Abstract
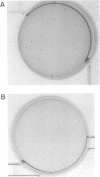
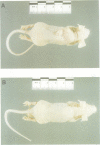
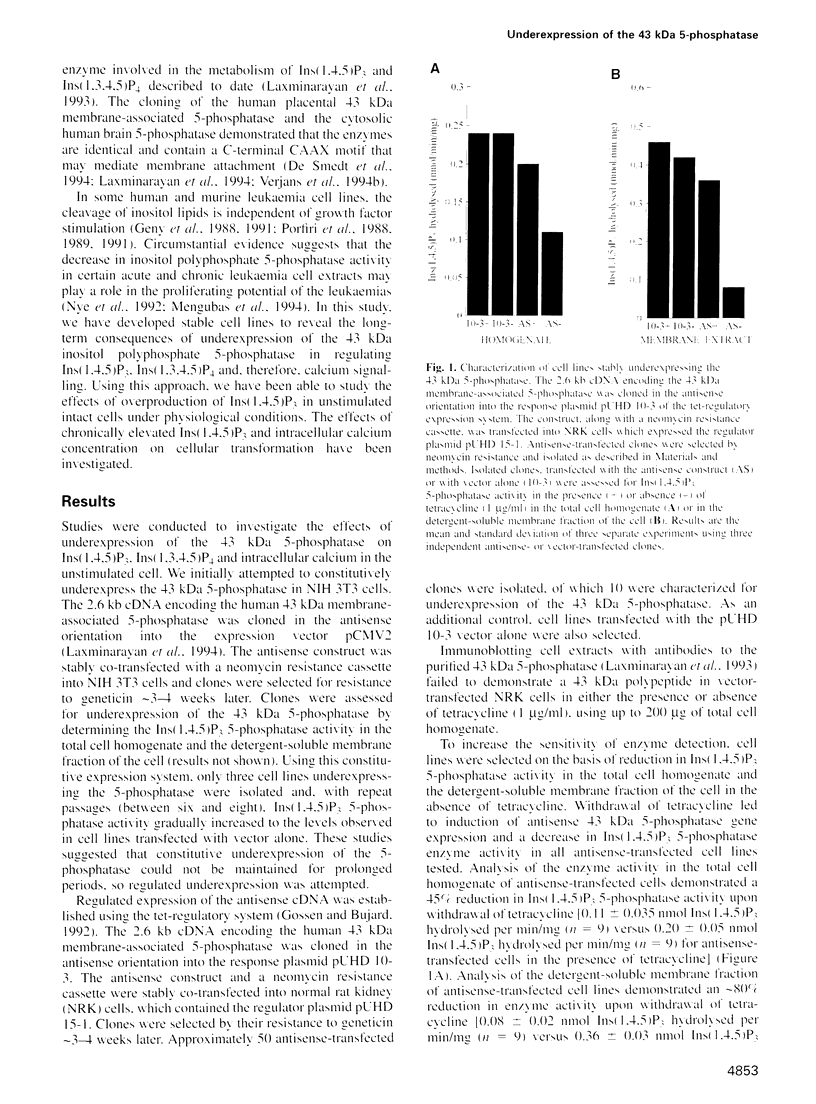
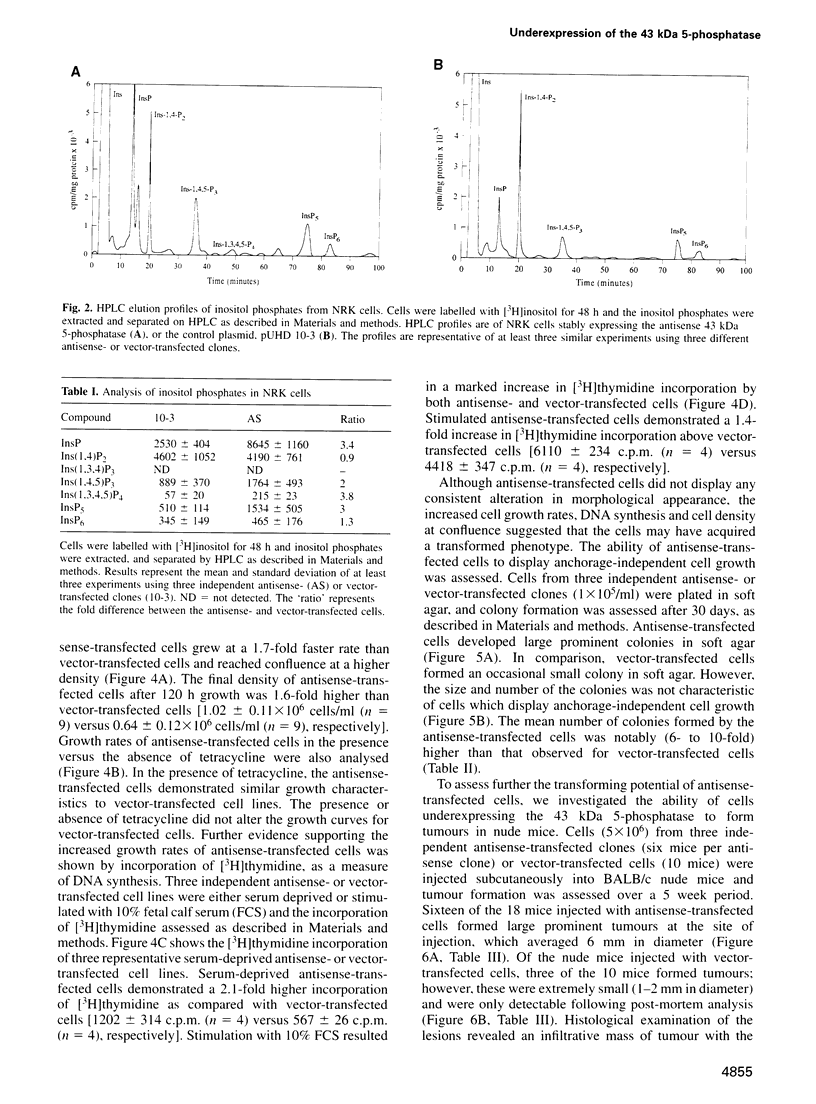
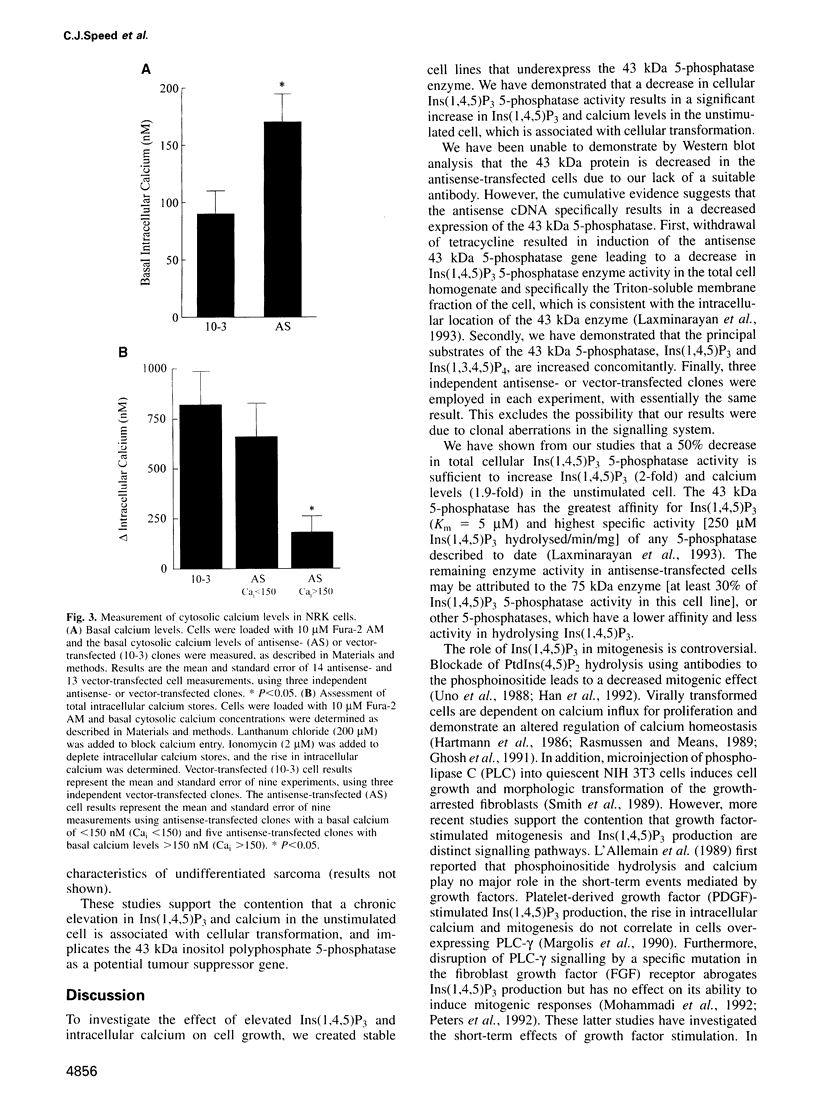
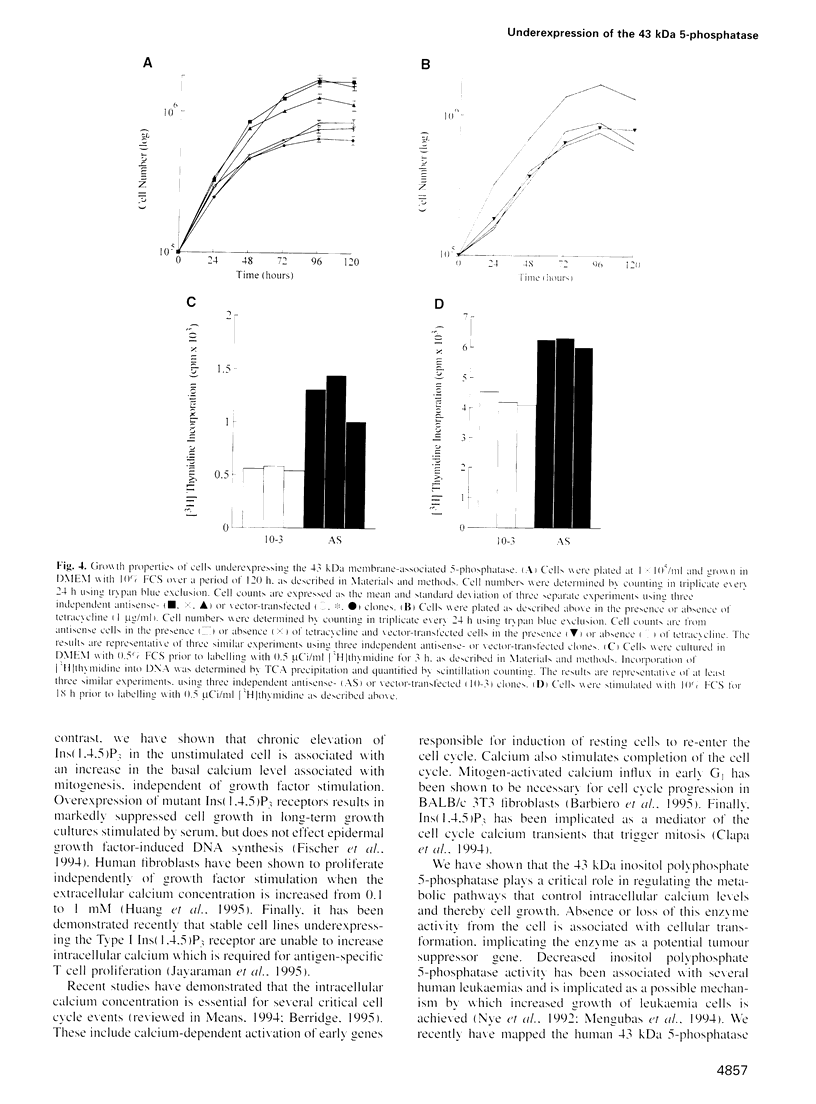
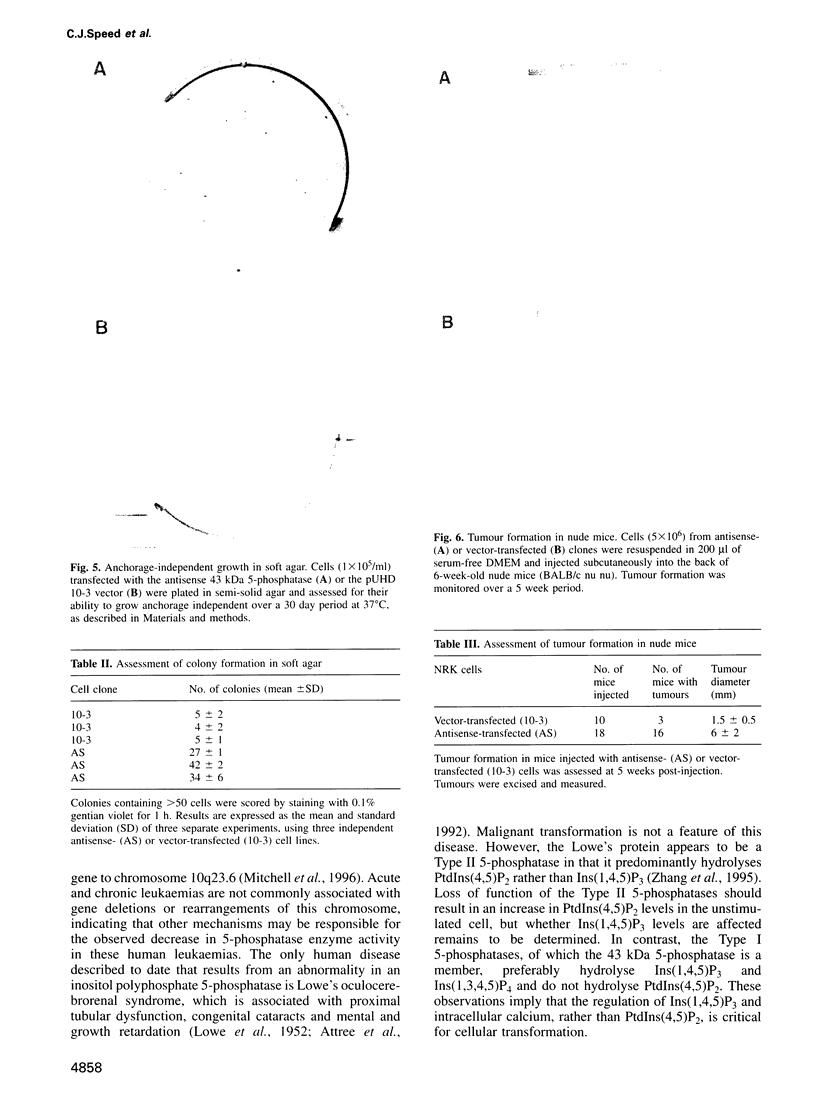
The 43 kDa inositol polyphosphate 5-phosphatase (5-phosphatase) hydrolyses the second messenger molecules inositol 1,4,5-trisphosphate [Ins(1,4,5)P3] and inositol 1,3,4,5-tetrakisphosphate [Ins(1,3,4,5)P4]. We have underexpressed the 43 kDa 5-phosphatase by stably transfecting normal rat kidney cells with the cDNA encoding the enzyme, cloned in the antisense orientation into the tetracycline-inducible expression vector pUHD10-3. Antisense-transfected cells demonstrated a 45% reduction in Ins(1,4,5)P3 5-phosphatase activity in the total cell homogenate upon withdrawal of tetracycline, and an approximately 80% reduction in the detergent-soluble membrane fraction of the cell, as compared with antisense-transfected cells in the presence of tetracycline. Unstimulated antisense-transfected cells showed a concomitant 2-fold increase in Ins(1,4,5)P3 and 4-fold increase in Ins(1,3,4,5)P4 levels. The basal intracellular calcium concentration of antisense-transfected cells (170 +/- 25 nM) was increased 1.9-fold, compared with cells transfected with vector alone (90 +/- 25 nM). Cells underexpressing the 43 kDa 5-phosphatase demonstrated a transformed phenotype. Antisense-transfected cells grew at a 1.7-fold faster rate, reached confluence at higher density and demonstrated increased [3H]thymidine incorporation compared with cells transfected with vector alone. Furthermore, antisense-transfected cells formed colonies in soft agar and tumours in nude mice. These studies support the contention that a decrease in Ins(1,4,5)P3 5-phosphatase activity is associated with cellular transformation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986 Sep;38(3):227–272. [PubMed] [Google Scholar]
- Attree O., Olivos I. M., Okabe I., Bailey L. C., Nelson D. L., Lewis R. A., McInnes R. R., Nussbaum R. L. The Lowe's oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature. 1992 Jul 16;358(6383):239–242. doi: 10.1038/358239a0. [DOI] [PubMed] [Google Scholar]
- Barbiero G., Munaron L., Antoniotti S., Baccino F. M., Bonelli G., Lovisolo D. Role of mitogen-induced calcium influx in the control of the cell cycle in Balb-c 3T3 fibroblasts. Cell Calcium. 1995 Dec;18(6):542–556. doi: 10.1016/0143-4160(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Calcium signalling and cell proliferation. Bioessays. 1995 Jun;17(6):491–500. doi: 10.1002/bies.950170605. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciapa B., Pesando D., Wilding M., Whitaker M. Cell-cycle calcium transients driven by cyclic changes in inositol trisphosphate levels. Nature. 1994 Apr 28;368(6474):875–878. doi: 10.1038/368875a0. [DOI] [PubMed] [Google Scholar]
- Connolly T. M., Bross T. E., Majerus P. W. Isolation of a phosphomonoesterase from human platelets that specifically hydrolyzes the 5-phosphate of inositol 1,4,5-trisphosphate. J Biol Chem. 1985 Jul 5;260(13):7868–7874. [PubMed] [Google Scholar]
- Damen J. E., Liu L., Rosten P., Humphries R. K., Jefferson A. B., Majerus P. W., Krystal G. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc Natl Acad Sci U S A. 1996 Feb 20;93(4):1689–1693. doi: 10.1073/pnas.93.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smedt F., Verjans B., Mailleux P., Erneux C. Cloning and expression of human brain type I inositol 1,4,5-trisphosphate 5-phosphatase. High levels of mRNA in cerebellar Purkinje cells. FEBS Lett. 1994 Jun 20;347(1):69–72. doi: 10.1016/0014-5793(94)00509-5. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erneux C., Lemos M., Verjans B., Vanderhaeghen P., Delvaux A., Dumont J. E. Soluble and particulate Ins(1,4,5)P3/Ins(1,3,4,5)P4 5-phosphatase in bovine brain. Eur J Biochem. 1989 May 1;181(2):317–322. doi: 10.1111/j.1432-1033.1989.tb14726.x. [DOI] [PubMed] [Google Scholar]
- Fischer G. A., Clementi E., Raichman M., Südhof T., Ullrich A., Meldolesi J. Stable expression of truncated inositol 1,4,5-trisphosphate receptor subunits in 3T3 fibroblasts. Coordinate signaling changes and differential suppression of cell growth and transformation. J Biol Chem. 1994 Jul 29;269(30):19216–19224. [PubMed] [Google Scholar]
- Geny B., Cost H., Barreau P., Basset M., Le Peuch C., Abita J. P., Cockcroft S. The differentiating agent, retinoic acid, causes an early inhibition of inositol lipid-specific phospholipase C activity in HL-60 cells. Cell Signal. 1991;3(1):11–23. doi: 10.1016/0898-6568(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Geny B., LePeuch C., Cost H., Basset M., Cockcroft S. Phorbol esters inhibit inositol phosphate and diacylglycerol formation in proliferating HL60 cells. Relationship to differentiation. FEBS Lett. 1988 Jun 20;233(2):239–243. doi: 10.1016/0014-5793(88)80434-4. [DOI] [PubMed] [Google Scholar]
- Ghosh T. K., Bian J. H., Short A. D., Rybak S. L., Gill D. L. Persistent intracellular calcium pool depletion by thapsigargin and its influence on cell growth. J Biol Chem. 1991 Dec 25;266(36):24690–24697. [PubMed] [Google Scholar]
- Gossen M., Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. K., Fukami K., Nuccitelli R. Reducing inositol lipid hydrolysis, Ins(1,4,5)P3 receptor availability, or Ca2+ gradients lengthens the duration of the cell cycle in Xenopus laevis blastomeres. J Cell Biol. 1992 Jan;116(1):147–156. doi: 10.1083/jcb.116.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansbro P. M., Foster P. S., Hogan S. P., Ozaki S., Denborough M. A. Purification and characterization of D-myo-inositol (1,4,5)/(1,3,4,5)- polyphosphate 5-phosphatase from skeletal muscle. Arch Biochem Biophys. 1994 May 15;311(1):47–54. doi: 10.1006/abbi.1994.1207. [DOI] [PubMed] [Google Scholar]
- Hansen C. A., Johanson R. A., Williamson M. T., Williamson J. R. Purification and characterization of two types of soluble inositol phosphate 5-phosphomonoesterases from rat brain. J Biol Chem. 1987 Dec 25;262(36):17319–17326. [PubMed] [Google Scholar]
- Hartmann T., Seuwen K., Adam G. Cellular compartmentation of calcium in mouse fibroblasts in vitro depends on cell density and transformation. Exp Cell Res. 1986 Mar;163(1):279–286. doi: 10.1016/0014-4827(86)90582-3. [DOI] [PubMed] [Google Scholar]
- Hejna J. A., Saito H., Merkens L. S., Tittle T. V., Jakobs P. M., Whitney M. A., Grompe M., Friedberg A. S., Moses R. E. Cloning and characterization of a human cDNA (INPPL1) sharing homology with inositol polyphosphate phosphatases. Genomics. 1995 Sep 1;29(1):285–287. doi: 10.1006/geno.1995.1247. [DOI] [PubMed] [Google Scholar]
- Hodgkin M., Craxton A., Parry J. B., Hughes P. J., Potter B. V., Michell R. H., Kirk C. J. Bovine testis and human erythrocytes contain different subtypes of membrane-associated Ins(1,4,5)P3/Ins(1,3,4,5)P4 5-phosphomonoesterases. Biochem J. 1994 Feb 1;297(Pt 3):637–645. doi: 10.1042/bj2970637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. R., Skryabin B. V., Belcher S. M., Zerillo C. A., Schmauss C. The responsiveness of a tetracycline-sensitive expression system differs in different cell lines. J Biol Chem. 1995 Jun 9;270(23):14168–14174. doi: 10.1074/jbc.270.23.14168. [DOI] [PubMed] [Google Scholar]
- Huang S., Maher V. M., McCormick J. J. Extracellular Ca2+ stimulates the activation of mitogen-activated protein kinase and cell growth in human fibroblasts. Biochem J. 1995 Sep 15;310(Pt 3):881–885. doi: 10.1042/bj3100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Stephens L. R., Musgrave A. Inositol polyphosphate metabolism and inositol lipids in a green alga, Chlamydomonas eugametos. Biochem J. 1992 Jan 1;281(Pt 1):261–266. doi: 10.1042/bj2810261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. Second messengers and Lowe syndrome. Nat Genet. 1992 Aug;1(5):315–316. doi: 10.1038/ng0892-315. [DOI] [PubMed] [Google Scholar]
- Jayaraman T., Ondriasová E., Ondrias K., Harnick D. J., Marks A. R. The inositol 1,4,5-trisphosphate receptor is essential for T-cell receptor signaling. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):6007–6011. doi: 10.1073/pnas.92.13.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson A. B., Majerus P. W. Properties of type II inositol polyphosphate 5-phosphatase. J Biol Chem. 1995 Apr 21;270(16):9370–9377. doi: 10.1074/jbc.270.16.9370. [DOI] [PubMed] [Google Scholar]
- L'Allemain G., Seuwen K., Velu T., Pouyssegur J. Signal transduction in hamster fibroblasts overexpressing the human EGF receptor. Growth Factors. 1989;1(4):311–321. doi: 10.3109/08977198909000255. [DOI] [PubMed] [Google Scholar]
- LOWE C. U., TERREY M., MacLACHLAN E. A. Organic-aciduria, decreased renal ammonia production, hydrophthalmos, and mental retardation; a clinical entity. AMA Am J Dis Child. 1952 Feb;83(2):164–184. doi: 10.1001/archpedi.1952.02040060030004. [DOI] [PubMed] [Google Scholar]
- Laxminarayan K. M., Chan B. K., Tetaz T., Bird P. I., Mitchell C. A. Characterization of a cDNA encoding the 43-kDa membrane-associated inositol-polyphosphate 5-phosphatase. J Biol Chem. 1994 Jun 24;269(25):17305–17310. [PubMed] [Google Scholar]
- Laxminarayan K. M., Matzaris M., Speed C. J., Mitchell C. A. Purification and characterization of a 43-kDa membrane-associated inositol polyphosphate 5-phosphatase from human placenta. J Biol Chem. 1993 Mar 5;268(7):4968–4974. [PubMed] [Google Scholar]
- Majerus P. W. Inositol phosphate biochemistry. Annu Rev Biochem. 1992;61:225–250. doi: 10.1146/annurev.bi.61.070192.001301. [DOI] [PubMed] [Google Scholar]
- Margolis B., Zilberstein A., Franks C., Felder S., Kremer S., Ullrich A., Rhee S. G., Skorecki K., Schlessinger J. Effect of phospholipase C-gamma overexpression on PDGF-induced second messengers and mitogenesis. Science. 1990 May 4;248(4955):607–610. doi: 10.1126/science.2333512. [DOI] [PubMed] [Google Scholar]
- Matzaris M., Jackson S. P., Laxminarayan K. M., Speed C. J., Mitchell C. A. Identification and characterization of the phosphatidylinositol-(4, 5)-bisphosphate 5-phosphatase in human platelets. J Biol Chem. 1994 Feb 4;269(5):3397–3402. [PubMed] [Google Scholar]
- McPherson P. S., Garcia E. P., Slepnev V. I., David C., Zhang X., Grabs D., Sossin W. S., Bauerfeind R., Nemoto Y., De Camilli P. A presynaptic inositol-5-phosphatase. Nature. 1996 Jan 25;379(6563):353–357. doi: 10.1038/379353a0. [DOI] [PubMed] [Google Scholar]
- Means A. R. Calcium, calmodulin and cell cycle regulation. FEBS Lett. 1994 Jun 20;347(1):1–4. doi: 10.1016/0014-5793(94)00492-7. [DOI] [PubMed] [Google Scholar]
- Menniti F. S., Oliver K. G., Putney J. W., Jr, Shears S. B. Inositol phosphates and cell signaling: new views of InsP5 and InsP6. Trends Biochem Sci. 1993 Feb;18(2):53–56. doi: 10.1016/0968-0004(93)90053-p. [DOI] [PubMed] [Google Scholar]
- Mitchell C. A., Connolly T. M., Majerus P. W. Identification and isolation of a 75-kDa inositol polyphosphate-5-phosphatase from human platelets. J Biol Chem. 1989 May 25;264(15):8873–8877. [PubMed] [Google Scholar]
- Mitchell C. A., Speed C. J., Nicholl J., Sutherland G. R. Chromosomal mapping of the gene (INPP5A) encoding the 43-kDa membrane-associated inositol polyphosphate 5-phosphatase to 10q26.3 by fluorescence in situ hybridization. Genomics. 1996 Jan 1;31(1):139–140. doi: 10.1006/geno.1996.0023. [DOI] [PubMed] [Google Scholar]
- Mohammadi M., Dionne C. A., Li W., Li N., Spivak T., Honegger A. M., Jaye M., Schlessinger J. Point mutation in FGF receptor eliminates phosphatidylinositol hydrolysis without affecting mitogenesis. Nature. 1992 Aug 20;358(6388):681–684. doi: 10.1038/358681a0. [DOI] [PubMed] [Google Scholar]
- Neylon C. B., Nickashin A., Little P. J., Tkachuk V. A., Bobik A. Thrombin-induced Ca2+ mobilization in vascular smooth muscle utilizes a slowly ribosylating pertussis toxin-sensitive G protein. Evidence for the involvement of a G protein in inositol trisphosphate-dependent Ca2+ release. J Biol Chem. 1992 Apr 15;267(11):7295–7302. [PubMed] [Google Scholar]
- Nye K. E., Riley G. A., Poulter L. W., Porfiri E., Hoffbrand A. V., Wickremasinghe R. G. Impaired degradation of Ca(2+)-regulating second messengers in myeloid leukemia cells. Implications for the regulation of leukemia cell proliferation. Leukemia. 1992 Aug;6(8):801–805. [PubMed] [Google Scholar]
- Palmer F. B., Théolis R., Jr, Cook H. W., Byers D. M. Purification of two immunologically related phosphatidylinositol-(4,5)- bisphosphate phosphatases from bovine brain cytosol. J Biol Chem. 1994 Feb 4;269(5):3403–3410. [PubMed] [Google Scholar]
- Peters K. G., Marie J., Wilson E., Ives H. E., Escobedo J., Del Rosario M., Mirda D., Williams L. T. Point mutation of an FGF receptor abolishes phosphatidylinositol turnover and Ca2+ flux but not mitogenesis. Nature. 1992 Aug 20;358(6388):678–681. doi: 10.1038/358678a0. [DOI] [PubMed] [Google Scholar]
- Porfiri E., Hoffbrand A. V., Wickremasinghe R. G. Granulocytic differentiation of HL60 human promyelocytic leukemia cells is preceded by a reduction in levels of inositol lipid-derived second messengers. Exp Hematol. 1988 Aug;16(7):641–646. [PubMed] [Google Scholar]
- Porfiri E., Hoffbrand A. V., Wickremasinghe R. G. Induction of macrophage-like differentiation of HL60 human myeloid leukemia cells by phorbol myristate acetate triggers an early decline in inositol lipid breakdown. Exp Hematol. 1989 May;17(4):344–350. [PubMed] [Google Scholar]
- Porfiri E., Hoffbrand A. V., Wickremasinghe R. G. Retinoic acid-induced granulocytic differentiation of HL60 human promyelocytic leukemia cells is preceded by downregulation of autonomous generation of inositol lipid-derived second messengers. Blood. 1991 Aug 15;78(4):1069–1077. [PubMed] [Google Scholar]
- Rasmussen C. D., Means A. R. Calmodulin, cell growth and gene expression. Trends Neurosci. 1989 Nov;12(11):433–438. doi: 10.1016/0166-2236(89)90092-1. [DOI] [PubMed] [Google Scholar]
- Resnitzky D., Gossen M., Bujard H., Reed S. I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994 Mar;14(3):1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross T. S., Jefferson A. B., Mitchell C. A., Majerus P. W. Cloning and expression of human 75-kDa inositol polyphosphate-5-phosphatase. J Biol Chem. 1991 Oct 25;266(30):20283–20289. [PubMed] [Google Scholar]
- Shears S. B. Metabolism of the inositol phosphates produced upon receptor activation. Biochem J. 1989 Jun 1;260(2):313–324. doi: 10.1042/bj2600313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., Ryu S. H., Suh P. G., Rhee S. G., Kung H. F. S-phase induction and transformation of quiescent NIH 3T3 cells by microinjection of phospholipase C. Proc Natl Acad Sci U S A. 1989 May;86(10):3659–3663. doi: 10.1073/pnas.86.10.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto K., Okada M., Nakagawa H. Purification and characterization of membrane-bound inositolpolyphosphate 5-phosphatase. J Biochem. 1989 Oct;106(4):684–690. doi: 10.1093/oxfordjournals.jbchem.a122917. [DOI] [PubMed] [Google Scholar]
- Verjans B., De Smedt F., Lecocq R., Vanweyenberg V., Moreau C., Erneux C. Cloning and expression in Escherichia coli of a dog thyroid cDNA encoding a novel inositol 1,4,5-trisphosphate 5-phosphatase. Biochem J. 1994 May 15;300(Pt 1):85–90. doi: 10.1042/bj3000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verjans B., Lecocq R., Moreau C., Erneux C. Purification of bovine brain inositol-1,4,5-trisphosphate 5-phosphatase. Eur J Biochem. 1992 Mar 15;204(3):1083–1087. doi: 10.1111/j.1432-1033.1992.tb16732.x. [DOI] [PubMed] [Google Scholar]
- Verjans B., Moreau C., Erneux C. The control of intracellular signal molecules at the level of their hydrolysis: the example of inositol 1,4,5-trisphosphate 5-phosphatase. Mol Cell Endocrinol. 1994 Jan;98(2):167–171. doi: 10.1016/0303-7207(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Zhang X., Jefferson A. B., Auethavekiat V., Majerus P. W. The protein deficient in Lowe syndrome is a phosphatidylinositol-4,5-bisphosphate 5-phosphatase. Proc Natl Acad Sci U S A. 1995 May 23;92(11):4853–4856. doi: 10.1073/pnas.92.11.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]