Abstract
Previous research has demonstrated that the nucleus accumbens is a site where opioids and cannabinoids interact to alter feeding behavior. However, the influence of the endocannabinoid 2-arachidonylglycerol (2-AG) on the well-characterized model of intra-accumbens opioid driven high-fat feeding behavior has not been explored. The present experiments examined high-fat feeding associated behaviors produced by the interaction of 2-AG and the µ-opioid receptor agonist DAla2,N,Me-Phe4,Gly-ol5-enkaphalin (DAMGO) administered into the nucleus accumbens. Sprague-Dawley rats were implanted with bilateral cannulae aimed at the nucleus accumbens and were co-administered both a sub-threshold dose of 2-AG (0 or 0.25µg/0.5µl/side) and DAMGO (0, 0.025µg or 0.25µg/0.5µl/side) in all dose combinations, and in a counterbalanced order. Animals were then immediately allowed a 2hr-unrestricted access period to a palatable high-fat diet. Consumption, number and duration of food hopper entries, and locomotor activity were all monitored. DAMGO treatment led to an increase in multiple behaviors, including consumption, duration of food hopper entry, and locomotor activity. However, combined intra-accumbens administration of DAMGO and a subthreshold dose of 2- AG led to a significant increase in number of food hopper entries and locomotor activity, compared to DAMGO by itself. The results confirm that intra-accumbens administration of subthreshold dose of the endogenous cannabinoid 2-AG increases the DAMGO-induced approach and locomotor behaviors associated with high-fat feeding.
Keywords: nucleus accumbens, 2-arachidonylglycerol, palatable food, cannabinoid, DAMGO, opioid, approach, consumption, reward, feeding, high fat
1. Introduction
The latest data indicate that 34.9% of the adult and 16.9% of the child U.S. population is obese (Ogden et al., 2014). Although obesity is certainly the result of a complex set of factors, including sedentary lifestyle, economic factors, genetic predisposition, and stressful life events, the over consumption of calorically dense palatable foods is involved. Research examining the nature of this critical behavior of overconsumption has revealed a distributed feeding network that includes many key regions of the brain and many neuromodulators that may contribute (Will et al., 2003; Berthoud 2012, for review). The nucleus accumbens in particular, and its associated circuitry, is a critical region that mediates the response to palatable food, most notably the actions of opioids (Pecina & Berridge, 2000; Will et al., 2003). For example, administration of the selective μ-opioid agonist D-Ala2, NMe-Phe4, Glyol5-enkephalin (DAMGO) into the nucleus accumbens produces a robust binge-like consumption of palatable diets such as those high in fat and/or sugar (Zhang et al., 2000; Pecina & Berridge, 2000). Behavioral and pharmacological characterizations of intra-accumbens DAMGO suggest that it does not induce a state of negative energy balance (i.e. “hunger”) (Hanlon et al., 2004; Will et al., 2009). Indeed, evidence suggests that the activation of the accumbens with DAMGO acts to increase the hedonic or rewarding nature of the food independent of negative energy balance, in turn producing increased consumption and associated food seeking behaviors (Kelley et al., 2002; Pecina & Berridge, 2000; Will et al., 2009).
Overlapping with the comparably longer period of research on opioids (Bodnar, 2013), research on endocannabinoids has led to promising targets that could lead to therapeutic advancements in the treatment of both obesity and drug addiction (Isoldi and Aronne, 2008; Bermudez-Silva et al., 2010). Systemic activation of the endocannabinoid system produces many of the same behavioral effects as the opioid system, including increased feeding behavior, and reinforcement of drug self-administration behavior (Maldonado and Rodriguez de Fonseca, 2002; Tanda and Goldberg, 2003; Silvestri and Di Marzo, 2013; Cristino et al., 2014; Jager and Witkamp, 2014). The endocannabinoids 2-arachidonoylglycerol (2-AG) (Mechoulam et al., 1995; Sugiura et al., 1995) and anandamide (Devane et al., 1992) have both been shown to increase feeding behavior when administered into the nucleus accumbens (Kirkham et al., 2002; Mahler et al., 2007). However, the combined influence of these endocannabinoids and opioid receptor agonists has not been explored in a palatable feeding model. It has been demonstrated that the behavioral effects of cannabinoids are partially dependent on co-activation of the opioid system (Williams and Kirkham, 2002; Maldonado and Rodriguez de Fonseca, 2002; Justinova et al., 2004; Skelly et al., 2010). For example, feeding increased by striatal infusions of cannabinoid and opioid agonists is blocked by prior administration of opioid and cannabinoid receptor antagonists, respectively (Williams and Kirkham, 2002; Skelly et al., 2010). Also, sub-threshold doses of opioid and cannabinoid antagonists that have little or no effect on feeding independently, demonstrate a potentiated effect when administered together (Kirkham and Williams, 2001; Chen et al., 2004; Tallett et al., 2009). Finally, intra-accumbens administration of a subthreshold dose of a selective CB1 agonist WIN55212-2 and DAMGO increased high-fat feeding above levels produced by DAMGO alone (Skelly et al., 2010).
The present study was designed to examine the potential interaction of the opioid and cannabinoid systems within the well-characterized model of intra-accumbens opioid-induced high-fat feeding. Specifically, a sub-threshold dose of 2-AG and multiple near-threshold doses of the μ-opioid agonist DAMGO were co-administered into the nucleus accumbens and multiple behaviors associated with high fat feeding were assessed, including food-directed approach (food hopper entries), consumption, and locomotor activity. Assessing approach, as well as consumption behaviors, has been shown to be critical in understanding the diverse effect of the interaction of opioids and cannabinoids (Tallett et al., 2009). Also, increased food-directed approach responses do not always predict a parallel increase in consumption measures (Will et al., 2009). Therefore, the current study investigated whether a sub-threshold dose of the endocannabinoid 2-AG alter the feeding behaviors driven by intra-accumbens DAMGO.
2. Results
2.1. Consumption
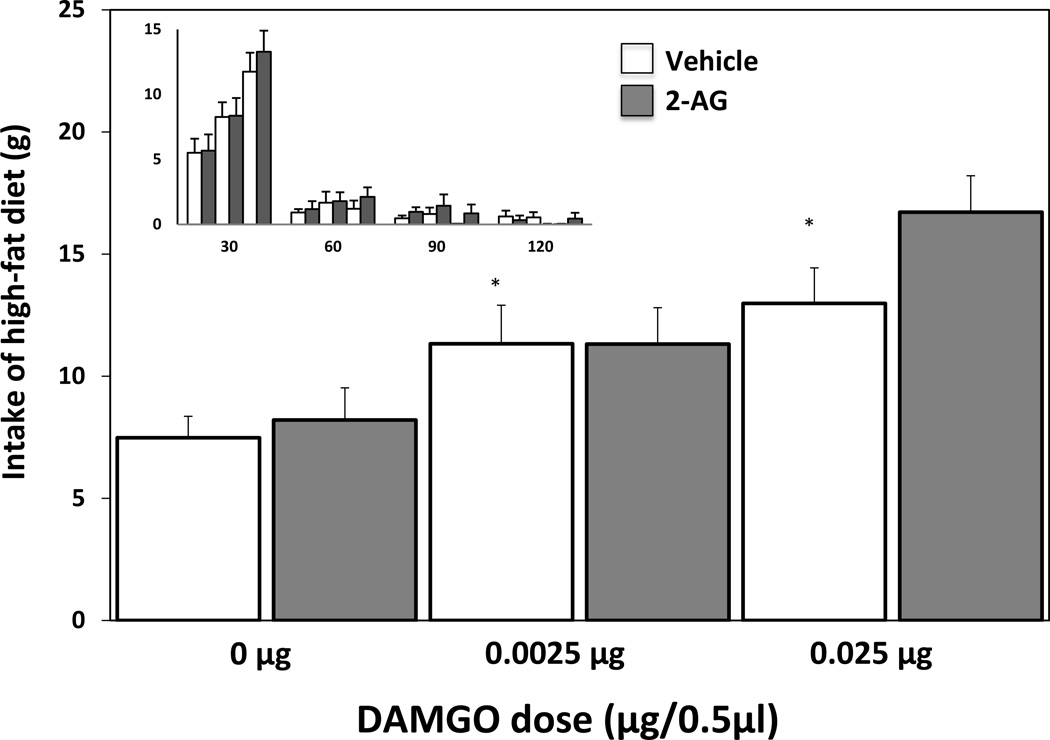
An ANOVA conducted on the food consumption during the total 2hr test session revealed no main effect of 2-AG pretreatment (vehicle or 0.25 µg) (F(1,36)=1.99, ns), a significant main effect of DAMGO treatment (saline, 0.025µg, or 0.0025µg) (F(2,36)=14.85, p < .0001), and no interaction (F(2,36)=1.20, ns) (Fig. 2). As can be observed in the smaller inset in Figure 2, there was a strong trend of decreasing consumption across time with the majority of consumption following all treatments occurring in the first 30 minutes. To further analyze this trend and treatment effects across time, an ANOVA examining 2-AG pretreatment×DAMGO treatment×time interval interaction was conducted. There was again no main effect of 2-AG pretreatment (F(1,144)=1.58, ns), yet a significant main effect of DAMGO treatment (F(2,144)=12.22, p < .0001), and time interval (F(3,144)=183.15, p < .0001). The only significant interaction observed was a DAMGO treatment×time interaction (F(6,144)=12.91, p < .0001). Post hoc comparisons of consumption during the first 30 min interval revealed that the highest dose of DAMGO, with or without 2-AG pretreatment, was significantly increased (p < .05) compared to control treatment (vehicle preatreatment + saline treatment). No other treatment comparison reached significance (p>.05).
Fig. 2.
Influence of intra-accumbens 2-AG (0.25μg/0.5μl) and DAMGO (0.0025μg or 0.025μg/0.5μl) on consumption levels (* = p < .05; compared to Vehicle – 0μg DAMGO coadministration). The inset graph depicts the same data across 30 min intervals; the x-axis represents 30-min time intervals across the 2hr testing period (30, 60, 90, and 120 min) and the y-axis represents the grams of high-fat consumed.
2.2. Food hopper entries
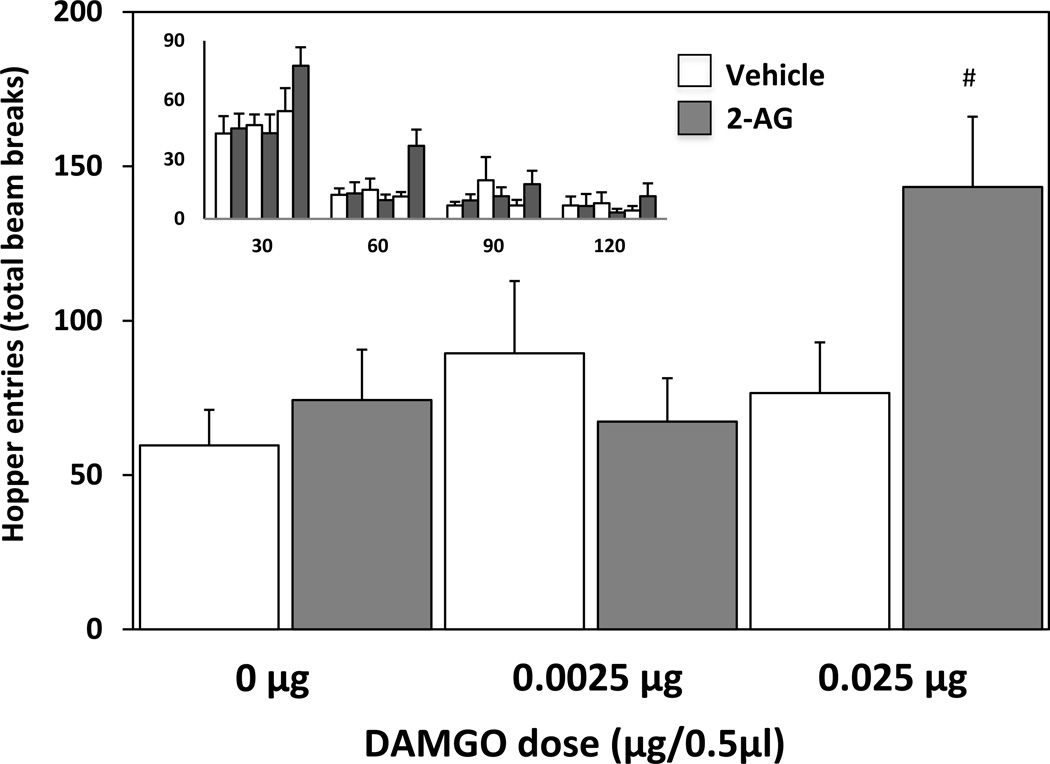
An ANOVA conducted on the total number of food hopper entries during the 2 hr test session revealed no main effect of 2-AG treatment (F(1,36)=2.12, ns), a significant main effect of DAMGO treatment (F(2,36)=3.59, p < .05), and a significant 2-AG×DAMGO treatment interaction (F(2,36)=3.60, p < .05). As displayed in Fig. 3, post-hoc comparisons revealed that intra-accumbens 2-AG and both DAMGO doses did not increase total hopper entries alone (p > .05). However, the combined administration of 2-AG and the high DAMGO dose produced a significant increase in total number of hopper entries compared to both vehicle treatment (p < 0.001) or the high DAMGO dose alone (p < 0.005). As can be observed in the smaller inset in Figure 3, there was a strong trend toward a decrease in hopper entries across time, with the majority occurring in the first 30 minutes. To further analyze this trend and treatment effects across time, an ANOVA examining 2-AG pretreatment×DAMGO treatment×time interval interaction was conducted. There was no main effect of 2-AG pretreatment (F(1,144)=3.05, ns), yet a significant main effect of DAMGO treatment (F(2,144)=6.12, p < .005), and main effect of time interval (F(3,144)=73.86, p < .0001). However, the only significant interaction observed was a 2-AG×DAMGO interaction (F(2,144)=7.53, p < .001). No significant interactions of either treatment across time were observed.
Fig. 3.
Influence of intra-accumbens 2-AG (0.25μg/0.5μl) and DAMGO (0.0025μg or 0.025μg/0.5μl) on food hopper entries (# = p < .05; compared to both Vehicle – 0μg DAMGO and Vehicle - 0.025μg DAMGO co-administration). The inset graph depicts the same data across 30 min intervals; the x-axis represents 30-min time intervals across the 2hr testing period (30, 60, 90, and 120 min) and the y-axis represents the total number of food hopper entries.
2.3. Duration of food hopper entries
An ANOVA conducted on total duration of food hopper entries during the 2 hr test session revealed no main effect of 2-AG treatment (F(1,36)=.018, ns), a significant main effect DAMGO treatment (F(2,36)=10.15, p < .0005), and no 2-AG×DAMGO treatment interaction (F(2,36)=.16, ns) (Fig. 4). As can be observed in the smaller inset in Figure 4, there was a strong trend toward a decrease in food hopper entry duration across time, with the majority occurring in the first 30 minutes. To further analyze this trend and treatment effects across time, an ANOVA examining 2-AG pretreatment×DAMGO treatment×time interval interaction was conducted. There was no main effect of 2-AG pretreatment (F(1,144)=0.012, ns), yet a significant main effect of DAMGO treatment (F(2,144)=6.7, p < .005), and time interval (F(3,144)=111.06, p < .0001). The only significant interaction observed was a DAMGO treatment×time interval interaction (F(6,144)=12.35, p < .0001). Post hoc comparisons showed that both low (p < .05) and high (p < .0001) doses of DAMGO produced significantly longer hopper entry durations during the first 30 min interval, compared to vehicle pretreatment×saline treatment. No significant effects of DAMGO treatment in other time intervals were observed.
Fig. 4.
Influence of intra-accumbens 2-AG (0.25μg/0.5μl) and DAMGO (0.0025μg or 0.025μg/0.5μl) on total food hopper entry duration (* = p <.05; compared to Vehicle – 0μg DAMGO co-administration). The inset graph depicts the same data across 30 min intervals; the x-axis represents 30-min time intervals across the 2hr testing period (30, 60, 90, and 120 min) and the y-axis represents the total duration of food hopper entries.
2.4. Locomotor Activity
An ANOVA conducted on the total horizontal beam breaks during the 2 hr test session revealed 2-AG treatment approached significance (F(1,36)=3.57, p = .066), while there was a main effect of intra-accumbens DAMGO treatment (F(2,36)=11.2, p < .0002), and a significant 2-AG×DAMGO treatment interaction (F(2,36)=4.18, p < .05). As displayed in Fig. 5, post-hoc comparisons revealed that intra-accumbens DAMGO administration produced significant increases in locomotor activity at both low (p < 0.05) and high (p < 0.05) doses, compared to vehicle treatment. The combined intra-accumbens treatment of 2-AG and the low dose of DAMGO led to similar activity levels as following the low dose of DAMGO by itself, however 2-AG and the high DAMGO dose combined treatment produced a significant increase in locomotor activity above that observed by the high DAMGO dose alone (p < 0.005). As can be observed in the smaller inset in Figure 5, there was a trend toward a decrease in locomotor activity across time. To further analyze this trend and treatment effects across time, an ANOVA examining 2-AG pretreatment×DAMGO treatment×time interval interaction was conducted. There was a main effect of 2-AG pretreatment (F(1,144)=7.95, p < .01), DAMGO treatment (F(2,144)=24.95, p < .0001), and time interval (F(3,144)=33.45, p < .0001). The only significant interaction observed was 2-AG×DAMGO treatment (F(2,144)=9.3, p < .001). No significant interactions of either treatment across time were observed.
Fig. 5.
Influence of intra-accumbens 2-AG and DAMGO on locomotor activity (# = p < .05; compared to Vehicle - 0.025μg DAMGO co-administration) (* = p <.05; compared to Vehicle – 0μg DAMGO co-administration). The inset graph depicts the same data across 30 min intervals; the x-axis represents 30-min time intervals across the 2hr testing period (30, 60, 90, and 120 min) and the y-axis represents the locomotor activity (beam breaks).
3. Discussion
The present study demonstrated that co-administration of the endocannabinoid 2-AG and μ-opioid receptor agonist DAMGO into the nucleus accumbens led to a potentiating influence on select food-directed approach and locomotor behaviors associated with high-fat feeding. The combined treatment of intra-accumbens 2-AG and DAMGO led to a significant increase in approach and general locomotor activity behavior yet produced only a marginal non-significant trend on increasing consumption. The majority of consumption following all treatments occurred in the first 30 minutes of the 2hr session. While endocannabinoid and opioid treatments have been shown to interact in the context of other behaviors, this is the first study to examine and demonstrate a significant interaction of the endocannabinoid 2-AG and DAMGO on high-fat feeding associated approach and locomotor behaviors.
In confirmation of previous reports, intra-accumbens DAMGO administration alone produced a significant increase in high-fat consumption at both the low and high doses used. These particular DAMGO doses were chosen as they represent the middle of the dose-response range shown to increase consumption of high-fat diet, representing doses near or just above threshold, and below the dose that produces maximal consumption (Zhang et al., 1998). In regard to 2-AG, a previous study examining chow intake, demonstrated that a 0.125μg dose of 2-AG was subthreshold, and a higher 0.5μg dose produced the maximal response (Kirkham et al., 2002; Deshmukh & Sharma, 2012). Therefore, we chose a 2-AG dose (0.25μg) that represented the middle of this range to examine its interaction with DAMGO, predicting it to have a marginal effect on consumption. However, the inability of combined administration of 2-AG and DAMGO to increase feeding above either treatment by itself could reflect differences in experimental conditions. Two of these differences were time of testing or diet, as Kirkham and colleagues (2002) assessed chow feeding during the onset of the dark cycle. This may suggest that homeostatic feeding processes are more sensitive to the influence of 2-AG than those modeled by DAMGO high-fat procedure (Baldo et al., 2013 for review), yet further study would be necessary to characterize.
The dose of 2-AG used in the current study was subthreshold by itself for all food-directed behaviors and locomotor activity assessed, yet exaggerated specific behavioral measures following co-administration of DAMGO. These effects were observed through the use of additional measures, including food hopper approach behaviors and locomotor activity. Approach behaviors included the number and duration of feeding bouts, as defined by total number and duration of beam breaks near the front entry of the food hopper over the 2hr feeding session. The combined administration of 2-AG and the high dose of DAMGO led to a 2-fold increase, compared to control treatment levels. The increase was most evident in the first 60 min of the 2hr test session then decreased to control levels, possibly through an influence of satiety factors. The total time of food hopper entry duration was significantly increased above control levels following the high DAMGO dose treatment, yet 2-AG treatment by itself or in combination with DAMGO had no effect on this measure.
While intra-accumbens administration of 2-AG has been shown to produce increased intake of palatable chow (Kirkham et al., 2002) and high-fat or high-carb diets (Deshmukh & Sharma, 2012), its interaction with ventral striatal opioids had not been explored. The current study targeted a region of the nucleus accumbens that DAMGO administration produces the largest consumption increase of a high-fat diet (Zhang & Kelley, 2000). CB1 and μ-opioid receptors have been shown to be co-localized on axons and dendrites (Pickel et al., 2004) and exhibit a functional interaction (Manzoni et al., 2001) within this region. A potentiated response was observed between opioid and cannabinoid systems on feeding using antagonists (Tallet et al., 2009), yet few studies have explored a similar response produced by activation of these two systems with agonists. One such study demonstrated that administration of the CB1 agonist WIN55212-2 and DAMGO into the medial shell of the accumbens led to an exaggerated intake of a palatable diet in a similar manner (Skelly et al., 2010). In contrast to the present findings, these authors did not observe an interaction on ambulation or approach behaviors, suggesting the pharmacological actions of the endocannabinoid 2-AG within the accumbens may be different compared to certain selective CB1 agonists. It may also be related to site of action, as Skelly and colleagues (2010) targeted the medial shell, where DAMGO effects on consumption of high-fat are less pronounced than those observed following administration into the core and lateral shell border (Zhang & Kelley, 2000). To our knowledge, the actions of 2-AG within the medial shell on appetitive approach or locomotor behaviors associated with a high-fat diet have not been examined. Future studies examining the other endogenous cannabinoid anandamide (Soria-Gómez et al., 2007), as well as the effect of cannabinoid receptor antagonists to assess the specificity of 2-AG acting on cannabinoid receptors, would aid in interpretation of the current findings. A recent review also raises the implication of endocannabinoids, 2-AG and anandamide, capable of having influences on behavior through the actions of their metabolites (Silvestri and Di Marzo, 2013).
In any model of feeding, an examination of all associated behaviors contributing to both the appetitive food-directed approach (i.e. hopper entries) and consummatory (i.e. consumption) phases of feeding has been proven critical to understanding the complex nature of this behavior. Examination of the neural circuitry of these two phases of feeding has demonstrated distinct circuits that mediate each, yet are both important for driving behaviors associated with food reinforcement (Will et al., 2009; Petrovich and Gallagher, 2003). In addition to the benefit of providing insight into each phase of feeding, it is critical to consider how changes in multiple behaviors expressed during feeding could influence the other. In the current study, the observed changes in locomotor activity could be interpreted to be a non-specific response or an anticipatory/appetitive behavior associated with and directed towards the food. A non-specific increase in locomotor activity could compete with and diminish consumption by decreasing sustained contact with food, yet the combined treatment of 2-AG and the high dose of DAMGO led to the highest level of both locomotor activity and consumption in the current study. This trend has been observed previously, as intra-accumbens DAMGO increased locomotor activity during a sucrose-drinking task and this increased activity did not interfere with the parallel increase in total sucrose intake (Zhang and Kelley, 1997).
In conclusion, the present findings provide a novel characterization of an opioid-cannabinoid interaction within the nucleus accumbens and its resulting influence on select behaviors within a model of high-fat feeding. They confirm that intra-accumbens administration of a subthreshold dose of the endogenous cannabinoid 2-AG increases DAMGO-induced approach and locomotor behaviors associated with high-fat feeding.
4. Experimental Procedure
4.1. Subjects
Subjects were 7 male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 250 to 300 g. Rats were housed in Plexiglas cages, 2–3 per cage throughout the entire experiment, in a temperature and humidity controlled room at 22 °C and maintained on a 12/12 light:dark cycle (lights on at 0700hr) with all experiments being conducted during the light phase (1100–1400hr). Throughout the experiment, animals were allowed unrestricted access to water and standard laboratory chow (Purina LabDiets, St. Louis, MO) in their home cages. Experimental procedures used were in accordance with the University of Missouri Institutional Animal Care and Use Committee guidelines and approved protocols.
4.2. Surgical placement of cannulae
Animals were anesthetized with a ketamine/xylazine mixture of 90 mg/kg ketamine/9 mg/kg xylazine (Sigma, St. Louis, MO) and stereotaxically implanted with bilateral guide cannulae (23 gauge, 10 mm) aimed at the nucleus accumbens using the following coordinates, from bregma: +1.4 AP, ±2.0 ML, −7.8 DV (Paxinos and Watson, 1998). Following standard flat skull procedures, the guide cannulae were secured to the skull using stainless steel screws and jet acrylic (Lang Dental Mfg. Co. Inc., Wheeling, IL). Following surgeries and throughout experiments, wire stylets (10.5 mm) were kept in guide cannulae to prevent blockage. Animals were allowed one week for recovery prior to treatment.
4.3. Drugs and microinjection procedure
The µ-opioid receptor agonist D-Ala2,N,Me-Phe4,Gly-ol5-enkaphalin (DAMGO) (Sigma Chemical Company, St. Louis, MO) was dissolved in sterile 0.9% saline and the cannabinoid receptor agonist 2-arachidonylglycerol (2-AG) (Sigma Chemical Company, St. Louis, MO) was dissolved in 10% DMSO. Animals were gently hand-held during the injection procedure. Infusions were administered using a microdrive pump (Harvard Apparatus, South Natick, MA) connected via polyethylene tubing (PE-10). After the stylets were removed, the drug or vehicle was infused through 12.5-mm 33 gauge injector cannulae, thus allowing the injector tips to extend 2.5 mm beyond the end of the 10 mm guide cannulae. The rate of injection was equated to produce an injection volume of 0.50µl for both 2-AG and DAMGO. The dose of 2-AG was 0.25μg/side and either 0.025µg or 0.0025µg/side of DAMGO over a 93 second duration. Injectors were removed and stylets replaced following infusion.
4.4. Specialized Diet
The high fat diet (HFD) was obtained from Teklad Diets (Madison, WI) and contained 278.3 g/kg vitamin free casein, 4.2 g/kg DL-methionine, 100.0 g/kg sucrose, 441.2 g/kg hydrogenated vegetable shortening, 77.7 g/kg linoleic safflower oil, 26.3 g/kg cellulose, 53.3 g/kg AIN-76 mineral mix, 15.2 g/kg AIN-76A vitamin mix, and 3.8 g/kg choline chloride. The diet consisted of 6.2 kilocalories/gram; 16.5% kcal from protein, 7.8% kcal from carbohydrates, and 75.6% of kcal from fat.
4.5. Apparatus and behavioral assessment of feeding behavior
Testing took place in a room separate from the colony room in eight Plexiglas (30.5 cm×24.1cm×21.0 cm) feeding chambers (Med Associates, St. Albans, VT). Feeding chambers were equipped with four infra-red photo-beams at intervals of 6 cm and positioned 4.3 cm above the bar floor to measure feeding associated locomotor activity across the chamber, an automated weigh scale for the food hopper to continuously monitor the weight of the hopper while automatically correcting for spillage, and a water bottle. The feeding hopper and water bottle were located on opposite corners of the same side of the chamber wall and a removable waste tray was located beneath the bar floor. Measurements were calculated based on the entire 2hr test session. These included locomotor activity (number of horizontal beam breaks), amount consumed (grams of diet consumed), hopper entries (number of times beam at entry point of recessed food hopper was broken), hopper entry duration (total duration beam at entry point of recessed food hopper was broken), duration per entry (hopper entry duration divided by number of hopper entries). Manual weights of the high fat diet were taken at the end of the session in addition to the automated measurements by the software to ensure accuracy. These two measures were very similar (i.e. differences ranging between only 0 – 0.4 grams for the full 2hr measurement), therefore, all data represents the automated measures. Measurements were calculated by monitoring software, Med-PC Version IV (Med Associates, St. Albans, VT).
4.6. General procedure timeline
Animals had ad libitum access to water and high fat diet (approximately 35 g) in the feeding chambers during all testing sessions. Subjects were placed in the feeding chambers for 2hr daily for 6 days. During the last 2 days of this habituation phase, animals were acclimated to the injection procedure. On Day 5, a 10.0 mm injector was inserted and left in place for 2 min, though no volume was administered. On Day 6, animals received an injection of saline into the accumbens with a 12.5 mm injector. Animals then received drug and vehicle treatments in a within-subjects, counter-balanced design. Immediately following each drug treatment, the animal was placed in the feeding chamber for 2hr of individual automated behavioral monitoring. At the end of the 2hr session, animals were returned to their home cages and returned to the colony room. One day separated treatment sessions.
4.7. Histology
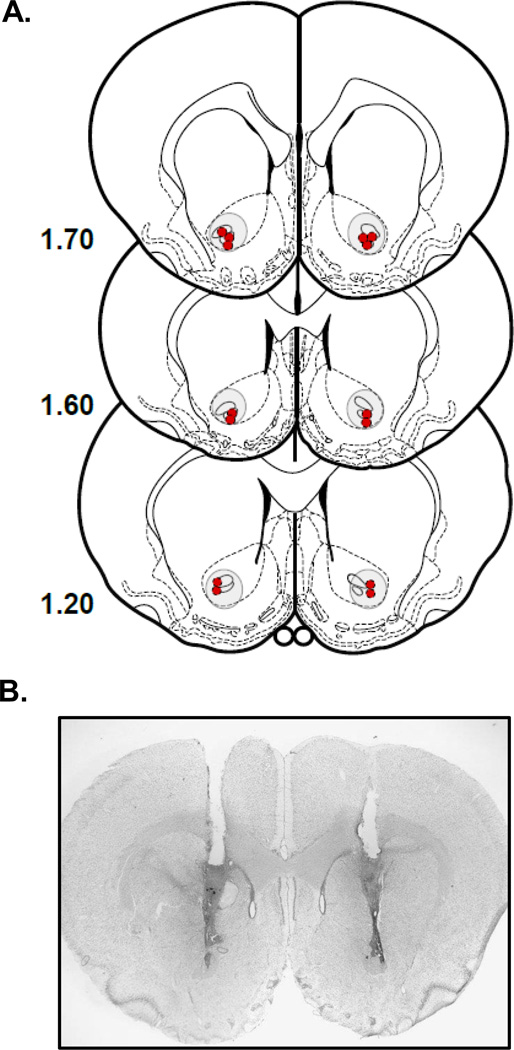
At the conclusion of the experiment, animals were overdosed with sodium pentobarbital and perfused transcardially using heparinized saline (200 ml) followed by 10% buffered formalin solution (200 ml). Brains were extracted and kept in 20% sucrose and 10% formalin mixture. Frozen serial sections (in 40 µm slices) of the injection site were collected and mounted on slides, stained with cresyl violet and cover slipped. Cannulae placements of all animals were assessed with a light microscopy for proper placement. No animals were excluded based on criteria of injector placement. A representative photomicrograph and schematic representing the tip of the injector track for all rats is represented in Fig. 1.
Fig. 1.
A) Bilateral locations of the most ventral track left by each injector for each rat. Number to left of each figure is distance (mm) anterior to bregma. (modified from Paxinos and Watson, 1998). (B) Photomicrograph depicting representative placement of bilateral cannulae and injector track.
4.8. Statistical Analysis
Data was analyzed using a 2 (2hr total) or 3-way (30 min intervals) ANOVA, followed by posthoc orthogonal contrasts of means when appropriate.
Acknowledgements
The authors would like to acknowledge the support of grant DA024829 to MJW from the National Institute of Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We. Examined Effect Of Opioid And The Endocannabinoids On high---fat feeding behaviours.
DAMGO And 2---Ag Were co---administered into the nucleus accumbens prior to feeding. Together They Increased Approach And Locomotor Activity Compared To Each Drug Alone.
References
- Abel EL. Cannabis: effects on hunger and thirst. Behav Biol. 1975;15:255–281. doi: 10.1016/s0091-6773(75)91684-3. [DOI] [PubMed] [Google Scholar]
- Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. Jama. 1999;282:1530–1538. doi: 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- Ameri A, Wilhelm A, Simmet T. Effects of the endogeneous cannabinoid, anandamide, on neuronal activity in rat hippocampal slices. Br J Pharmacol. 1999;126:1831–1839. doi: 10.1038/sj.bjp.0702478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfelbaum M, Mandenoff A. Naltrexone suppresses hyperphagia induced in the rat by a highly palatable diet. Pharmacol Biochem Behav. 1981;15:89–91. doi: 10.1016/0091-3057(81)90344-0. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Pratt WE, Will MJ, Hanlon EC, Bakshi VP, Cador M. Principles of motivation revealed by the diverse functions of neuropharmacological and neuroanatomical substrates underlying feeding behavior. Neurosci Biobehav Rev. 2013 Nov;37(9 Pt A):1985–1998. doi: 10.1016/j.neubiorev.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Berry EM, Mechoulam R. Tetrahydrocannabinol and endocannabinoids in feeding and appetite. Pharmacol Ther. 2002;95:185–190. doi: 10.1016/s0163-7258(02)00257-7. [DOI] [PubMed] [Google Scholar]
- Bermudez-Silva FJ, Viveros MP, McPartland JM, Rodriguez de Fonseca F. The endocannabinoid system, eating behavior and energy homeostasis: the end or a new beginning? Pharmacol Biochem Behav. 2010 Jun;95(4):375–382. doi: 10.1016/j.pbb.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Mind versus metabolism in the control of food intake and energy balance. Physiol Behav. 2004;81:781–793. doi: 10.1016/j.physbeh.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc. 2012. 2012 Nov;71(4):478–487. doi: 10.1017/S0029665112000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar RJ. Endogenous opiates and behavior. Peptides. 2013 Dec;50:55–95. doi: 10.1016/j.peptides.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Huang RC, Shen C, MacNeil DJ, Fong TM. Synergistic effects of cannabinoid inverse agonist AM251 and opioid antagonist nalmefene on food intake in mice. Brain Research. 2004 Mar 5;999(2):227–230. doi: 10.1016/j.brainres.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Cooper SJ. Endocannabinoids and food consumption: comparisons with benzodiazepine and opioid palatability-dependent appetite. Eur J Pharmacol. 2004;500:37–49. doi: 10.1016/j.ejphar.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Lutz B, Vicennati V, Stalla GK, Pasquali R, Pagotto U. Endogenous cannabinoid system as a modulator of food intake. Int J Obes Relat Metab Disord. 2003;27:289–301. doi: 10.1038/sj.ijo.0802250. [DOI] [PubMed] [Google Scholar]
- Cristino L, Becker T, Di Marzo V. Endocannabinoids and energy homeostasis: an update. Biofactors. 2014 Jul-Aug;40(4):389–397. doi: 10.1002/biof.1168. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Le Moal M, Piazza PV, Soubrie P. SR141716, a CB1 receptor antagonist, decreases the sensitivity to the reinforcing effects of electrical brain stimulation in rats. Psychopharmacology (Berl) 2001;157:254–259. doi: 10.1007/s002130100804. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Diana G, Malloni M, Pieri M. Effects of the synthetic cannabinoid nabilone on spatial learning and hippocampal neurotransmission. Pharmacol Biochem Behav. 2003;75:585–591. doi: 10.1016/s0091-3057(03)00127-8. [DOI] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Graceffo TJ, Robinson JK. Delta-9-tetrahydrocannabinol (THC) fails to stimulate consumption of a highly palatable food in the rat. Life Sci. 1998;62:PL85–PL88. doi: 10.1016/s0024-3205(97)01176-4. [DOI] [PubMed] [Google Scholar]
- Hanlon EC, Baldo BA, Sadeghian K, Kelley AE. Increases in food intake or food-seeking behavior induced by GABAergic, opioid, or dopaminergic stimulation of the nucleus accumbens: is it hunger? Psychopharmacology (Berl) 2004 Mar;172(3):241–247. doi: 10.1007/s00213-003-1654-0. [DOI] [PubMed] [Google Scholar]
- Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, Kustanovich I, Mechoulam R. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci U S A. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299:853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- Isoldi KK, Aronne LJ. The challenge of treating obesity: the endocannabinoid system as a potential target. J Am Diet Assoc. 2008 May;108(5):823–831. doi: 10.1016/j.jada.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Jager G, Witkamp RF. The endocannabinoid system and appetite: relevance for food reward. Nutr Res Rev. 2014. 2014 Jun;27(1):172–185. doi: 10.1017/S0954422414000080. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Munzar P, Goldberg SR. The opioid antagonist naltrexone reduces the reinforcing effects of Delta 9 tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology (Berl) 2004 Apr;173(1–2):186–194. doi: 10.1007/s00213-003-1693-6. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM. Synergistic effects of opioid and cannabinoid antagonists on food intake. 2001 Jan 1;153(2):267–270. doi: 10.1007/s002130000596. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances 'liking' of a sweet reward. Neuropsychopharmacology. 2007 Nov;32(11):2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Rodriguez de Fonseca F. Cannabinoid addiction: behavioral models and neural correlates. J Neurosci. 2002;22:3326–3331. doi: 10.1523/JNEUROSCI.22-09-03326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares J, Corchero J, Romero J, Fernandez-Ruiz JJ, Ramos JA, Fuentes JA. Chronic administration of cannabinoids regulates proenkephalin mRNA levels in selected regions of the rat brain. Brain Res Mol Brain Res. 1998;55:126–132. doi: 10.1016/s0169-328x(97)00371-9. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Bockaert J. Cannabinoids inhibit GABAergic synaptic transmission in mice nucleus accumbens. Eur J Pharmacol. 2001;412:R3–R5. doi: 10.1016/s0014-2999(01)00723-3. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Miller CC, Murray TF, Freeman KG, Edwards GL. Cannabinoid agonist, CP 55,940, facilitates intake of palatable foods when injected into the hindbrain. Physiol Behav. 2004;80:611–616. doi: 10.1016/j.physbeh.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Navarro M, Carrera MR, Fratta W, Valverde O, Cossu G, Fattore L, Chowen JA, Gomez R, del Arco I, Villanua MA, Maldonado R, Koob GF, Rodriguez de Fonseca F. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci. 2001;21:5344–5350. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH Report. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- Paxinos G, Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014 Feb 26;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th Ed. San Diego: Academic Press; 1998. [Google Scholar]
- Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic 'liking' for food: map based on microinjection Fos plumes. Brain Res. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kash TL, Rodriguez JJ, MackKie K. Compartment-specific localization of cannabinoid 1 (CB1) and mu-opioid receptors in rat nucleus accumbens. Neuroscience. 2004;127:101–112. doi: 10.1016/j.neuroscience.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Gallagher M. Amygdala subsystems and control of feeding behavior by learned cues. Ann N Y Acad Sci. 2003 Apr;985:251–262. doi: 10.1111/j.1749-6632.2003.tb07086.x. Review. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Mukherjee M, Robertson K. Effects of the cannabinoid receptor antagonist SR 141716, alone and in combination with dexfenfluramine or naloxone, on food intake in rats. Psychopharmacology (Berl) 2001;159:111–116. doi: 10.1007/s002130100910. [DOI] [PubMed] [Google Scholar]
- Salzet M, Breton C, Bisogno T, Di Marzo V. Comparative biology of the endocannabinoid system possible role in the immune response. Eur J Biochem. 2000;267:4917–4927. doi: 10.1046/j.1432-1327.2000.01550.x. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Skelly MJ, Guy EG, Howlett AC, Pratt WE. CB1 receptors modulate the intake of a sweetened-fat diet in response to μ-opioid receptor stimulation of the nucleus accumbens? Pharmacology Biochemistry and Behavior. 2010;97(1):144–151. doi: 10.1016/j.pbb.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013 Apr 2;17(4):475–490. doi: 10.1016/j.cmet.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Tian Q, Valenti M, Di Marzo V. Endocannabinoids link feeding state and auditory perception-related gene expression. J Neurosci. 2004;24:10013–10021. doi: 10.1523/JNEUROSCI.3298-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofia RD, Knobloch LC. Comparative effects of various naturally occurring cannabinoids on food, sucrose and water consumption by rats. Pharmacol Biochem Behav. 1976;4:591–599. doi: 10.1016/0091-3057(76)90202-1. [DOI] [PubMed] [Google Scholar]
- Soria-Gómez E, Matias I, Rueda-Orozco PE, Cisneros M, Petrosino S, Navarro L, Di Marzo V, Prospéro-García O. Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-Fos expression in the hypothalamus. Br J Pharmacol. 2007 Aug;151(7):1109–1116. doi: 10.1038/sj.bjp.0707313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CJ, Colditz GA. The epidemic of obesity. J Clin Endocrinol Metab. 2004;89:2522–2525. doi: 10.1210/jc.2004-0288. [DOI] [PubMed] [Google Scholar]
- Tallett AJ, Blundell JE, Rodgers RJ. Effects of acute low-dose combined treatment with naloxone and AM251 on food intake, feeding behavior and weight gain in rats. Pharmacol Biochem Behav. 2009 Jan;91(3):358–366. doi: 10.1016/j.pbb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Tanda G, Goldberg SR. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms--a review of recent preclinical data. Psychopharmacology (Berl) 2003;169:115–134. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003 Apr 1;23(7):2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. The amygdala is critical for opioid-mediated binge eating of fat. Neuroreport. 2004;15:1857–1860. doi: 10.1097/00001756-200408260-00004. [DOI] [PubMed] [Google Scholar]
- Will MJ, Pritchett CE, Parker KE, Sawani AM, Ma H, Lai AY. Behavioral characterization of amygdala involvement in mediating intra-accumbens opioid-driven feeding behavior. Behav Neurosci. 2009 Aug;123(4):781–793. doi: 10.1037/a0016060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Observational analysis of feeding induced by Delta9-THC and anandamide. Physiol Behav. 2002;76:241–250. doi: 10.1016/s0031-9384(02)00725-4. [DOI] [PubMed] [Google Scholar]
- Wright CI, Beijer AV, Groenewegen HJ. Basal amygdaloid complex afferents to the rat nucleus accumbens are compartmentally organized. J Neurosci. 1996;16:1877–1893. doi: 10.1523/JNEUROSCI.16-05-01877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99:267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther. 1998;285:908–914. [PubMed] [Google Scholar]