Abstract
Erythroid cell maturation and diseases affecting erythrocytes are frequently accompanied by morphologic and immunophenotypic changes to these cells. In the past, these changes have been assessed primarily through the use of manual microscopy, which substantially limits the statistical rigor, throughput, and objectivity of these studies. Imaging flow cytometry provides a technology to examine both the morphology of cells as well as to quantify the staining intensity and signal distribution of numerous fluorescent markers on a cell-by-cell basis with high throughput in a statistically robust manner, and thus is ideally suited to studying erythroid cell biology. To date imaging flow cytometry has been used to study erythrocytes in three areas: 1) erythroid cell maturation, 2) sickle cell disease, and 3) infectious diseases such as malaria. In the maturation studies, imaging flow cytometry can closely recapitulate known stages of maturation and has led to the identification of a new population of erythroid cell precursors. In sickle cell disease, imaging flow cytometry provides a robust method to quantify sickled erythrocytes and to identify cellular aggregates linked to morbidities, and in malaria, imaging flow cytometry has been used to screen for new chemotherapeutic agents. These studies have demonstrated the value of imaging flow cytometry for investigations of erythrocyte biology and pathology.
Keywords: Imaging flow cytometry, erythrocyte, red blood cell, sickle cell, malaria
Introduction
Even though they are among the more numerous cells in the peripheral circulation, erythrocytes (red blood cells – RBCs) are not studied as often as leukocytes using flow cytometry. This is largely due to the latter being mediators of immune responses and thus playing significant roles in the pathogenesis of numerous diseases, and that malignancies of these cells are common and can have fatal outcomes. However, erythrocytes can also be involved in pathological aspects of many diseases, including leukemia (erythroleukemias), infectious diseases, and genetic anomalies. Therefore, studies on these cells are equally important in order to understand their role in various diseases. Human erythrocytes are distinctive from other cells present in the human circulation due to their lack of a nucleus when mature and their discoid morphology, however, these unique morphologic features can change as the result of various perturbations or diseases. For example, an intracellular pathogen in mature erythrocytes will result in the presence of intracellular DNA in these cells due to the pathogen. Similarly, a disruption in maturation may result in more immature cells in the circulation which express either DNA or RNA. The distinctive disc shape of erythrocytes can also change with maturation, as can the expression of distinctive surface and intracellular markers, and morphologic changes can also arise as a result of genetic anomalies.
Imaging flow cytometry (IFC), which can provide information regarding cellular morphology in addition to the detection of multiple fluorescent markers, is ideally suited to the study of erythrocytes and their characteristics in various diseases. Quite often, diseases involving erythrocytes induce changes in morphology or physical characteristics, such as the presence of intracellular objects, which can be detected by microscopy. Manual microscopy is often used for these studies but has limited statistical power due to the low numbers of cells that can be examined and can be subject to user bias in the selection of cells to be counted. Imaging flow cytometry overcomes these limitations and can provide statistically robust, unbiased data in a rapid manner. In addition, powerful analysis software (IDEAS, Amnis-EMD-Millipore, Seattle, WA), allows for the customized creation and use of features (which can be based on cell size, shape, signal intensity, signal location, and other cellular attributes) and masks (which define the region of interest in the cell such as membrane, cytoplasm, or nucleus, etc.). Combining masks and features allows for a pixel by pixel calculation of cellular information and quantitation of these measurements over defined cell populations. For example, one could calculate the total intensity of a protein found in a cell, as well as the intensity of that protein found only in the nucleus or lysosomes.
This manuscript will provide an overview of studies performed in erythrocyte biology using imaging flow cytometry. These studies have been grouped into three general categories classified broadly based on the diseases or processes being studied. The methodologies of each will be discussed and compared, with the goal of presenting the reader a brief, yet comprehensive overview of red cell studies by IFC.
Sickle Cell Disease
It is estimated that approximately 300,000 individuals, mainly of African descent but also including those of Mediterranean ancestry, are born each year with sickle cell disease (SCD) (1, 2), with millions suffering from this disease worldwide. SCD is primarily caused by a mutation in the β-globin gene, resulting in abnormal hemoglobin, called Hemoglobin-S (HbS), in erythrocytes. Under hypoxic conditions, HbS can polymerize, become rigid, and distort erythrocytes into abnormal shapes. The morphology of mature erythrocytes is generally a biconcave disc, but in hypoxic conditions, red cells with polymerized HbS can uniquely display a crescent, or sickled, shape. This shape change can result in vaso-occlusion in the capillaries of patients with SCD and lead to pain crisis, morbidity, and a reduced lifespan (3, 4). Other complications of SCD include acute chest syndrome, pulmonary hypertension, and endothelial dysfunction, among others (2, 5).
Numerous therapeutic approaches to the treatment of SCD involve inhibiting the polymerization of HbS either with agents that directly target HbS (3), or through agents that reduce polymerization of HbS by inducing fetal hemoglobin (HbF), which is known to have a protective effect in SCD (4). The efficacy of these approaches is determined, in part, on laboratory methods for enumerating the number or percentage of sickled erythrocytes. The most widely used method is the manual scoring of morphology by microscopy of a few hundred cells to assess for an abnormal or “sickled” phenotype in erythrocytes after treatment (6 7). Major limitations to this approach lay in its laboriousness, subjectivity due to operator bias, low sensitivity, and high variability.
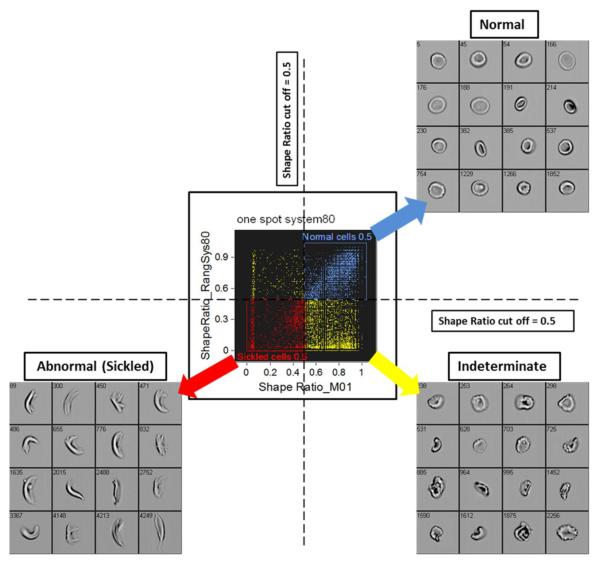
Using imaging flow cytometry for the enumeration of sickled cells provides a vast improvement over manual methods currently in use. Van Beers and colleagues from our own laboratory (8), describe a robust, high throughput, automated IFC-based assay using blood from sickle cells patients to distinguish sickled cells from normal red blood cells (RBC) by counting large numbers of cells with high specificity. In this assay, the investigators were able to image approximately 500-1000 red blood cells per second and employ a series of features and masks to enumerate the percentage of sickled and normal shaped cells based on brightfield imagery. Patient blood was subjected to hypoxic conditions to induce sickling, then fixed, and subsequently imaged by IFC. In IDEAS analysis software (Amnis- EMD-Millipore, Seattle, WA), tight masks were developed on brightfield imagery to increase the likelihood of analyzing only one cell per frame. This also reduced artifacts arising from objects which had altered feature values resulting from more than one cell in a frame, or from a cell with a small particle in the same frame as the cell. Since normal erythrocytes are round discs but sickled erythrocytes are elongated crescents, it was possible to use the shape ratio feature (minimum thickness of the cell divided by its length) calculated on brightfield imagery of single, focused erythrocytes to enumerate these cells. Cut off values were empirically determined for these shape ratio features which classified cells as abnormal/sickled, normal/round, or undefined/indeterminate (Figure 1). This yielded extremely high specificity and sensitivity, as well as the ability to quantify the continuum between extremes in morphology. The robustness of this assay was tested in three manners: by spiking experiments in which known quantities of SCD blood were mixed into healthy blood; by treating SCD blood with an anti-sickling agent; and by correlating the amount of sickling detected in blood subjected to conditions known to affect sickling such as pH and HbF with these treatments. All experimental data showed strong correlations between the percentage of sickled red cells and the sickling expected under the condition tested. The rigor of this assay suggested this assay’s potential use in SCD drug discovery and research, in monitoring erythrocyte responses to treatment, and in evaluating the state of disease.
Figure 1.
Sickle cell identification by IFC
Shape ratio cut off values used for classifying cells as normal/round (upper right quadrant), abnormal/sickled (lower left quadrant), or indeterminate (lower right quadrant), and associated representative brightfield imagery. van Beers (8) found that using a shape ratio cut off value of 0.5 yielded the highest sensitivity and specificity as compared to other cut off values.
Measurement of the levels of HbF in the blood, which is known to be correlated with disease severity and to have a protective effect from HbS polymerization, is another area of potential importance in assessing the severity of SCD and the efficacy of therapy. However, there is still a great deal unknown about the quantity of HbF in an individual erythrocyte necessary to provide a protective effect to inhibit sickling. Progress toward the goal of developing a robust method for detecting and measuring HbF in human samples was demonstrated by our group (Fertrin and colleagues, 9), who assessed the presence of HbF in individual red blood cells and correlated the HbF measurement with the cellular shape, with both measurements occurring simultaneously by IFC. Patient blood was subjected to hypoxic conditions and labeled with antibodies against HbF-phycoerythrin (PE), followed by imaging of 20,000 of these cells using IFC. This assay builds upon the IFC based sickling assay described by van Beers et al (8), but in addition to classifying erythrocytes as sickled, normal, or indeterminate based on brightfield imagery, this method also measures the fluorescence intensity of HbF in erythrocytes and correlated this with cell shape. These data demonstrated that the F-cell (erythrocytes containing HbF) count determined by IFC strongly correlated with the F-cell count determined by conventional flow cytometry, and with the percentage of HbF determined by high performance liquid chromatography (HPLC), a rapid and precise method for determining HbF concentrations (10). Further, their studies showed a positive correlation between the percentage of HbF and the percentage of erythrocytes retaining normal red cell shape after deoxygenation as determined by IFC, as well as a concomitant negative correlation between the percentage of HbF and the percentage of sickled cells. Interestingly, Fertrin and colleagues observed detectable HbF in both sickled and normal shaped F-cells, as well no detectable HbF in both sickled and normal shaped non-F-cells, demonstrating that erythrocytes which contain HbF may display sickling, while some morphologically normal cells may contain no detectable HbF, suggesting that the presence or absence of HbF alone is not the gatekeeper on whether or not a cell may sickle. Not surprisingly, a greater proportion of sickling was observed in non-F-cells than in F-cells, and significantly less sickling was observed in F-cells in patients on hydroxyurea (HU) compared to patients not on HU. IFC may not be adequate to quantitate the absolute amount of HbF per cell, however it may be a powerful tool for studying the correlation between HbF and red cell morphology in individual cells and additional cellular features of F-cells.
The pathology of sickle cell disease extends to cell types other than just the erythrocyte. One of the major complications of SCD arises from vaso-occlusion of rigid and distorted erythrocytes in small capillaries, which can potentially lead to death. However, elevated levels of leukocytes in the blood of SCD patients can also relate to disease severity and lead to an increased occurrence of vaso-occlusive events (11), as can leukocyte-RBC aggregation (11, 12). These circulating aggregates of cells have not been well documented in humans, partially due to the lack of acceptable methodology to explore this question. It is thought that aberrations in the interactions between leukocytes and red cells may contribute to the vaso-occlusive process. Two groups of investigators utilized the ability of IFC to identify, quantify, and characterize cell conjugates to assess the level and types of leukocyte-RBC aggregates in SCD and to examine the cellular adhesion markers involved in the cell-to-cell interactions in SCD.
Chaar and coworkers (12) isolated peripheral blood mononuclear cells (PBMCs) from the blood of patients with SCD by Ficoll-Paque density gradient separation. The PMBCs were labeled with the panleukocyte marker CD45 and glycophorin-A (GPA), a known erythrocyte marker, and were analyzed by traditional flow cytometry as well as by IFC. By traditional flow cytometry, an unusually high percentage of GPA positive events and CD45 GPA double positive events were seen in the PBMCs of SCD patients, as compared to the PBMCs of normal subjects. These CD45+GPA+ events from the PBMCs of SCD patients were identified as cellular aggregates by IFC imagery of both brightfield and fluorescence, and were found to consist of one PBMC adhered to one or more erythrocyte. Not only did IFC allow for visual confirmation of aggregates, it also permitted the identification of a population of RBC-derived microparticles in these patients. Exclusion of this microparticle population from the analysis provided a more accurate enumeration of PBMC:erythrocyte aggregates, thus improving upon the specificity possible by traditional flow cytometry. Interestingly, through the use of CD71 as a maturation marker, both reticulocytes and mature erythrocytes were found as components of these aggregates.
Dominical and colleagues (11), in collaboration with our laboratory, expanded upon the ability of IFC to identify leukocyte:RBC aggregates and examined neutrophil:RBC aggregates in patient samples and the role of platelets in the formation of those aggregates. The granulocytic fraction obtained by Ficoll sedimentation of peripheral blood from healthy subjects and SCD patients was labeled with antibodies for CD66b (neutrophils), CD71 (reticulocytes), and CD235a (RBC). CD66b positive events were analyzed by IFC using an acquisition gate on CD66b intensity to include only CD66b positive events in the data file. Neutrophils which were aggregated to RBC were identified through both brightfield imagery and CD66b+CD235a+ cell clusters. This group found that the percentage of neutrophil:RBC aggregates was significantly higher in SCD patients than healthy subjects, and that within these aggregates, more of the erythrocytes were determined to be reticulocytes (immature RBCs identified as being CD71+ CD235a+) than were found to be mature RBCs (CD71− CD235a+). When comparing the percentage of neutrophil:reticulocyte aggregates identified by IFC with clinical laboratory blood tests, no correlation was found between the total reticulocyte count, but a negative correlation with HbF was seen. The authors suggest that considering these data together, it may be alterations in the reticulocytes that are responsible for increased neutrophil:reticulocyte aggregates (rather the increased number of reticulocytes). Thus reduced HbS polymerization (caused by the presence of HbF) may prevent alterations in RBCs which lead to aggregate formation. Due to a positive correlation found between neutrophil:reticulocyte aggregates and platelets, the investigators examined whether platelets were directly involved in these aggregates. When incubated in the presence of antibodies against activated platelets (CD62P, P-selectin), granulocyte suspensions from blood of SCD patients had decreases in aggregation of both reticulocytes and RBCs. Further, analysis of the imagery of these aggregates showed that the platelets appeared to be between the neutrophil and RBC, possibly linking the two cells together (Figure 2). Since the number and activation state of platelets may have a significant impact on aggregate formation, and since these aggregates may increase vaso-occlusion, the authors suggested further studies targeting the reduction of platelet activity may have clinical utility. The addition of fluorochrome conjugated antibodies to CD11a and CD11b into this staining permitted evaluation of the role of these adhesion molecules in the formation of the aggregates by IFC. No differences in expression were observed for either CD11a or CD11b between SCD and healthy control blood, suggesting that these molecules did not play a crucial role in the increased neutrophil-red cell aggregation. Functional blocking assays did suggest a role for the adhesion molecules VLA-4 on reticulocytes and ICAM-4 on mature red cells in the formation of neutrophils-red cell aggregates.
Figure 2.
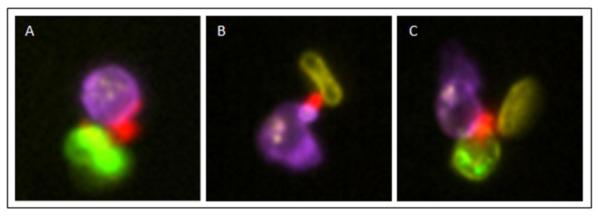
Cellular aggregation in sickle cells
Representative imagery of platelet-neutrophil-RBC aggregates. A. Platelet (CD62P+; red) aggregated to a neutrophil (CD66b+; purple) and to a reticulocyte (CD235a+ CD71+ merge; green). B. Platelet (CD62P+; red) aggregated to a neutrophil (CD66b+; purple) and to a mature RBC (CD235a+CD71−; yellow). C. Platelet (CD62P+; red) aggregated to a neutrophil (CD66b+; purple), reticulocyte (CD235a+ CD71+ merge; green), and to a mature RBC (CD235a+CD71−; yellow). Intensity values were gained for visualization of staining, but raw feature values were not changed.
By combining the speed and statistical power of flow cytometry with the morphological characterizations possible by microscopy, IFC is well suited to play a prominent role in the study of erythrocyte biology in sickle cell disease, particularly in identification of the distinctive shape changes that occur in red blood cells as a consequence of this disease. The existence of a high throughput, highly sensitive, and automated assay to classify erythrocyte shape changes upon deoxygenation provides researchers with a powerful tool to be utilized in drug discovery and monitoring disease severity. IFC also will facilitate elucidation of more details concerning the protective effect that HbF has on HbS polymerization and red cell distortion, and of the role leukocyte:RBC aggregates play in the painful and potentially lethal vaso-occlusive process.
Erythroid cell maturation
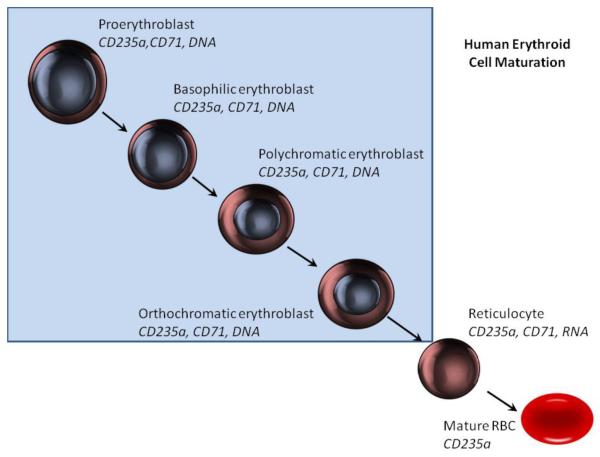
Erythroid cell maturation is a well-defined process in which primitive proerythroblasts in the bone marrow go through a series of morphologic changes including the shedding of their nuclei to emerge into the peripheral circulation with the typical biconcave disc appearance of mature red cells (Figure 3). These cells also display phenotypic changes as they mature, with the expression of transferring receptor (CD71) decreasing as erythroid cells mature while glycophorin A (CD235a) (human) or Ter119 (mouse) expression remains constant. Imaging flow cytometry can concomitantly assess the cell morphology, nucleation of a cell, the presence of RNA, and the intensity of surface markers, thus making it an excellent technology for studies of erythroid cell maturation.
Figure 3.
Human erythroid cell maturation
Erythroid cells display a number of changes as they mature. Early cells are nucleated and display CD71 and CD235a. As cell mature they lose CD71 and eventually become enucleated. After enucleation RNA is retained in reticulocytes but is also lost as cells become mature erythrocytes. Shaded area show cells with a nucleus.
McGrath and coworkers (13) explored the use of IFC for delineating stages of erythroid cell maturation in mice using a combination of size (brightfield area), thiazole orange (for RNA), Ter119, ckit (which is highly expressed on proerythroblasts but is down regulated during maturation), and DRAQ5 for DNA. In this approach, cells were first gated on brightfield area versus brightfield aspect ratio to remove clumps of cells, and the out of focus cells were eliminated from analysis. Cells were then gated on brightfield area versus Ter119 mean intensity to identify erythrocytes, followed by gating on Draq5 intensity versus Draq5 aspect ratio to identify whether or not the cells were nucleated. Non-nucleated cells were then examined for thiazole orange intensity to delineate reticulocytes, which have residual RNA, from mature RBCs, which lack RNA. The nucleated RBCs were then gated on Draq5 area versus Draq5 mean intensity/area and Ter119 area versus Ter119 mean intensity/area to subset the stages of erythroblasts. CD71 was not found to be a good discriminator of the intermediate stages of maturation, although it is useful in the latter stages to differentiate mature RBCs from reticulocytes. This technique thus permitted recapitulation of the stages of erythropoiesis previously identified by morphology, with objective criteria and collection of a statistically significant number of events. As this identification was performed with only four colors of fluorescence, it then becomes possible to further characterize the cells in each stage using additional antibodies or stains.
This group of investigators (14) also used IFC to further study erythropoiesis in mice and identified a transient population in the erythroid cell maturation process that they termed pyrenocytes. Using a fetal mouse model in which erythroid cells were obtained from liver or peripheral blood, these cells were stained with combinations of Ter119, anti-εγ-globin and Draq5. Fixation of the cells prior to staining was necessary and was accomplished through the use of 4% paraformaldehyde followed by acetone at −20°C. Pyrenocytes were identified as positive for εγ-globin and a nucleus, together with having a small cellular size identified using brightfield. These cells were generated from erythroid cell precursors and characterized as small nucleated cells with only a small rim of cytoplasm. A relatively high percentage, 35%, of the pyrenocytes were positive for annexin V, suggesting these cells are a transient population representing the nucleated offspring of the recently enucleated erythrocytes. This novel population helped to define the mechanisms underlying enucleation of erythroblasts and clearly illustrated the utility of IFC for this type of study. Here morphology, nucleation, and surface markers were used to define a novel population and to measure apoptosis within it, with a large, statistically significant number of events being collected.
Further work to understand the process of erythroblast enucleation in mice was performed by Konstantinidis and colleagues (15) using IFC. Specifically, this group was seeking to better understand the role of the cytoskeleton and membrane signaling molecules in the enucleation process. Using an approach similar to McGrath, this group characterized erythroblasts based on the expression of Ter119 and nucleation, and identified cells in the process of enucleation by examining the delta centroid of Ter119/Draq5 staining. Enucleating cells identified using this gating were then examined for the distribution of F-actin and myosin in a contractile actomyosin ring (CAR) during this process. By this technique, it was demonstrated that the association of myosin with F-actin permits the CAR to form and contract between the forming reticulocyte and the nucleus in enucleating erythroblasts.
The studies described above highlight the utility of imaging flow cytometry for studies related to erythroid cell maturation. In addition to these studies, numerous additional investigations have also applied imaging flow cytometry to the study various aspects of erythroid cell maturation such as cell cycling or death and organelle loss (16-19).
Infectious disease
Erythrocytes can be the direct targets for infection in several diseases; foremost among these being malaria. Malaria is caused by several species of the parasitic protozoan Plasmodium. In the initial infection the Plasmodium parasites in the form of sporozoites travel to the liver, multiply, and are released back into the blood as merozoites which directly infect erythrocytes. The merozoites proliferate in the red cells using hemoglobin as a source of amino acids, until they reach a density at which they cause destruction of the erythrocyte. The Plasmodium merozoites within the erythrocytes are of sufficient size that they can be visualized by microscopy and also by imaging flow cytometry. The key advantage of imaging flow cytometry studies in malaria is not only that erythrocytes containing parasites can be directly detected but also that these cells can be examined for additional morphologic features and fluorescent stains in a high throughput manner.
Safeukui, et. al. (20) used IFC to study the morphology and cell dimensions of human red cells to determine the impact of these features in the process of splenic entrapment of erythrocytes. Using malaria-infected RBCs, these authors also studied whether the morphology and dimensional changes induced upon infection played a role in the ability of these cells to traverse microsphilters (an array of metal microspheres used to filter erythrocytes for studies of erythrocyte deformability) serving as surrogates to mimic the filtering function of human spleens (21). In elegantly simple experiments, paraformaldehyde-fixed erythrocytes were examined using only brightfield imaging with analysis of the diameter, perimeter, area, aspect ratio and circularity of each cell. The loss of the area of the cells upon infection was calculated as: 1 - (mean area infected cells/mean area healthy cells) × 100, and the aspect ratio of the cells was used to estimate the relative spherical nature of the cell. In this manner Safeukui and colleagues were able to demonstrate that erythrocytes with ring stage infection by Plasmodium displayed reduced area and a more spherical shape. Furthermore, these morphologic changes in the infected cells were shown to contribute to the cells being retained in the microsphilters, and by extension, in the human spleen. This same technique was used to measure the reduction of surface area of erythrocytes after artesunate treatment of infected cells (Artesunate is a potent anti-malarial compound that is a derivative of artemisinin. Artesunate, whose precise mechanism of action remains unclear, is active against the ring stage of the malaria parasite). The treated cells expelled their parasite and subsequently demonstrated a nearly 9% reduction in area (22). By this method, this group was able to correlate the presence of once infected erythrocytes with an increased incidence of hemolysis after artesunate treatment, providing a potential explanation for the occurrence of this post-therapy side effect.
Mantel and colleagues (23) used IFC to study erythrocyte-derived microvesicles in human malaria infections. Using brightfield, side scatter, and two channels of fluorescence (one for annexin and one for calsein-AM), erythrocyte microvesicles were identified in various stages of formation in infected RBCs and were classified by size. The effects of chemotherapeutic agents for malaria have also been examined using IFC. Based on the observation that chloroquine treatment of P. falciparum exhibits features resembling programmed cell death which is associated with permeabilization of the parasite’s digestive vacuoles, Lee and coworkers (24) developed a high content method of screening new compounds for therapy in human erythrocytes. Working with infected erythrocytes, an assay was devised to detect leakage of the digestive vacuoles as a surrogate for the death of the parasite. Cells are labeled with Hoechst 3342 and Fluo-4 AM; with the latter being a probe for calcium. The Fluo-4 fluoresces green when bound to calcium, and as the vacuoles are calcium stores, in untreated, infected erythrocytes, the staining is brightly localized the site of the digestive vacuole. Leakage of the digestive vacuoles results in calcium being released from the stores and the green fluorescence becomes more diffuse. Thus, cells treated with compounds inducing leakage of the digestive vacuoles will be characterized by a more diffuse pattern of green fluorescence than would be observed in untreated cells. Co-staining with Hoechst permits localization of the parasite, as the mature erythrocyte will be enucleated. In this assay using IFC, the stained cells are first gated to include single round cells (area brightfield vs aspect ratio brightfield) followed by including only cells that were in focus (gradient RMS brightfield). The cells were then gated to include cells with trophozoite-infected erythrocytes (intensity Hoechst vs intensity Fluo-4) and then the intactness of the digestive vacuole was assessed by the area of Fluo-4 staining (Area Fluo-4 vs normalized frequency). This method was successfully used to identify several compounds with therapeutic potential among twenty five pharmacologically active compounds.
IFC has also been used to demonstrate the association of another pathogen, Brucella meliensis, with murine red blood cells (25). Brucella is a Gram negative coccobacillus capable of causing disease in both man and animals. Bacteremia is common in brucellosis and is closely associated with relapse of this disease. Vitry et al (25) used IFC to demonstrate that Brucella bacteria are present in the erythrocytes of infected patients. Using m-Cherry labeled bacteria together with brightfield and looking for overlap between the brightfield cell mask and the mCherry signal, this group was able to delineate extracellular bacteria from those which overlapped the brightfield image (and presumably many of these were intracellular). Intracellular Brucella bacteria were found in both erythrocytes and phagocytic white cells. In time-associated experiments, the internalization of the Brucella appeared to be rapid. Although the bacteria did not appear to divide within the cells (based upon only finding one bacterium per erythrocyte), the authors suggest that the presence of the bacteria within the erythrocyte might immunologically protect the bacteria from the immune response as well as diminish the efficacy of antibiotics.
Conclusions
In the current text, we have attempted to present the reader with a brief outline of how imaging flow cytometry may be of use in studying the biology and various pathologies of erythrocytes. The somewhat unique enucleation process in mammalian erythrocyte maturation is easily detected using imaging flow cytometry, as are accompanying morphologic and immunophenotypic changes. Similarly, intracellular pathogens in erythrocytes often bring DNA and shape changes into the red cells. While the examples of using IFC in the study of intracellular pathogens focused mainly on Plasmodium, a number of other pathogens such as Babesia (babesiosis), Francisella tularensis (tularemia); Bartonella (cat scratch disease), and Streptococcus pneumonia, have also been demonstrated to infect erythrocytes and it is likely that studies using IFC could shed new insight into these diseases. The dramatic shape changes in erythrocytes after deoxygenation in sickle cell disease are particularly amenable to detection by IFC and provide a more robust manner for quantification of the sickled cells than do manual methods, and this may yield a reliable technique for use in drug discovery or monitoring therapy in this disease.
Despite the numerous advantages IFC provides over traditional microscopy discussed in this review, there are some limitations as well. IFC is based on running cells in a suspension, rather than imaging cells on a slide or adhered to a plate. Care should be taken to ensure that if adherent cell lines are used, that the trypsinization process does not damage or alter receptors to be analyzed. Similarly, if the sample type to be analyzed has undergone fixation, steps should be taken to ensure that normal cellular morphology has not been altered to a degree which greatly affects any cellular measurements made. This can be accomplished by comparing morphological measurements made on fixed and unfixed cells, when possible. IFC can routinely image approximately 1000 cells per second, which, while a vast improvement over traditional microscopy, is much slower than high throughput flow cytometers. Further, the sizes of files containing imagery can be quite large (approximately 1Gb for 10,000 events), thus necessitating adequate storage space. These latter two limitations can be an issue when acquiring enough total events to adequately identify a rare population of cells. And, finally, use of custom masks and features should be carefully validated to ensure the masks chosen and regions selected for quantitation are appropriate for the majority of the cells.
To date, studies performed on erythrocytes by IFC have relied heavily on morphology and have made limited use of the many flouorescence channels available. Taking full advantage of all available detectors for these studies will enable far more sophisticated studies to be undertaken generating statistically robust data. The studies described in this manuscript represent only the tip of the iceberg in terms of potential applications of IFC in the study of red cells biology and disease. For example, there are other diseases in addition to sickle cell anemia that are characterized by abnormal shaped erythrocytes. Examples of these include hereditary elliptocytosis, hereditary spherocytosis, and poikilocytosis. IFC assays could be developed to study the biology of these diseases or responses to therapy. Similarly, the quest to manufacture blood in vitro that is suitable for transfusions might be aided by IFC studies of erythroid cell maturation. This unique technology will thereby foster a deeper understanding of erythrocyte biology and the sequelae associated with infection or diseases involving these cells.
Highlights.
Imaging flow cytometry can be used to assess shape changes of erythrocytes cells that are associated with diseases.
Studies of erythroid cell maturation are feasible using imaging flow cytometry.
Parasitic diseases of erythrocytes such as Plasmodium falciparum can be studied by imaging flow cytometry and may prove useful in elucidating mechanisms of anti-malarial therapies.
ACKNOWLEDGMENT
This work was supported by the Intramural Research Program, National Heart, Lung, and Blood Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008;86(6):480–487. doi: 10.2471/BLT.06.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yusuf HR, Lloyd-Puryear MA, Grant AM, Parker CS, Creary MS, Atrash HK. Sickle Cell Disease: The Need for a Public Health Agenda. American J Preventive Med. 2011;(41):S376–S383. doi: 10.1016/j.amepre.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Abdulmalik O, Safo MK, Chen Q, Yang J, Brugnara C, Ohene-Frempong K, Abraham DJ, Asakura T. 5-hydroxymethyl-2-furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells. Br J Haematol. 2005;128(4):552–561. doi: 10.1111/j.1365-2141.2004.05332.x. [DOI] [PubMed] [Google Scholar]
- 4.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337(11):762–9. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 5.Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. N Engl J Med. 2008;359(21):2254–65. doi: 10.1056/NEJMra0804411. [DOI] [PubMed] [Google Scholar]
- 6.Horiuchi K, Ohata J, Hirano Y, Asakura T. Morphologic studies of sickle erythrocytes by image analysis. J Lab Clin Med. 1990;115(5):613–620. [PubMed] [Google Scholar]
- 7.Chang H, Ewert SM, Boochin RM, Nagel RL. Comparative Evaluation of Fifteen Anti-Sickling Agents. Blood. 1983;61(4):693–704. [PubMed] [Google Scholar]
- 8.van Beers EJ, Samsel L, Mendelsohn L, Saiyed R, Fertrin KY, Brantner CA, Daniels MP, Nichols J, McCoy JP, Kato GJ. Imaging flow cytometry for automated detection of hypoxia-incuded erythrocyte shape change in sickle cell disease. Am J Hematol. 2014;89(6):598–603. doi: 10.1002/ajh.23699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fertrin KY, van Beers EJ, Samsel L, Mendelsohn LG, Saiyed R, Nichols JS, Hepp DA, Brantner CA, Daniels MP, McCoy JP, Kato GJ. Imaging flow cytometry documents incomplete resistance of human sickle F-cells to ex vivo hypoxia-induced sickling. Blood. 2014;124(4):658–660. doi: 10.1182/blood-2014-03-559054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan GB, Aw TC, Dunstan RA, Lee SH. Evaluation of high performance liquid chromatography for routine estimation of hemoglobins A2 and F. J Clin Pathol. 1993;46(9):852–856. doi: 10.1136/jcp.46.9.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dominical VM, Samsel L, Nichols JS, Saad STO, Costa FF, McCoy JP, Conran N, Kato GJ. Prominent role of platelets in the formation of circulating neutrophil-red cell heterocellular aggregates in sickle cell anemia. Haematologica. 2014;99(10):1–4. doi: 10.3324/haematol.2014.108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaar V, Picot J, Renaud O, Bartolucci P, Nzouakou R, Bachir D, Galactéros F, Colin Y, Le Van Kim C, El Nemer W. Aggregation of mononuclear and red blood cells through an α4β1-Lu/basal cell adhesion molecule interaction in sickle cell disease. Haematologica. 2010;95(11):1841–1848. doi: 10.3324/haematol.2010.026294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGrath KE, Bushnell TP, Palis J. Multispectral imaging of hematopoietic cells: where flow meets morphology. J Immunol Methods. 2008;336(2):91–7. doi: 10.1016/j.jim.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGrath KE, Kingsley PD, Koniski AD, Porter RL, Bushnell TP, Palis J. Enucleation of primitive erythroid cells generates a transient population of “pyrenocytes” in the mammalian fetus. Blood. 2008;111(4):2409–17. doi: 10.1182/blood-2007-08-107581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konstantinidis DG, Pushkaran S, Johnson JF, Cancelas JA, Manganaris S, Harris CE, Williams DA, Zheng Y, Kalfa TA. Signaling and cytoskeletal requirements in erythroblast enucleation. Blood. 2012;119(25):6118–27. doi: 10.1182/blood-2011-09-379263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Getman M, England SJ, Malik J, Peterson K, Palis J, Steiner LA. Extensively self-renewing erythroblasts derived from transgenic beta-yac mice is a novel model system for studying globin switching and erythroid maturation. Exp Hematol. 2014;42:536–546.e538. doi: 10.1016/j.exphem.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malik J, Kim AR, Tyre KA, Cherukuri AR, Palis J. Erythropoietin critically regulates the terminal maturation of murine and human primitive erythroblasts. Haematologica. 2013;98:1778–1787. doi: 10.3324/haematol.2013.087361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peslak SA, Wenger J, Bemis JC, Kingsley PD, Frame JM, Koniski AD, Chen Y, Williams JP, McGrath KE, Dertinger SD, et al. Sublethal radiation injury uncovers a functional transition during erythroid maturation. Exp Hematol. 2011;39:434–445. doi: 10.1016/j.exphem.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thom CS, Traxler EA, Khandros E, Nickas JM, Zhou OY, Lazarus JE, Silva AP, Prabhu D, Yao Y, Aribeana C, et al. Trim58 degrades Dynein and regulates terminal erythropoiesis. Dev Cell. 2014;30:688–700. doi: 10.1016/j.devcel.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safeukui I, Buffet PA, Deplaine G, Perrot S, Brousse V, Ndour A, Nguyen M, Mercereau-Puijalon O, David PH, Milon G, Mohandas N. Quantitative assessment of sensing and sequestration of spherocytic erythrocytes by the human spleen. Blood. 2012;120(2):424–30. doi: 10.1182/blood-2012-01-404103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safeukui I, Buffet PA, Perrot S, Sauvanet A, Aussilhou B, Dokmak S, Couvelard A, Hatem DC, Mohandas N, David PH, Mercereau-Puijalon O, Milon G. Surface area loss and increased sphericity account for the splenic entrapment of subpopulations of Plasmodium falciparum ring-infected erythrocytes. PLoS One. 2013;8(3):e60150. doi: 10.1371/journal.pone.0060150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jauréguiberry S, Ndour PA, Roussel C, Ader F, Safeukui I, Nguyen M, Biligui S, Ciceron L, Mouri O, Kendjo E, Bricaire F, Vray M, Angoulvant A, Mayaux J, Haldar K, Mazier D, Danis M, Caumes E, Thellier M, Buffet P, French Artesunate Working Group Postartesunate delayed hemolysis is a predictable event related to the lifesaving effect of artemisinins. Blood. 2014;124(2):167–75. doi: 10.1182/blood-2014-02-555953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantel PY, Hoang AN, Goldowitz I, Potashnikova D, Hamza B, Vorobjev I, Ghiran I, Toner M, Irimia D, Ivanov AR, Barteneva N, Marti M. Malaria-Infected Erythrocyte-Derived Microvesicles Mediate Cellular Communication within the Parasite Population and with the Host Immune System. Cell Host and Microbe. 2013;13(5):521–534. doi: 10.1016/j.chom.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YQ, Goh AS, Ch’ng JH, Nosten FH, Preiser PR, Pervaiz S, Yadav SK, Tan KS. A high-content phenotypic screen reveals the disruptive potency of quinacrine and 3′,4′-dichlorobenzamil on the digestive vacuole of Plasmodium falciparum. Antimicrob Agents Chemother. 2014;58(1):550–8. doi: 10.1128/AAC.01441-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vitry MA, Hanot Mambres D, Deghelt M, Hack K, Machelart A, Lhomme F, Vanderwinden JM, Vermeersch M, De Trez C, Pérez-Morga D, Letesson JJ, Muraille E. Brucella melitensis Invades Murine Erythrocytes during Infection. Infect Immun. 2014;82(9):3927–38. doi: 10.1128/IAI.01779-14. [DOI] [PMC free article] [PubMed] [Google Scholar]