiolipin in aging. Free Radic. Biol. Med. 2010;48:1286–1295. doi: 10.1016/j.freeradbiomed.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Pamplona R. Membrane phospholipids, lipoxidative damage and molecular integrity: A causal role in aging and longevity. Biochim. Biophys. Acta - Bioenerg. 2008;1777:1249–1262. doi: 10.1016/j.bbabio.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Mejia EM, Nguyen H, Hatch GM. Mammalian cardiolipin biosynthesis. Chem. Phys. Lipids. 2014;179:11–16. doi: 10.1016/j.chemphyslip.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y. Emerging roles of cardiolipin remodeling in mitochondrial dysfunction associated with diabetes, obesity, and cardiovascular diseases. J. Biomed. Res. 2010;24:6–15. doi: 10.1016/S1674-8301(10)60003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whited K, Baile MG, Currier P, Claypool SM. Seven functional classes of barth syndrome mutation. Hum. Mol. Genet. 2013;22:483–492. doi: 10.1093/hmg/dds447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y, Malhotra A, Ren M, Schlame M. The enzymatic function of tafazzin. J. Biol. Chem. 2006;281:39217–39224. doi: 10.1074/jbc.M606100200. [DOI] [PubMed] [Google Scholar]
- 18.Valianpour F, Wanders RJA, Barth PG, Overmars H, Van Gennip AH. Quantitative and compositional study of cardiolipin in platelets by electrospray ionization mass spectrometry: Application for the identification of Barth syndrome patients. Clin. Chem. 2002;48:1390–1397. [PubMed] [Google Scholar]
- 19.Clarke SLN, Bowron A, Gonzalez IL, Groves SJ, Newbury-Ecob R, Clayton N, et al. Barth syndrome. Orphanet J. Rare Dis. 2013;8:23. doi: 10.1186/1750-1172-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makaryan V, Kulik W, Vaz FM, Allen C, Dror Y, Dale DC, et al. The cellular and molecular mechanisms for neutropenia in Barth syndrome. Eur. J. Haematol. 2012;88:195–209. doi: 10.1111/j.1600-0609.2011.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan RO, Forte TM, Oda MN. Optimized bacterial expression of human apolipoprotein A-I. Protein Expr. Purif. 2003;27:98–103. doi: 10.1016/s1046-5928(02)00568-5. [DOI] [PubMed] [Google Scholar]
- 22.Forte TM, Nordhausen RW. Electron microscopy of negatively stained lipoproteins. Methods Enzymol. 1986:442–457. doi: 10.1016/0076-6879(86)28086-6. [DOI] [PubMed] [Google Scholar]
- 23.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 24.Garrett TA, Kordestani R, Raetz CRH. Quantification of Cardiolipin by Liquid Chromatography-Electrospray Ionization Mass Spectrometry. Methods Enzymol. 2007;433:213–230. doi: 10.1016/S0076-6879(07)33012-7. [DOI] [PubMed] [Google Scholar]
- 25.Schakowski F, Buttgereit P, Mazur M, Märten A, Schöttker B, Gorschlüter M, et al. Novel non-viral method for transfection of primary leukemia cells and cell lines. Genet. Vaccines Ther. 2004;2:1. doi: 10.1186/1479-0556-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 2006;1:1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- 27.Ryan RO. Nanodisks: hydrophobic drug delivery vehicles. Expert Opin. Drug Deliv. 2008;5:343–351. doi: 10.1517/17425247.5.3.343. [DOI] [PubMed] [Google Scholar]
- 28.Ryan RO. Nanobiotechnology applications of reconstituted high density lipoprotein. J. Nanobiotechnology. 2010;8:28. doi: 10.1186/1477-3155-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houtkooper RH, Vaz FM. Cardiolipin, the heart of mitochondrial metabolism. Cell. Mol. Life Sci. 2008;65:2493–2506. doi: 10.1007/s00018-008-8030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paradies G, Petrosillo G, Paradies V, Ruggiero FM. Role of cardiolipin peroxidation and Ca2+ in mitochondrial dysfunction and disease. Cell Calcium. 2009;45:643–650. doi: 10.1016/j.ceca.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Valianpour F, Wanders RJ, Overmars H, Vaz FM, Barth PG, van Gennip AH. Linoleic acid supplementation of Barth syndrome fibroblasts restores cardiolipin levels: implications for treatment. J. Lipid Res. 2003;44:560–566. doi: 10.1194/jlr.M200217-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Megli FM, Selvaggi M, De Lisi A, Quagliariello E. EPR study of annexin V-cardiolipin Camediated interaction in phospholipid vesicles and isolated mitochondria. Biochim. Biophys. Acta - Biomembr. 1995;1236:273–278. doi: 10.1016/0005-2736(95)00057-a. [DOI] [PubMed] [Google Scholar]
- 33.van Raam BJ, Kuijpers TW. Mitochondrial defects lie at the basis of neutropenia in Barth syndrome. Curr. Opin. Hematol. 2009;16:14–19. doi: 10.1097/MOH.0b013e32831c83f3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.