Abstract
Purpose
Limbal epithelial stem cell deficiency is caused by exposure of the cornea to thermal, chemical, or radiation burns or by diseases (aniridia and Stevens-Johnson syndrome). Autologous cell transplantation is a widely used therapeutic modality for restoring the corneal surface in such pathological conditions. Ex vivo cultured limbal, conjunctival, and oral biopsies have been widely used to reconstruct the corneal surface with variable outcomes. Culture characterization of the ex vivo cultured cells would provide insight and clues into the underlying signaling mechanisms that would aid in determining the probable transplantation outcome. Comparison of the vital proteins and genes among the three ex vivo cultured tissues has implications in clinical practice. To address this issue, we characterized and compared the proliferative and differentiated properties of ex vivo cultured limbal, conjunctival, and oral biopsies used for cell-based therapy for corneal surface restoration.
Methods
Limbal, conjunctival, and oral biopsies were collected with informed patient consent. Explant cultures were established on the denuded human amniotic membrane with corneal lineage differentiation medium. The day 14 cultures were characterized for epithelial and corneal lineage-specific markers using reverse transcription (RT)–PCR for cytokeratin 3, 4, 12, 13, 15, connexin 43, vimentin, p63α, and ABCG2 markers. mRNA expression was estimated in day 14 cultures with real-time quantitative real time (qRT)-PCR for pluripotency markers (OCT4, SOX2, NANOG), putative corneal stem cell markers (ABCG2 and p63α), proliferation markers (cyclin d1, Ki-67, PCNA, and CDC20), apoptotic markers (BCL2, BAX, caspase 3, and caspase 9), Notch signaling pathway markers (Notch1, Jagged1, Hes1, Hes3, Hes5, and Hey1), and autophagic markers (LC3A, LC3B, ATG7, RAB7, LAMP1, and LAMP2). Fluorescence-activated cell sorter profiling was performed for pluripotent markers and putative corneal stem cell markers ABCG2 and p63α.
Results
The protein and mRNA expression levels of the pluripotent markers were lower, whereas those of the putative stem/progenitor markers ABCG2, ΔNp63α, and Notch signaling molecules (Notch1 and Jagged1) were elevated in limbal cultures. The gene expression levels of the autophagy markers (LC3A, LC3B, and LAMP1) were significantly increased in the limbal cultures compared to the oral and conjunctival cultures.
Conclusions
In conclusion, the limbal epithelial cultures showed higher expression of proliferative, limbal stem cell marker, Notch signaling, and autophagy markers suggesting a role in stem cell maintenance and differentiation. This implicates the probable factors that might drive a successful transplantation. Our findings provide the initial steps toward understanding transplantation medicine in an ex vivo model.
Introduction
Limbal stem cell deficiency (LSCD) leads to the loss of limbal epithelial stem cells (LESCs) caused by congenital or acquired factors. The damage to the corneal surface leads to conjunctivalization and eventual partial or complete blindness depending on the extent of the damage of the corneal surface. Congenital factors leading to LSCD are pathological conditions driven by genetic and autoimmune disorders. Whereas acquired factors such as exposure to thermal, chemical, or ultraviolet rays and contact lens can lead to LSCD. Patients with LSCD are classified as having unilateral or bilateral LSCD based on the eyes affected [1-3].
Autologous limbal epithelial stem cell transplantation is the preferred treatment protocol for corneal surface reconstruction in patients with LSCD [4]. Though cells of various origins have been used, the most commonly used cell types for restoring the damaged corneal surface include limbal, conjunctival, and oral tissues [2].
The widely accepted treatment modality for unilateral LSCD disease is autologous LESC transplantation followed by conjunctival epithelial cells, whereas in bilateral cases cultured oral mucosal cells are used for treatment [5-9]. Transplantation of these cultured cells has shown promising results with variable success rates [4].
Reports that show higher rates of success with LESC transplantation in patients with LSCD are increasing [10,11]. Studies have revealed that the autologous cultured conjunctival and oral cells used in transplantation also improve and restore visual acuity in patients with LSCD [12,13]. Though limbal and conjunctival cells are of ocular origin, they have variable outcomes in terms of transplantation success. On another front, ex vivo cultured oral mucosal cells showed good transplantation efficiency in some studies [9,14]. For corneal surface reconstruction, cultivated limbal epithelial transplantation (CLET) is performed for unilateral LSCD, whereas cultivated oral mucosal epithelial transplantation (COMET) is widely used for bilateral LSCD. The reported success rate for CLET clinically has been around 77%. COMET, however, has shown an early decline in the efficiency of the transplanted cells that was stabilized within a year [8]. In one of the longest follow-up studies, the transplantation success of COMET was 53% based on the measurement of visual acuity [15]. In an attempt to improve the success rate of CLET, cocultures of conjunctival and limbal autologous transplantation have been attempted in several cases of unilateral LSCD. The outcome has been variable [8].
Surprisingly, though three different cell types have been used in the treatment of patients with LSCD, reports of the clinical outcome remain unclear. The underlying molecular signaling mechanisms that dictate the successful outcome of transplantation among the three tissues are unknown. Though the inherent cell-specific properties might have a role in dictating the clinical outcome, there are not many studies. Notch signaling plays a crucial role in stem cell maintenance, proliferation, apoptosis, and differentiation [16]. However, not much is known in the activity of Notch signaling during ex vivo culture of limbal, conjunctival, and oral epithelial cells. The Notch family has four transmembrane receptors (Notch 1–4) and five ligands (Jagged 1–2, delta like 1, 3, 4) as members. On ligand-based activation, Notch releases the Notch-intracellular domain (NICD) that goes to the nucleus and binds to the CBF-1, Suppressor of Hairless, Lag-2 (CSL/Rbpj) domain in the DNA. Thus, this interaction initiates transcription of Notch downstream targets, such the basic-helix–loop–helix family of proteins such as Hes1, Hes3, Hes5, Hey1, and Hey2. These proteins are the driver of the effects of active Notch signaling [16].
Notch signaling has been shown to play a decisive role in corneal wound healing, and the expression of receptors and ligands have been observed in corneal suprabasal epithelial layers [17,18]. Active Notch signaling promotes corneal epithelial proliferation, and abrogation of Notch signaling in mice prevented differentiation of corneal epithelial cells [18,19]. Thus, Notch signaling might have an important role for assessing the clinical status of the transplantation. The regulation of Notch signaling in cultured limbal, conjunctival, and oral epithelial cells is not well known.
Thus, we used ex vivo cultured limbal, conjunctival, and oral mucosal cells to identify the cell-inherent properties and the role of Notch signaling pathway in an attempt to understand the variable transplantation success rate among the three types of cells in patients with LSCD. We also studied the autophagy pathway, since in adult stem cells autophagy plays a crucial role in deciphering the regenerative potential [20]. Moreover, autophagy signaling is critical in the homeostatic control of stem cell functions during aging, tissue regeneration, and cellular reprogramming [21]. Autophagy clears away damaged proteins and organelles such as defective mitochondria, thus decreasing reactive oxygen species (ROS) levels and reducing genomic damage and cellular senescence, and playing a crucial role in enhancing stem cell longevity or maintenance [22,23]. These findings provide clues to improving the existing treatment strategy and understanding the regulation of vital signaling pathways in corneal surface restoration.
Methods
Ex vivo culture of limbal, conjunctival, and oral cell biopsies
All the patients recruited in the study were limbal stem cell deficiency patients who visited Narayana Nethralaya, Bommasandra, Bangalore, India for treatment. In total 26 patients were recruited for the study, with 13 for limbal and conjunctival samples and 13 for oral biopsies. Limbal and conjunctival samples were collected from 9 males and 5 females with unilateral limbal stem cell deficiency, whereas oral biopsies were obtained from 10 males and 3 female patients with bilateral limbal stem cell deficiency. Ages of the males and females recruited in the study were within 35–50 years old. This study was approved by the Narayana Nethralaya Review Board and the Narayana Nethralaya Ethics Board. The study was conducted in accordance with the ARVO statement for the use of human subjects in Ophthalmic and Vision research. Samples were collected following the Declaration of Helsinki after patient consent was obtained. Biopsies were collected from male and female patients within the age group of 35–50 years. Feeder-free culture methods for the limbal, conjunctival, and oral biopsies were followed as mentioned previously [24]. Briefly, explant cultures were established from patients who underwent transplantation treatment for LSCD. Back-up culture plates not used for transplantation were used for this study. The biopsies were chopped into tiny pieces under sterile conditions, placed on deepithelialized human amniotic membrane (HAM), and incubated at 37 °C with 5% CO2. All cultures were maintained with growth medium contained 1:1 Dulbecco’s Modified Eagle Medium/F12 (1:1; Gibco, Grand Island, New York, NY), 10% autologous serum, human recombinant epidermal growth factor (EGF; 10 ng/ml; Gibco), insulin (5 μg/ml; Gibco), and antibiotics (1%; penicillin, streptomycin, and amphotericin B; HiMedia, Mumbai, India). All analyses were performed on day 0 and day 14 cultures.
RNA extraction and PCR
Limbal, conjunctival, and oral cultured cells were trypsinized with 0.25% trypsin (HiMedia, Mumbai, India); the total RNA was extracted using the RNeasy Micro Kit (Qiagen, Hilden, Germany) and quantified. A total of 1 μg RNA was converted to cDNA using the high-capacity cDNA reverse transcription kit (Life Technologies, Carlsbad, NM) and stored at −20 °C. Reverse transcription polymerase chain reaction (RT–PCR) was performed on Applied Biosystems™ Veriti (Life Technologies, Foster City, CA) as described previously [25]. Briefly, PCR was set in a total reaction volume of 25 μl containing 300 ng of cDNA, 10X PCR buffer, 0.5 mM dNTPs, 1U Taq DNA polymerase, and 0.5 nmol of gene-specific primer. The RT–PCR cycle conditions were set with initial denaturation at 95 °C for 2min followed by 30 cycles of 94 °C for 30 s, annealing temperature specific to the primers as mentioned in Table 1, 72 °C for 30 s, and final extension at 72 °C for 5 min. The PCR products were resolved on ethidium bromide (final concentration of 0.5 μg/ml) containing 1% agarose gel and documented.
Table 1. List of primers used in RT- PCR.
| S. No | Primer name | Sequence (5′-3′) | Tm | Size (bp) | Gene acc no |
|---|---|---|---|---|---|
| 1 |
Gapdh |
F: GCCAAGGTCATCCATGACAAC |
60 |
498 |
NM_001256799 |
| P: GTCCACCACCCTGTTGCTGTA |
|||||
| 2 |
Cytokeratin 3 |
P: GGCAGAGATCGAGGGTGTC |
58 |
145 |
NM_057088 |
| R: GTCATCCTTCGCCTGCTGTAG |
|||||
| 3 |
Cytokeratin 4 |
F: GCCATGATTGCCAGACAGCAGTGT |
58 |
408 |
NM_002272 |
| R: GGGGGTGAGCAAGCTATGGTTG |
|||||
| 4 |
Cytokeratin 12 |
F: ACATGAAGAAGAACCACGAGGATG |
62.7 |
150 |
NM_000223 |
| R: TCTGCTCAGCGATGGTTTCA |
|||||
| 5 |
Cytokeratin 13 |
F: GATCCAGGGACTCATCAGCA |
58 |
290 |
NM_153490 |
| R: AAGGCCTACGGACATCAGAA |
|||||
| 6 |
Cytokeratin 15 |
F: GGAGGTGGAAGCCGAAGTAT |
64 |
194 |
NM_002275 |
| R GAGAGGAGACCACCATCGCC |
|||||
| 7 |
Connexin 43 |
F: CCTTCTTGCTGATCCAGTGGTAC |
60 |
145 |
NM_000165 |
| R: ACCAAGGACACCACCAGCAT |
|||||
| 8 |
p63α specific |
F: AGGGGCTGACCACCATCTAT |
59 |
196 |
NM_003722 |
| R: GTCTCACTGGAGCCCACACT |
|||||
| 9 |
Abcg2 |
F: ACCATTGCATCTTGGCTGTC |
56.5 |
181 |
NM_001257386 |
| R CGATGCCCTGCTTTACCAAA |
|||||
| 10 |
Vimentin |
F: TGGCCGACGCCATCAACACC |
60 |
257 |
NM_003380 |
| R: CACCTCGACGCGGGCTTTGT |
qRT-PCR
Quantitative real time PCR was performed in a 10 μl reaction volume containing 5 μl 2X Power SYBER Green PCR Master Mix (Life Technologies, Foster City, CA), 50 pmol of primer and 1 μl cDNA on the Applied Biosystems™ 7500 real-time PCR system (Life Technologies, Foster City, CA). Triplicate reactions were performed for each sample. Each qRT-PCR cycle consisted denaturation for 5 min at 95 °C, followed by 40 cycles at 95 °C for 15 s and 1min at 60 °C. Data were analyzed according to the manufacturer’s instructions using 7500 Software Version 2.0.1. The list of details of the gene specific primers is provided in Table 2.
Table 2. List of primers used in real-time qRT-PCR.
| S. No | Gene name | Sequence (5′- 3′) | Size (bp) | Gene acc no |
|---|---|---|---|---|
| 1 |
Oct4 |
F: TGTACTCCTCGGTCCCTTTC |
150 |
NM_203289 |
| R: TCCAGGTTTTCTTTCCCTAGC |
||||
| 2 |
Sox2 |
F: GCTAGTCTCCAAGCGACGAA |
144 |
NM_003106 |
| R: GCAAGAAGCCTCTCCTTGAA |
||||
| 3 |
Nanog |
F: CAGTCTGGACACTGGCTGAA |
149 |
NM_024865 |
| R: CTCGCTGATTAGGCTCCAAC |
||||
| 4 |
Notch 1 |
F: ATCCAGAGGCAAACGGAG |
106 |
NM_017617 |
| R: CACATGGCAACATCTAACCC |
||||
| 5 |
Jagged 1 |
F: AAGGCTTCACGGGAACATAC |
120 |
NM_000214 |
| R: AGCCGTCACTACAGATGCAC |
||||
| 6 |
Hes1 |
F: GAGAGGCGGCTAAGGTGTTT |
118 |
NM_005524 |
| R: GTGTAGACGGGGATGACAGG |
||||
| 7 |
Hes3 |
F: GAAAGTCTCCCTGGCTCGTC |
146 |
NM_001024598 |
| R: CCAAATAGGGAGCGCCTTCA |
||||
| 8 |
Hes5 |
F: AGAGAATGTGTGTGCAGAGTCC |
70 |
NM_001010926 |
| R: GGTCAGACACTTGGCAGAAGA |
||||
| 9 |
Hey1 |
F: GACCGTGGATCACCTGAAAA |
91 |
NM_012258 |
| R: TCCCAAACTCCGATAGTCCA |
||||
| 10 |
Abcg2 |
F: GAGCCTACAACTGGCTTAGACTCAA |
85 |
NM_004827 |
| R: TGATTGTTCGTCCCTGCTTAGAC |
||||
| 11 |
Δnp63α |
F: AGCCAGAAGAAAGGACAGCA |
104 |
NM_001114980 |
| R: CAGGTTCGTGTACTGTGGCT |
||||
| 12 |
Pcna |
F: GCCAGAGCTCTTCCCTTACG |
87 |
NM_002592 |
| R: TAGCTGGTTTCGGCTTCAGG |
||||
| 13 |
Cyclin D1 |
F: TCTACACCGACAACTCCATCCG |
133 |
NM_053056 |
| R: TCTGGCATTTTGGAGAGGAAGTG |
||||
| 14 |
Ki67 |
F: CTTTGGGTGCGACTTGACG |
199 |
NM_002417 |
| R: GTCGACCCCGCTCCTTTT |
||||
| 15 |
Cdc20 |
F: GTTCGGGTAGCAGAACACCA |
187 |
NM_001255 |
| R: CCCCTTGATGCTGGGTGAAT |
||||
| 16 |
Bcl2 |
F: TGGCCAGGGTCAGAGTTAAA |
143 |
NM_000633 |
| R: TGGCCTCTCTTGCGGAGTA |
||||
| 17 |
Bax |
F: TTGCTTCAGGGTTTCATCCA |
113 |
NM_138761 |
| R: AGACACTCGCTCAGCTTCTTG |
||||
| 18 |
Caspase 3 |
F: TGTGGCATTGAGACAGAC |
159 |
NM_004346 |
| R: CATGGCACAAAGCGACTG |
||||
| 19 |
Caspase 9 |
F: CCAGAGATTCGCAAACCAGAGG |
88 |
NM_001229 |
| R: GAGCACCGACATCACCAAATCC |
||||
| 20 |
Lc3a |
F: CGTCCTGGACAAGACCAAGT |
181 |
NM_181509 |
| R: CTCGTCTTTCTCCTGCTCGT |
||||
| 21 |
Lc3b |
F: AGCAGCATCCAACCAAAA |
187 |
NM_022818 |
| R: CTGTGTCCGTTCACCAACAG |
||||
| 22 |
Atg-7 |
F: GGATGAAGCTCCCAAGGACAT |
54 |
NM_001144912 |
| R: CCAGCAGAGTCACCATTGTAGTA |
||||
| 23 |
Rab 7 |
F: AGTACAAAGCCACAATAGGAGC |
116 |
NM_004637 |
| R: ACCGAGAGACTGGAACCGT |
||||
| 24 |
Lamp-1 |
F: AGTGGCCCTAAGAACATGACC |
128 |
NM_005561 |
| R: AGTGTATGTCCTCTTCCAAAAGC |
||||
| 25 |
Lamp-2 |
F: GAAAATGCCACTTGCCTTTATGC |
173 |
NM_002294 |
| R: GGTCCGAACTGCACTGCTATT |
||||
| 27 |
β-actin |
F: ACAGGGGAGGTGATAGCATT |
100 |
NM_001101 |
| R: GACCAAAAGCCTTCATACATCTC |
FACS staining
To perform fluorescent activated cell sorting (FACS) staining, the day 14 cultured limbal, conjunctival, and oral epithelial cells were trypsinized with 0.25% trypsin and fixed with 4% paraformaldehyde on ice for 10 min. The cells were then washed twice with PBS (1X; 155 mM NaCl, 2.9 mM Na2HPO4, 1.05 mM KH2PO4, pH 7.4), permeabilized with 0.1% Triton X (staining buffer) and stained for rabbit anti-human OCT4, rabbit anti-human SOX2, rabbit anti-human NANOG (Imgenex India, Bhubaneswar, India), rabbit anti-human p63α (Cell Signaling, Beverly, MA), and mouse anti-human ABCG2-APC (BioLegend, San Diego, CA) antibodies [16]. Anti-rabbit Alexa 488 secondary antibody (Jackson Immuno-research, West Grove, PA) was used for all antibodies except rabbit anti-ABCG2-APC, by diluting with staining buffer containing 0.1% Triton X per the manufacturer’s instructions. Unstained cells and cells stained with secondary antibody alone were used as controls. The fluorescence emitted by 10,000 cells in the FL-1 channel was recorded and analyzed using BD CellQuest Pro software (FACSCaliber, San Jose, CA).
Immunohistochemistry
The mouse anti-human ABCG2 (used at 1:20), mouse anti-human cytokeratin 3+12 antibody (used at 1:50), and rabbit anti-human connexin 43 (used at 1:100) were obtained from Abcam (Cambridge, MA). The ready-to-use rabbit anti-human cytokeratin 4 and mouse anti-human cytokeratin 13 antibodies were obtained from BioGenex (Fremont, CA). Cultured epithelial cells on the amniotic membrane were subjected to immunohistochemistry. Briefly, the amniotic membrane embedded cells were fixed with 4% paraformaldehyde (Sigma-Aldrich, Steinheim, Germany) and blocked for endogenous peroxidase using 3% H2O2 in methanol for 20 min. Nonspecific sites were blocked using peroxidase-blocking solution buffer for 15 min (Dako REAL, Glostrup, Denmark) after which the cells were incubated overnight at 4 °C with the primary antibody. Detection of the bound antibody was performed using EnVision Detection Systems Peroxidase/DAB Rabbit/Mouse (Dako, Glostrup, Denmark) according to the manufacturer’s instructions. Cells were counterstained with hematoxylin (Fisher Scientific, Waltham, MA) mounted in a Distrene, Plasticiser, Xylene (DPX; Merck, Darmstadt, Germany) mounting medium, and observed under a light microscope (Nikon, Eclipse E200, Tokyo, Japan) using software (NIS Element D, Tokyo, Japan).
Statistical analysis
The results of three independent experiments (n=3) were used for statistical analysis. Data are represented as the mean ± SD and were analyzed with the Student t test. Significance is denoted with p *<0.05, **<0.01, and ***<0.005.
Results
Morphological analysis and characterization of epithelial cell-specific marker expression
Phase-contrast microscopic images of ex vivo cultured limbal, conjunctival, and oral biopsies on day 14 did not reveal any gross morphological differences at lower magnification (Appendix 1A,B,D,E,G,H). However, the day 14 cultured limbal epithelial cells (used for transplantation) illustrated hexagonal morphology at a higher magnification (40X). Similar to the limbal cells, the conjunctival cells showed a hexagonal morphology but much smaller, whereas the oral cultures showed elongated cell structures (Appendix 1C,F,I). Cell type–specific gene expression was analyzed for the ex vivo cultured day 14 cultures with RT–PCR. The results showed expression of cytokeratin 3, 4, 12, 13, and 15, connexin 43, p63α, ABCG2, and vimentin in the limbal cultures (Appendix 1J). Cultured conjunctival cells on day 14 expressed cytokeratin 4, 13, and 15 (Appendix 1 K) whereas the cultured oral cells showed expression of cytokeratin 4, 13, and 15, connexin 43, p63α, and ABCG2 (Appendix 1L). Immunohistochemistry with connexin 43, cytokeratin 3/12, cytokeratin 4, and cytokeratin 13 revealed positivity except ABCG2 in day 14 cultured limbal epithelial cells (Appendix 2B–F). In the positive cells in the day 14 cultured conjunctival cells, ABCG2, connexin 43, and cytokeratin 3/12 markers could not be detected (Appendix 2H–J). Cultured conjunctival cells showed positive staining for cytokeratin 4 and cytokeratin 13 (Appendix 2 K–L). Connexin 43, cytokeratin 4, and cytokeratin 13-positive cells were detected in the day 14 cultured oral mucosal cells (Appendix 2O,Q,R). Cytokeratin 3/12 and ABCG2-positive cells were not detected in cultured oral mucosal cells (Appendix 2N,P). The unstained controls for the limbal, conjunctival, and oral cells are shown in Appendix 2A, G, and M.
Differential expression of pluripotent markers
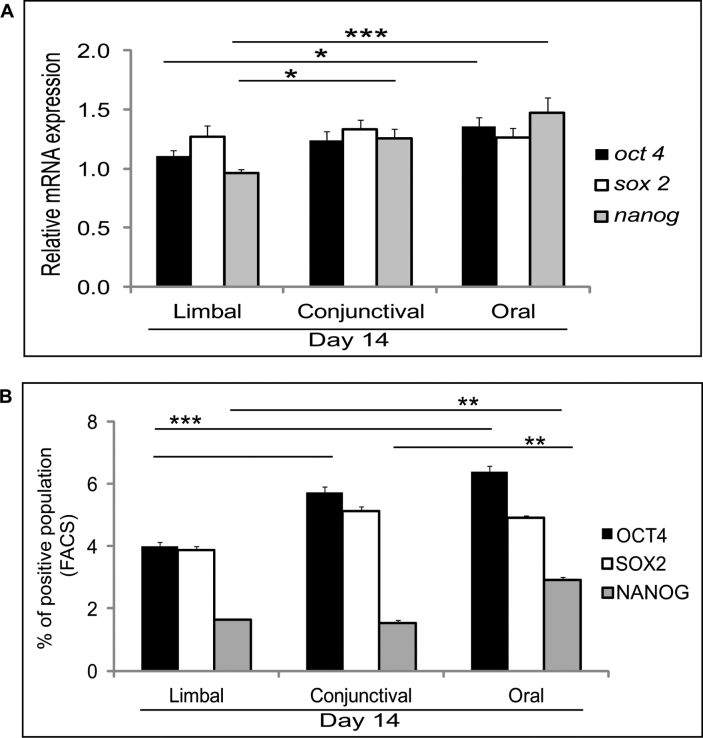
We then checked the pluripotent marker gene expression profile (OCT4, SOX2, and NANOG) in day 0 and day 14 ex vivo cultured limbal, conjunctival, and oral epithelial cells. The mRNA expression profile of the day 0 cells was comparable across the different cell types (Appendix 3A) except a significant decrease in SOX2 expression in the oral biopsy (p=0.0286). Interestingly, the pluripotent markers were decreased in the day 14 limbal cultures compared to the conjunctival and oral cultures. There was a significant decrease in the expression of OCT4 (p=0.028) and NANOG (p=0.005), but no difference was observed in level of SOX2 expression in the limbal cultured cells compared to the oral cultures. The day 14 cultured conjunctival cells did not show any differences in the expression levels of the OCT4 and SOX2 genes, but NANOG (p=0.0085) was significantly elevated in comparison to the cultured limbal epithelial cells (Figure 1A).
Figure 1.
Expression of pluripotent markers. A: Quantitative PCR results for day 14 cultured limbal, conjunctival, and oral cells for OCT4, SOX2, and NANOG mRNA expression. Results were normalized with β-actin. All experiments were performed in triplicate using six samples per group (limbal, conjunctival, and oral). B: Graphical representation of the fluorescent activated cell sorting (FACS) data obtained for three different tissue origins, showing positive staining with antibodies against OCT4, SOX2, and NANOG. Statistical significance denoted, p *<0.05, **<0.01, ***<0.005.
FACS analysis was performed to validate the pluripotent marker (OCT4, SOX2, and NANOG) protein expression levels. The analysis revealed a decrease in OCT4-positive cells in the cultured limbal epithelial cells compared to the conjunctival (p=0.018) and oral (p=0.0009) epithelial cultures. Although not significant, the number of SOX2-positive cells was also lower in the cultured limbal cells compared to the cultured conjunctival (p=0.05) and oral (p=0.06) cells. The number of NANOG-positive cells also decreased in the cultured limbal cells compared to the cultured conjunctival (p=0.56) and oral (p=0.002) cells (Figure 1B and Appendix 3B). Overall, the number of cells positive for the pluripotent markers was highest in the day 14 oral cultures followed by the conjunctival and limbal cultures, respectively.
Expression of Notch signaling–related markers
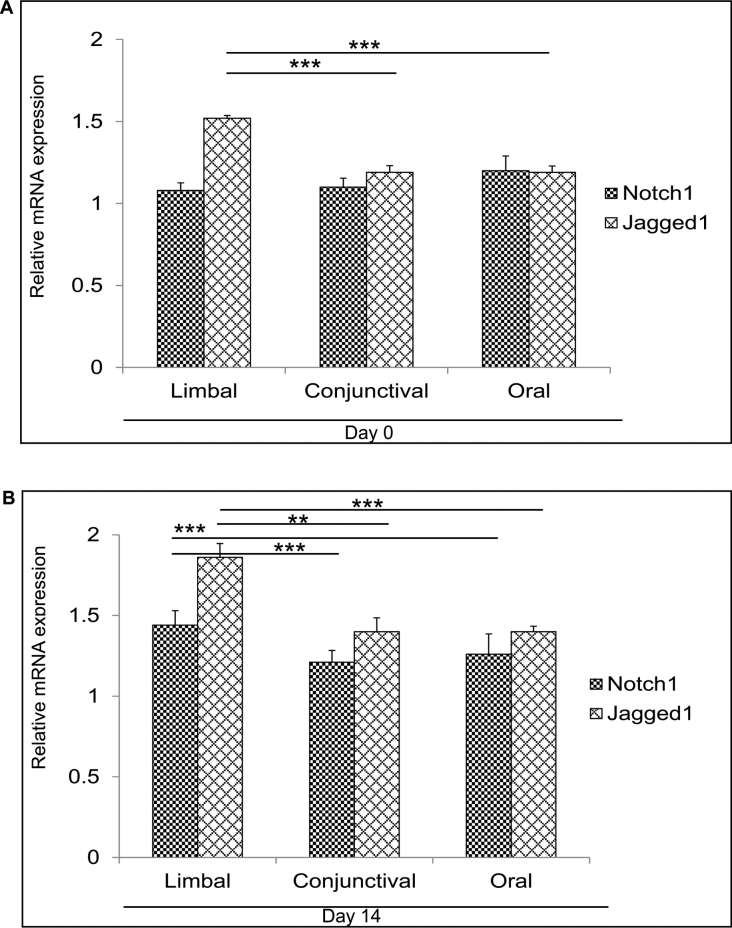
Notch signaling has been shown to have an important role during corneal epithelial proliferation and differentiation. Abnormal differentiation has been detected in the mouse corneal epithelium on deletion of Notch1 in the epidermal layer [19]. Recently, it has been proposed that blocking Notch signaling with dominant negative Mastermind did not have an effect on corneal epithelial differentiation [26]. Moreover, Notch activity is downregulated during the initial phase of corneal epithelial proliferation, whereas active Notch signaling is necessary for corneal epithelial differentiation [18,27]. There are contradictory reports on the role of Notch signaling in the epithelial differentiation of the cornea. Thus, we were interested in comparing Notch signaling by looking at the expression of Notch1, Jagged1, and Notch downstream targets Hes1, 3, and 5, and Hey1 in the day 0 (Figure 2A and Appendix 4A) and day 14 (Figure 2B and Appendix 4B) cultures. Cultured day 0 limbal cells showed elevated expression of Jagged1 compared to the cultured conjunctival (p=0.0002) and oral (p≤0.001) cells, but no difference in Notch1 expression was detected among all three cultures. The day 0 limbal epithelial cultures revealed a significantly lower expression of Hes1 compared to the conjunctival (p=0.002) and oral (p≤0.001) epithelial cultures. Moreover, Hes3 expression in the day 0 cultured limbal epithelial cells was significantly lower than in the day 0 cultured oral epithelial cells (p=0.0001). The day 14 cultured limbal epithelial cells revealed elevated mRNA expression of Notch1 (p=0.0004; p<0.005) and Jagged1 (p=0.0022; p<0.005) compared to the conjunctival and oral cultures, respectively. Expression of Notch1 was elevated significantly (p=0.0005) in the day 14 cultured oral mucosal epithelial cells compared to the cultured conjunctival cells. Concurrently, the elevated mRNA expression of Notch downstream targets Hes1, Hes3, Hes5, and Hey1 was noted in the day 14 cultures of all three biopsies. The day 14 cultured limbal epithelial cells revealed significantly higher Hes1 expression compared to the cultured conjunctival (p=0.008) and oral (p≤0.001) epithelial cells. Hey1 expression was also elevated in the day 14 limbal cultures compared to the conjunctival (p=0.0006) and oral (0.001) cultures. The day 14 cultured oral epithelial cells showed higher expression of Notch1 (p=0.0005), Jagged1 (p=0.0003), Hes1 (p=0.002), Hes5 (p=0.009), and Hey1 (p=0.001) expression compared to the conjunctival cultures. As a result, the day 14 limbal and oral cultures showed the highest expression of Notch signaling components compared to the day 14 cultured conjunctival cells.
Figure 2.
Expression of Notch1 and Jagged1 expression. A: Quantitative PCR results for Notch1 and Jagged1 mRNA expression in the day 0 cultured limbal, conjunctival, and oral biopsies. B: mRNA expression of Notch1 and Jagged1in the day 14 limbal conjunctival and oral cultures. Results were calibrated with β-actin. All experiments were performed in triplicate using six samples per group (limbal, conjunctival, and oral). Statistical significance denoted, p *<0.05, **<0.01, ***<0.005.
Putative stem/progenitor cell marker expression
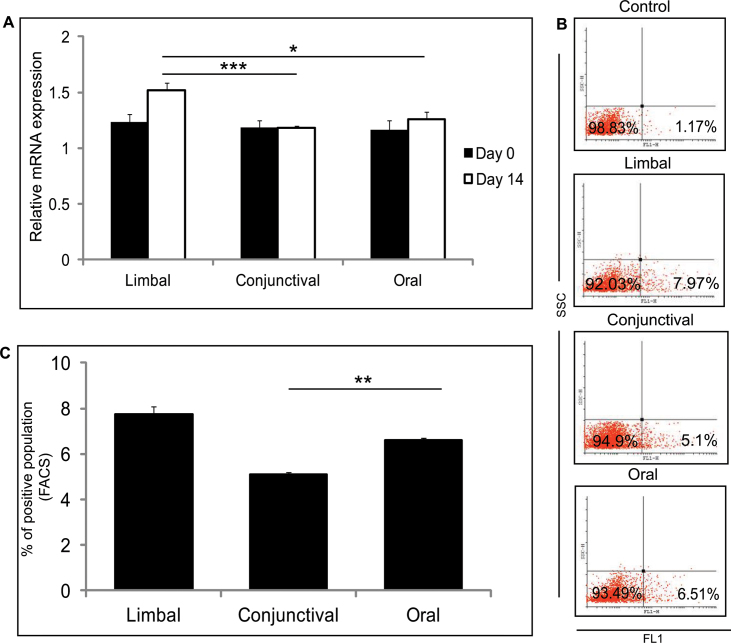
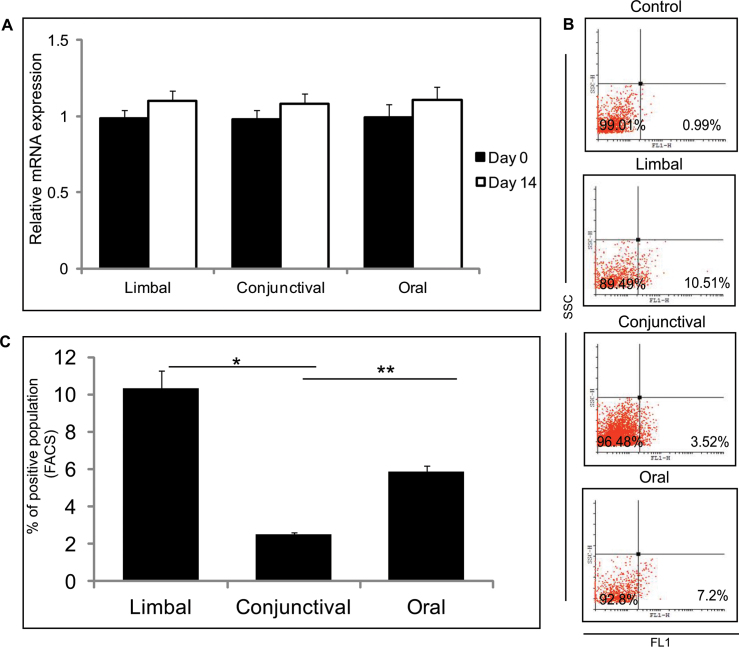
An ABC transporter protein is encoded by ABCG2, which showed no difference in mRNA expression level in the day 0 limbal, conjunctival, and oral cultures (Figure 3A). The day 14 limbal cultures showed higher expression of ABCG2 (p=0.0246 and p=0.0021) compared to the conjunctival and oral cultures, respectively (Figure 3A). FACS analysis revealed an increased number of ABCG2-positive cells in the day 14 limbal cultures compared to the conjunctival and oral cultures (Figure 3B,C). The number of ABCG2-positive cells was higher in the day 14 cultured oral cells compared to the cultured conjunctival cells (p=0.006). No difference was observed in the mRNA expression level of the ΔNp63α levels for day 0 and day 14 in the three cultured biopsies (Figure 4A). However, when compared the day 14 cultured limbal, conjunctival, and oral biopsies for p63 positivity with FACS staining, a significant difference was observed. The day 14 cultured limbal epithelial cells showed significant upregulation of p63α positivity compared to the day 14 cultured conjunctival cells (p=0.01) and the day 14 cultured oral epithelial cells (p=0.004; Figure 4B,C).
Figure 3.
Putative stem/progenitor cell marker expression, ABCG2. A: Quantitative PCR expression levels of ABCG2 in limbal, conjunctival, and oral cultured cells from day 0 and day 14 cultures. Results were normalized with β-actin. All experiments were performed in triplicate using six samples per group (limbal, conjunctival, and oral). B: Representative fluorescent activated cell sorting (FACS) analysis of ABCG2-positive population in limbal, conjunctival, and oral cultured cells from day 14 cultures. C: Summarizing graph represents the results of the FACS analysis of ABCG2 in the limbal, conjunctival, and oral cultured cells, performed in triplicate. Significance denoted, p *<0.05, **<0.01, ***<0.005.
Figure 4.
Putative limbal stem/progenitor cell marker expression p63α. A: Quantitative PCR expression levels of ΔNp63 in limbal, conjunctival, and oral cultured cells from day 0 and day 14 cultures. Results were calibrated with β-actin. All experiments were performed in triplicate using six samples per group (limbal, conjunctival, and oral). B: Representative fluorescent activated cell sorting (FACS) analysis of the p63α-positive population in the limbal, conjunctival, and oral cultured cells from day 14 cultures. C: Summarizing graph represents the results of the FACS analysis of p63α in the limbal, conjunctival, and oral cultured cells, performed in triplicate. Significance denoted, p *<0.05, **<0.01, ***<0.005.
Status of proliferation and apoptosis
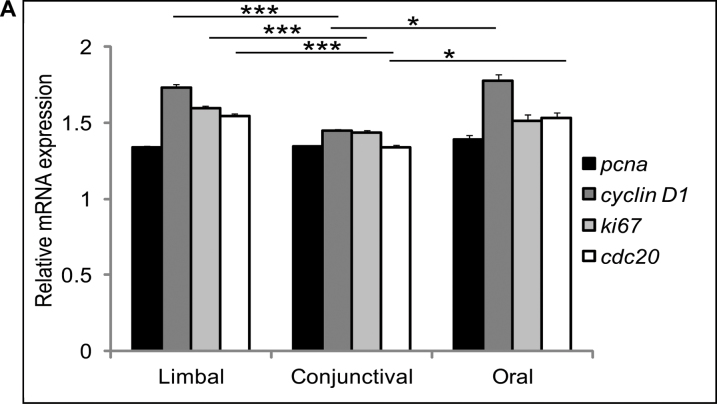
Cell proliferation and apoptotic levels were estimated for the day 14 limbal, conjunctival, and oral cultured cells. Proliferative markers (cyclin D1 (p=0.0039; p=0.0205), Ki-67 (p=0.0021) and CDC20 (p=0.0020; p=0.0485) showed significantly elevated expression levels in the cultured limbal epithelial cells compared to the conjunctival and oral cultured cells. Based on the expression level, the conjunctival cells showed the lowest proliferative status among all three types of ex vivo cultured epithelial cells (Figure 5).
Figure 5.
mRNA expression of proliferative markers. mRNA expression profile of proliferative markers, PCNA, cyclin D1, Ki-67, and CDC20 genes of day 14 limbal, conjunctival, and oral cultured cells. Results were normalized with β-actin. All experiments were performed in triplicate using six samples per group (limbal, conjunctival, and oral). Significance denoted, p *<0.05, **<0.01, ***<0.005.
Then we further investigated the gene expression levels of apoptotic markers (caspase 3, caspase 9, BCL2, and BAX) of day 14 cultured limbal, conjunctival, and oral cells. The results showed that the proapoptotic markers caspase 3 and 9 expression levels were marginally elevated in the day 14 cultured oral epithelial cells. There was no difference in the expression profile of BCL2 (antiapoptotic) and BAX (proapoptotic) among the day 14 cultured limbal, conjunctival, and oral epithelial cells (Appendix 5).
Expression of macroautophagy-related markers
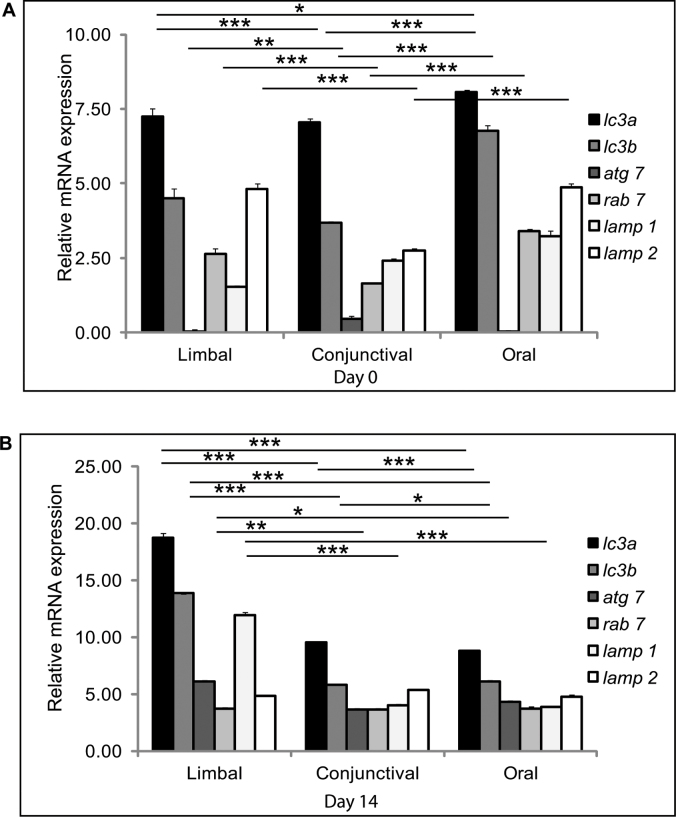
Autophagy signaling tightly regulates stem cells between their self-renewal and differentiation potential [20]]. During differentiation, autophagy regulates cellular remodeling whereas in stem cell self-renewal is maintained in the quiescent stage [21]]. The autophagy-related markers (LC3A, LC3B, ATG7, RAB7, LAMP1, LAMP2) were analyzed with real-time qRT-PCR in day 0 and 14 limbal, conjunctival, and oral cultures. Interestingly, LC3 gene expression (autophagosome markers LC3A, LC3B) levels in the day 14 in the limbal cultures were elevated compared to the day 0 cultures. In addition, lysosomal-associated membrane protein LAMP1 also showed increased expression in the day 14 limbal cultures compared to the conjunctival and oral cultures. The overall expression of the autophagy markers was significantly higher in the limbal cultures on day 14 compared to the oral and conjunctival cultures (Figure 6A,B).
Figure 6.
Autophagy regulation of stem cells. Quantitative PCR analysis of autophagy markers LC3A, LC3B, ATG7, RAB7, LAMP1, and LAMP2 was performed on day 0 and day 14. A: Relative mRNA expression of autophagy genes on 0 day culture of the limbal, conjunctival, and oral explant cultures. B: Gene expression of autophagy genes in the day 14 limbal, conjunctival, and oral cultures. Results were calibrated with β-actin. All experiments were performed in triplicate using six samples per group (limbal, conjunctival, and oral). Significance denoted, p *<0.05, **<0.01, ***<0.005.
Discussion
Corneal surface damage following an insult by exposure to chemical, thermal, or radiation rays or acquired by disease leads to LSCD. The treatment modality for LSCD involves reconstruction of the damaged corneal surface using autologous cell transplantation [1]. Sources of cells that have been widely reported for restoring corneal surface reconstruction are autologous limbal, conjunctival, and oral mucosal tissues [28]. One of the major challenges of the cell-based therapy strategy has been to minimize the variability in the clinical outcome.
Thus far, in most LSCD cases, ex vivo cultured limbal transplantation has shown a higher rate of success in corneal surface reconstruction compared to cultured conjunctival and oral epithelial cells [5,29,30]. Studying the underlying properties of the cultured limbal, conjunctival, and oral epithelial cells would provide a better insight into the differences in the clinical outcomes of cell transplantation. Although several studies have characterized cultured limbal, conjunctival, or oral cells, no studies have delineated comparative characteristics of cultured limbal, conjunctival, and oral epithelial cells in terms of putative stem cell and pluripotent markers, Notch signaling, autophagy regulation, proliferation, and apoptosis levels.
To identify the inherent vital properties of the cells from the three sources, we compared the characteristics of ex vivo cultured limbal, conjunctival, and oral epithelial cells in terms of the expression of stem cell markers, pluripotent marker, proliferative, apoptotic, Notch signaling, and autophagy markers. Studies have shown that culturing the cells on irradiated/mitomycin C–treated 3T3 cells would support the maintenance of LESC-like features [31,32]. Whereas culturing the cells on deepithelialized HAM promotes differentiation of LESC to the corneal lineage [32]. Moreover, the relative percentage of corneal progenitors plays an important role in transplantation success. Thus, all biopsies were cultured on deepithelialized HAM and not 3T3 to obtain a higher corneal progenitor population, since the primary objective of the study was to evaluate the cell-inherent property that plays a role in transplantation success in an in vitro study [33].
In agreement with previous reports, the limbal and conjunctival cultures showed cuboidal-shaped cells, whereas oral cells were more polygonal shaped [34-36]. Cultured cells were characterized by the gene expression of cytokeratins and tight junction markers on day 14 as shown earlier [37]. The presence or absence of these markers ascertains the source of the cells as well as indicates the differentiation potential. Pluripotency is a major attribute of stemness in stem cells to differentiate into multiple lineage specifications; well differentiated cells show lower expression of pluripotent markers [38]. We were interested to know among the three cell types which cell type expressed pluripotent markers the most. This study demonstrated pluripotent protein markers at low levels in cultured limbal cells compared to cultured conjunctival and oral mucosal cells, implicating the loss of pluripotency and a simultaneous progression in differentiation on day 14. However, the gene as well as the protein expression level of pluripotent markers was high in the cultured conjunctival and oral cultures in day 14. This suggested poor differentiation of cultured conjunctival and oral cells. Notch signaling is a fundamental pathway that controls the proliferation and differentiation of the corneal epithelium [19]. Using a conditional knockout model for Notch1, it was established that in the absence of Notch1 differentiation of corneal epithelial cells was blocked [18]. Conditional blockage of Notch signaling in the corneal epithelium using dominant negative Mastermind with the Cytokeratin 12 (CK12; corneal epithelial specific) promoter did not show any abnormal development of the cornea. This might be because the CK12 promoter drove expression only in the differentiated corneal epithelium. Blockage of Notch signaling driven with dominant negative Mastermind through a Cytokeratin 14 (CK14; marker of basal cells of stratified epithelia) promoter on a ROSA locus showed that Notch signaling was vital for the differentiation of conjunctival goblet cells but not corneal epithelial differentiation. Researchers concluded that the loss of the corneal epithelium differentiation marker (CK12 expression) was secondary due to the loss of conjunctival goblet cells by ablation and interruption of Notch signaling. Studies are needed to delineate the function of Notch signaling in normal differentiation and differentiation induced by corneal debridement [26]. Because of the proven role in proliferation and differentiation of corneal epithelial cells, the active status of Notch signaling was further investigated. The mRNA expression levels of Notch members Notch1 and Jagged1 and downstream targets hes1 and hes3, was performed on day 0 and day 14 cultured limbal, conjunctival, and oral epithelial cells. Significant upregulation of Notch1 and Jagged1 expression was detected in the day 14 limbal epithelial cultures. Enhanced active Notch signaling was detected in the day 14 cultures of all biopsies compared to the day 0 cultures. Moreover, active Notch signaling was highest in the day 14 cultured limbal and oral biopsies compared to the conjunctival cultures. This implies a definitive role of Notch signaling in promoting differentiation of limbal epithelial cells as has been reported previously [18,27,39] and a probable crucial role in the transplantation success. It has been also reported that elevated Notch signaling prevents epithelial-mesenchymal transition in limbal epithelial cells, thus retaining the limbal progeny population in ex vivo cultures [40]. There is dynamic bidirectional signaling crosstalk between Notch signaling and p63 [41]. In one aspect, p63 has been shown to suppress Notch signaling in cells thus reducing high proliferative potential, and p63 works in conjunction with Notch signaling during the early stages of differentiation. At the latter stages of differentiation of epidermal cells, Notch signaling downmodulates p63 [41]. Six transcripts are generated from the p63 gene, three from an upstream promoter and three from a downstream promoter lacking the trans-activating domain (ΔN isoforms) [42]. ΔNp63 expression is a characteristic of stem cells and undergoes alternative splicing to generate α, β, and γ forms [42]. We then looked at the expression of mRNA as well as the protein levels of the stem cell markers ABCG2 and ΔNp63α [31]. This indicates the propensity of different cell types to differentiate into corneal epithelial cells. Elevated expression of ABCG2 was observed in the cultured limbal cells on day 14, but no difference was seen in the mRNA levels of ΔNp63α expression. ABCG2, a member of the ATP-binding cassette protein family, is a downstream target of Notch signaling [43]. Thus, we observed upregulation of the ABCG2 protein, and mRNA was upregulated in the day 14 cultured limbal epithelial cells compared to the conjunctival and oral cultures. The lack of a difference in the mRNA transcripts of the ΔNp63α levels among the three cultures indicated that most probably there are no differences in the proliferation and migration properties among limbal, conjunctival, and oral epithelial stem cells. Di Lorio et al. showed that the α isoform dictates the proliferation of human LESCs whereas the β and γ isoforms regulate the differentiation toward corneal regeneration using corneal wound models [44]. FACS analysis revealed significant upregulation of ABCG2 and p63α in the day 14 cultured limbal epithelial cultures compared to the conjunctival and oral cultures. Though there was no difference in the mRNA expression of ΔNp63α among the three cultures, there was a significant increase in the number of p63α-positive cells in the day 14 cultured limbal epithelial cells compared to the conjunctival and oral cultures. In a recent study, Pellegrini et al. demonstrated that the success of limbal transplantation correlated with ΔNp63α expression [29]. Another study showed that cultures with more than 3% of cells positive for ΔNp63α have better prognosis post-transplantation [4]. In the present study, vital cellular properties, such as proliferation and apoptosis, were investigated by studying the mRNA expression levels for specific markers. A balance between the proliferative and apoptotic markers will eventually provide clues for healthy cell status. Higher expression of proliferative markers in limbal cultures is a good indicator of higher proliferation. There was no detectable change in the expression level of PCNA among the three cultured biopsies. However, upregulation of the Ki-67 and cyclin D1 transcripts was observed. The cell cycle marker Ki-67 marker is positive for all stages of the cell cycle, indicating the population of all dividing cells [45]. Thus, the transcript level also reflects all dividing cells in a population, implying that there are a higher number of dividing cells in cultured limbal epithelial cells compared to cultured conjunctival and oral biopsies. Most likely, the non-stem cell population undergoes proliferation, since the ΔNp63α transcript levels were similar in all three cultures. Cyclin D1 is cell cycle check point regulator of cells entering from the G1 to S phase [46]. The elevated mRNA expression of cyclin D1 indicated that a higher number of limbal epithelial cells (most likely non-stem cell population) entered the cell cycle at a particular stage. The PCNA marker is for mostly the cells in the S-phase. Thus, the gene expression profile of PCNA indicates the number of cells in the DNA synthesis stage [47]. The results in the study indicate elevated expression of Ki-67 and cyclin D1, implying that more cells divide and move through the cell check gate G1/S in faster numbers, respectively. The lack of difference in the PCNA expression profile indicates that there is no difference in the synthesis stage of the cell cycle. Similar expression levels of PCNA and elevated expression of cyclin D1 indicated a similar number of cells exited the S-phase by crossing the G2/S check gate, and a higher number of cells entered the S-phase by crossing the G1/S cell cycle check gate, respectively. In other words, the cells entering the S-phase and exiting the S-phase might be similar, as indicated by the similar gene expression level of PCNA. However, conjunctival cells showed lower proliferative potential. The mRNA expression of the apoptotic markers remained unchanged in the day 14 limbal, conjunctival, and oral cultures. This suggests that most likely apoptosis may not have a lead role in classifying the three cell types presently used to reconstruct the corneal surface. In addition, macroautophagy regulation was studied, as it has a vital role in maintaining and regulating stem cell properties [21,48]. The proper functional clearing of unwanted toxic proteins (the autophagy lysosomal pathway) is a prerequisite for a successful transplantation [49]. On the 14th day, the expression pattern of LC3A/B, a microtubule-associated protein important for the induction of autophagy, through elongation and expansion of the phagophore formed the autophagosome [50]. Similarly high expression of LC3 by the autophagy activator promotes the differentiation of adipose-derived stromal cells into neuronal-like cells [51]. High autophagic activity was observed in adult skin and blood stem cells suggesting that autophagy could be a general phenomenon under physiologic conditions. This is in contrast to differentiated cells, where autophagy is usually induced as a consequence of stress [20]. We observed higher expression of LAMP1 in the day 14 cultured limbal epithelial cells compared to the conjunctival and oral epithelial cells, implicating the differentiation regime is upregulated. The expression of LAMP1 is higher when human salivary gland cells differentiate into acinar-like structures [52].
The similar expression pattern of autophagic markers in limbal, oral, and conjunctival cells could be a reason for the steady-state level or basal of the autophagic lysosomal pathway in the three different cell types. This requires further exploration with drug studies that use modulation of autophagy. Additionally, this suggests healthy cell physiology is maintained in cultured limbal epithelial cells compared to cultured conjunctival and oral epithelial cells. In the future, pharmacological modulation of autophagy in oral and conjunctival cultures might help in improvising their efficacy in transplantation.
Our findings provide an insight into the three different cell types that are used to reconstruct the corneal surface. The results suggest ex vivo limbal epithelial cultures plausibly have a higher probability of success in transplantation compared to conjunctival and oral epithelial cells. Moreover, the results provide clues for modulation of signaling pathways for improving conjunctival and oral cultures, so they can be equally efficient in transplantation. All three biopsies were processed following one standardized protocol to avoid the interfering factors of culture medium, the scaffold used for growth, and environmental factors such as the oxygen level, humidity, carbon dioxide levels, and so on. The vital role of host factor interplay was not accounted for in the present study.
Limbal epithelial cell transplantation has shown remarkable clinical success in corneal surface reconstruction surgery [30]. In an attempt to enhance transplantation efficiency recently, corneal surface reconstruction was attempted using a mixed population of cells of limbal and conjunctival origin. As expected, the results improved success in corneal restoration compared to the use of conjunctival cells alone [53,54]. Ricardo et al. in a recent study transplanted cultured conjunctival cells in 12 eyes of patients with LSCD. With a short-term follow-up, the curative status of the transplantation showed promising results [55]. In another study, Ang et al. in an animal study found a similar clinical outcome for limbal and conjunctival cell transplantation [56]. However, Liu et al. attempted oral epithelial cell transplantation in seven eyes of patients with LSCD and during the long-term follow-up found that five of the seven eyes developed peripheral corneal neovascularization [57].
Although the oral mucosal epithelial cells in our study showed a better outcome, with lower apoptotic and higher proliferative potential, their non-ocular origin prevailed [12]. Post-transplantation of ex vivo cultured oral mucosal cells showed neovascularization in corneas with the expression of FGF2, VEGF, and PEDF [58,59]. This has always remained at the forefront of an issue to be resolved before attempting to use oral mucosal epithelial cells for corneal surface reconstruction. In this study, we observed cultured oral epithelial cells showed higher expression of stem cell marker (ABCG2 and p63α) positivity compared to conjunctival cells. This might suggest that following ex vivo cultured limbal epithelial cells, oral epithelial cells might be better than the cultured conjunctival epithelial cells, though some additional treatment might be necessary to avoid neovascularization. The findings from our study imply that apart from the angiogenic properties, stem/progenitor cell, proliferation, and apoptosis properties also play a decisive role in the success or failure of a transplantation of ocular surface reconstruction.
Further studies are required to investigate the intricacies of the cell potential and determine the factors that could be modulated in an attempt to bring uniformity in clinical outcome. This would not only provide modalities in the branch of regenerative medicine for ophthalmology but also contribute in other branches of medicine.
Several mitigating factors can influence the success of cell transplantation [3]. Cell extrinsic and intrinsic factors such as inflammatory response, purity and identity of the cells, mode of transplantation, unwanted dedifferentiation potential post-transplantation, presence of specific markers such as ABCG2, ABCB5, and the ΔNp63α transcription factor, presence or absence of antiangiogenic factors such as FLT1, TIMP3, and TSP1, biomaterial scaffolds used as substrates, signaling pathways such as WNT7A and its regulation during differentiation through PAX6, and the number of stem cells transplanted play a crucial role in transplantation success [4,60-66].
Proliferation and differentiation play a crucial role in the success of cell transplantation [66]. Apart from having a confirmed role in cell proliferation and differentiation, Notch signaling induces corneal lineage–specific differentiation [27]. The number of stem cells expressing p63α positivity in cultured limbal epithelial cells before transplantation was correlated with the clinical outcome [29]. Low levels of pluripotent markers indicate higher differentiation state, which has been correlated with better transplantation success [67]]. Results of our study show that certain cell vital factors differ among the three biopsy sources. Additionally, the data also provide clues to some of the underlying components such as proliferation, stem cell marker expression, Notch signaling, and autophagy pathway differences among the three biopsy sources. In our study, we attempted to determine if there are any cell inherent properties along with several signaling pathways (Notch and autophagy pathways) between the three biopsy sources with the assumption that these could be the additional factors that might have a role in the transplantation outcome. Further studies are required to confirm and validate the role of each factor as well as identify additional factors that predict the treatment outcome.
Taken together, our results show that limbal cells are the best source for corneal surface reconstruction followed by oral mucosal cells and then conjunctival cells. Moreover, the clinical applicability of conjunctival and oral cultures could be enhanced using pharmacological modulators. This study sheds further light on understanding transplantation and regenerative medicine for ocular surface reconstruction.
Acknowledgments
The authors would like to thank Narayana Nethralaya Foundation, Department of Science and Technology, Govt of India (SRSOHS2282012) for providing the financial support to conduct this study. The authors would like to thank Dr. P. Narendra and Dr. Arkasubhra Ghosh for their administrative support. The authors would also like to convey their gratitude to Dr. K Bhujang Shetty for providing all the needed logistics for this study.
Appendix 1. Culture and characterization of limbal, conjunctival and oral mucosal biopsies.
Explants from all the three tissue origins were cultured for 14 days. Day 0 explants cultures at 10X magnification (A, D, G). Day 14 cultures at lower (10X; B, E, H) and higher (40X; C, F, I) magnification. Gel documentation image showing RT–PCR results for characterizing the cells of limbal (J), conjunctival (K) and oral (L) cultures of day 14 using primers for gapdh, cytokeratin 3, 4, 12, 13, 14, 15, connexin 43, p63α, abcg2 and vimentin. To access the data, click or select the words “Appendix 1.”
Appendix 2. Immunostaining of cultured limbal, conjunctival and oral biopsies.
Immunohistochemistry staining with anti-ABCG2, CX43, K3/K12 and K4/K13 on day 14 cultured limbal (A-F), conjunctival (G-L) and oral epithelial cells (M-R). The black arrows are used for showing the positive staining. To access the data, click or select the words “Appendix 2.”
Appendix 3. Expression of pluripotent markers.
Quantitative PCR results for oct4, sox2 and nanog markers on day 0 (A) cultured limbal, conjunctival and oral biopsies. Results were calibrated with β-actin. All the experiments were performed in triplicate using 6 samples per group (limbal, conjunctival and oral). Representative FACS plots of day 14 cultured limbal conjunctival and oral mucosal epithelial cells stained with antibodies against OCT4, SOX2 and NANOG (B). To access the data, click or select the words “Appendix 3.”
Appendix 4. Expression of Notch pathway molecules.
Quantitative PCR results of hes1, hes3, hes5 and hey1 mRNA expression in day 0 cultured limbal, conjunctival and oral biopsies (A). mRNA expression of hes1, hes3, hes5 and hey1 in day 14 limbal conjunctival and oral cultures (B). Results were calibrated with β-actin. All the experiments were performed in triplicate using 6 samples per group (limbal, conjunctival and oral). Statistical significance denoted, p *<0.05, **<0.01, ***<0.005. To access the data, click or select the words “Appendix 4.”
Appendix 5. Apoptotic status.
mRNA expression profile of apoptotic markers, bcl2, bax, caspase 3 and caspase 9 genes of day 14 limbal, conjunctival and oral cultured cells (B). Results were normalized with β-actin. All the experiments were performed in triplicate using 6 samples per group (limbal, conjunctival and oral). Significance denoted, p *<0.05, **<0.01, ***<0.005. To access the data, click or select the words “Appendix 5.”
References
- 1.Dhamodaran K, Subramani M, Ponnalagu M, Shetty R, Das D. Ocular stem cells: a status update! Stem Cell Res Ther. 2014;5:56. doi: 10.1186/scrt445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhamodaran K, Shetty R, Subramani M, Das D. Ocular Stem Cells: An Overview. In: Dimitrova G, editor. Recent Advances in Ophthalomology Research. 1st ed. New York: Nova Science Publishers Inc; 2013. p. 103–35 [Google Scholar]
- 3.Pellegrini G, De Luca M. Eyes on the prize: limbal stem cells and corneal restoration. Cell Stem Cell. 2014;15:121–2. doi: 10.1016/j.stem.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–55. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 5.Shortt AJ, Tuft SJ, Daniels JT. Ex vivo cultured limbal epithelial transplantation. A clinical perspective. Ocul Surf. 2010;8:80–90. doi: 10.1016/s1542-0124(12)70072-1. [DOI] [PubMed] [Google Scholar]
- 6.Tan DT, Ficker LA, Buckley RJ. Limbal transplantation. Ophthalmology. 1996;103:29–36. doi: 10.1016/s0161-6420(96)30737-9. [DOI] [PubMed] [Google Scholar]
- 7.Gaddipati S, Muralidhar R, Sangwan VS, Mariappan I, Vemuganti GK, Balasubramanian D. Oral epithelial cells transplanted on to corneal surface tend to adapt to the ocular phenotype. Indian J Ophthalmol. 2014 doi: 10.4103/0301-4738.109517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eslani M, Baradaran-Rafii A, Ahmad S. Cultivated limbal and oral mucosal epithelial transplantation. Semin Ophthalmol. 2012;27:80–93. doi: 10.3109/08820538.2012.680641. [DOI] [PubMed] [Google Scholar]
- 9.Burillon C, Huot L, Justin V, Nataf S, Chapuis F, Decullier E, Damour O. Cultured autologous oral mucosal epithelial cell sheet (CAOMECS) transplantation for the treatment of corneal limbal epithelial stem cell deficiency. Invest Ophthalmol Vis Sci. 2012;53:1325–31. doi: 10.1167/iovs.11-7744. [DOI] [PubMed] [Google Scholar]
- 10.Sangwan VS, Basu S, Vemuganti GK, Sejpal K, Subramaniam SV, Bandyopadhyay S, Krishnaiah S, Gaddipati S, Tiwari S, Balasubramanian D. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. Br J Ophthalmol. 2011;95:1525–9. doi: 10.1136/bjophthalmol-2011-300352. [DOI] [PubMed] [Google Scholar]
- 11.Sangwan VS, Matalia HP, Vemuganti GK, Fatima A, Ifthekar G, Singh S, Nutheti R, Rao GN. Clinical outcome of autologous cultivated limbal epithelium transplantation. Indian J Ophthalmol. 2006;54:29–34. doi: 10.4103/0301-4738.21611. [DOI] [PubMed] [Google Scholar]
- 12.Sotozono C, Inatomi T, Nakamura T, Koizumi N, Yokoi N, Ueta M, Matsuyama K, Miyakoda K, Kaneda H, Fukushima M, Kinoshita S. Visual improvement after cultivated oral mucosal epithelial transplantation. Ophthalmology. 2013;120:193–200. doi: 10.1016/j.ophtha.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 13.Ang LP, Tanioka H, Kawasaki S, Ang LP, Yamasaki K, Do TP, Thein ZM, Koizumi N, Nakamura T, Yokoi N, Komuro A, Inatomi T, Nakatsukasa M, Kinoshita S. Cultivated human conjunctival epithelial transplantation for total limbal stem cell deficiency. Invest Ophthalmol Vis Sci. 2010;51:758–64. doi: 10.1167/iovs.09-3379. [DOI] [PubMed] [Google Scholar]
- 14.Satake Y, Higa K, Tsubota K, Shimazaki J. Long-term outcome of cultivated oral mucosal epithelial sheet transplantation in treatment of total limbal stem cell deficiency. Ophthalmology. 2011;118:1524–30. doi: 10.1016/j.ophtha.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura T, Takeda K, Inatomi T, Sotozono C, Kinoshita S. Long-term results of autologous cultivated oral mucosal epithelial transplantation in the scar phase of severe ocular surface disorders. Br J Ophthalmol. 2011;95:942–6. doi: 10.1136/bjo.2010.188714. [DOI] [PubMed] [Google Scholar]
- 16.Das D, Lanner F, Main H, Andersson ER, Bergmann O, Sahlgren C, Heldring N, Hermanson O, Hansson EM, Lendahl U. Notch induces cyclin-D1-dependent proliferation during a specific temporal window of neural differentiation in ES cells. Dev Biol. 2010;348:153–66. doi: 10.1016/j.ydbio.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Lu H, Lu Q, Zheng Y, Li Q. Notch signaling promotes the corneal epithelium wound healing. Mol Vis. 2012;18:403–11. [PMC free article] [PubMed] [Google Scholar]
- 18.Djalilian AR, Namavari A, Ito A, Balali S, Afshar A, Lavker RM, Yue BY. Down-regulation of Notch signaling during corneal epithelial proliferation. Mol Vis. 2008;14:1041–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Ma A, Boulton M, Zhao B, Connon C, Cai J, Albon J. A role for notch signaling in human corneal epithelial cell differentiation and proliferation. Invest Ophthalmol Vis Sci. 2007;48:3576–85. doi: 10.1167/iovs.06-1373. [DOI] [PubMed] [Google Scholar]
- 20.Salemi S, Yousefi S, Constantinescu MA, Fey MF, Simon HU. Autophagy is required for self-renewal and differentiation of adult human stem cells. Cell Res. 2012;22:432–5. doi: 10.1038/cr.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan JL, Simon AK, Prescott M, Menendez JA, Liu F, Wang F, Wang C, Wolvetang E, Vazquez-Martin A, Zhang J. Autophagy in stem cells. Autophagy. 2013;9:830–49. doi: 10.4161/auto.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami M, Ichisaka T, Maeda M, Oshiro N, Hara K, Edenhofer F, Kiyama H, Yonezawa K, Yamanaka S. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol. 2004;24:6710–8. doi: 10.1128/MCB.24.15.6710-6718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Liu Y, Zheng P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matalia H, Shetty R, Dhamodaran K, Subramani M, Arokiaraj V, Das D. Potential apoptotic effect of ultraviolet-A irradiation during cross-linking: a study on ex vivo cultivated limbal epithelial cells. Br J Ophthalmol. 2012;96:1339–45. doi: 10.1136/bjophthalmol-2012-301811. [DOI] [PubMed] [Google Scholar]
- 25.Shetty R, Matalia H, Nuijts R, Subramani M, Dhamodaran K, Pandian R, Jayadev C, Das D. Safety profile of accelerated corneal cross-linking versus conventional cross-linking: a comparative study on ex vivo-cultured limbal epithelial cells. Br J Ophthalmol. 2015 doi: 10.1136/bjophthalmol-2014-305495. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Lam O, Nguyen MT, Ng G, Pear WS, Ai W, Wang IJ, Kao WW, Liu CY. Mastermind-like transcriptional co-activator-mediated Notch signaling is indispensable for maintaining conjunctival epithelial identity. Development. 2013;140:594–605. doi: 10.1242/dev.082842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xin Y, Lu Q, Li Q. 14–3–3sigma controls corneal epithelial cell proliferation and differentiation through the Notch signaling pathway. Biochem Biophys Res Commun. 2010;392:593–8. doi: 10.1016/j.bbrc.2010.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhamodaran K, Shetty R, Subramani M, Das D. Ocular Stem Cells: An Overview. In: Dimitrova G, editor. Recent Advances in Ophthalomology Research. New York: Nova Science Publishers Inc; 2013 [Google Scholar]
- 29.Pellegrini G, Rama P, Matuska S, Lambiase A, Bonini S, Pocobelli A, Colabelli RG, Spadea L, Fasciani R, Balestrazzi E, Vinciguerra P, Rosetta P, Tortori A, Nardi M, Gabbriellini G, Traverso CE, Macaluso C, Losi L, Percesepe A, Venturi B, Corradini F, Panaras A, Di Rocco A, Guatelli P, De Luca M. Biological parameters determining the clinical outcome of autologous cultures of limbal stem cells. Regen Med. 2013;8:553–67. doi: 10.2217/rme.13.43. [DOI] [PubMed] [Google Scholar]
- 30.Menzel-Severing J, Kruse FE, Schlötzer-Schrehardt U. Stem cell-based therapy for corneal epithelial reconstruction: present and future. Can J Ophthalmol. 2013;48:13–21. doi: 10.1016/j.jcjo.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Sudha B, Sitalakshmi G, Iyer GK, Krishnakumar S. Putative stem cell markers in limbal epithelial cells cultured on intact & denuded human amniotic membrane. Indian J Med Res. 2008;128:149–56. [PubMed] [Google Scholar]
- 32.Tseng SC, Meller D, Anderson DF, Touhami A, Pires RT, Grüterich M, Solomon A, Espana E, Sandoval H, Ti SE, Goto E. Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane for treating corneal diseases with total limbal stem cell deficiency. Adv Exp Med Biol. 2002;506:1323–34. doi: 10.1007/978-1-4615-0717-8_192. [DOI] [PubMed] [Google Scholar]
- 33.Balasubramanian S, Jasty S, Sitalakshmi G, Madhavan HN, Krishnakumar S. Influence of feeder layer on the expression of stem cell markers in cultured limbal corneal epithelial cells. Indian J Med Res. 2008;128:616–22. [PubMed] [Google Scholar]
- 34.Jeon S, Choi SH, Wolosin JM, Chung SH, Joo CK. Regeneration of the corneal epithelium with conjunctival epithelial equivalents generated in serum- and feeder-cell-free media. Mol Vis. 2013;19:2542–50. [PMC free article] [PubMed] [Google Scholar]
- 35.Koizumi N, Cooper LJ, Fullwood NJ, Nakamura T, Inoki K, Tsuzuki M, Kinoshita S. An evaluation of cultivated corneal limbal epithelial cells, using cell-suspension culture. Invest Ophthalmol Vis Sci. 2002;43:2114–21. [PubMed] [Google Scholar]
- 36.Sen S, Sharma S, Gupta A, Gupta N, Singh H, Roychoudhury A, Mohanty S, Sen S, Nag TC, Tandon R. Molecular characterization of explant cultured human oral mucosal epithelial cells. Invest Ophthalmol Vis Sci. 2011;52:9548–54. doi: 10.1167/iovs.11-7946. [DOI] [PubMed] [Google Scholar]
- 37.Fatima A, Sangwan VS, Iftekhar G, Reddy P, Matalia H, Balasubramanian D, Vemuganti GK. Technique of cultivating limbal derived corneal epithelium on human amniotic membrane for clinical transplantation. J Postgrad Med. 2006;52:257–61. [PubMed] [Google Scholar]
- 38.Pauklin M, Thomasen H, Pester A, Steuhl KP, Meller D. Expression of pluripotency and multipotency factors in human ocular surface tissues. Curr Eye Res. 2011;36:1086–97. doi: 10.3109/02713683.2011.608238. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura T, Ohtsuka T, Sekiyama E, Cooper LJ, Kokubu H, Fullwood NJ, Barrandon Y, Kageyama R, Kinoshita S. Hes1 regulates corneal development and the function of corneal epithelial stem/progenitor cells. Stem Cells. 2008;26:1265–74. doi: 10.1634/stemcells.2007-1067. [DOI] [PubMed] [Google Scholar]
- 40.Tsai TH, Sun MH, Ho TC, Ma HI, Liu MY, Tsao YP. Notch prevents transforming growth factor-beta-assisted epithelial-mesenchymal transition in cultured limbal progenitor cells through the induction of Smad7. Mol Vis. 2014;20:522–34. [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen BC, Lefort K, Mandinova A, Antonini D, Devgan V, Della Gatta G, Koster MI, Zhang Z, Wang J, Tommasi di Vignano A, Kitajewski J, Chiorino G, Roop DR, Missero C, Dotto GP. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev. 2006;20:1028–42. doi: 10.1101/gad.1406006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao JY, Chen JK. Roles of p63 in epidermal development and tumorigenesis. Biom J. 2012;35:457–63. doi: 10.4103/2319-4170.104410. [DOI] [PubMed] [Google Scholar]
- 43.Bhattacharya S, Das A, Mallya K, Ahmad I. Maintenance of retinal stem cells by Abcg2 is regulated by notch signaling. J Cell Sci. 2007;120:2652–62. doi: 10.1242/jcs.008417. [DOI] [PubMed] [Google Scholar]
- 44.Di Iorio E, Barbaro V, Ruzza A, Ponzin D, Pellegrini G, De Luca M. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci USA. 2005;102:9523–8. doi: 10.1073/pnas.0503437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 46.Yang K, Hitomi M, Stacey DW. Variations in cyclin D1 levels through the cell cycle determine the proliferative fate of a cell. Cell Div. 2006;1:32. doi: 10.1186/1747-1028-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kisielewska J, Lu P, Whitaker M. GFP-PCNA as an S-phase marker in embryos during the first and subsequent cell cycles. Biol Cell. 2005;97:221–9. doi: 10.1042/BC20040093. [DOI] [PubMed] [Google Scholar]
- 48.Phadwal K, Watson AS, Simon AK. Tightrope act: autophagy in stem cell renewal, differentiation, proliferation, and aging. Cellular and molecular life sciences. Cell Mol Life Sci. 2013;70:89–103. doi: 10.1007/s00018-012-1032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan H, Cai N, Li M, Liu GH, Izpisua Belmonte JC. Autophagic control of cell 'stemness'. EMBO Mol Med. 2013;5:327–31. doi: 10.1002/emmm.201201999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujita N, Itoh T, Omori H, Fukuda M, Noda T, Yoshimori T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol Biol Cell. 2008;19:2092–100. doi: 10.1091/mbc.E07-12-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu Y, Yuan X, Sun Q, Ou Y. Autophagy activator promotes neuronal differentiation of adult adipose-derived stromal cells. Neural Regen Res. 2013;8:882–9. doi: 10.3969/j.issn.1673-5374.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janebodin K, Reyes M. Neural Crest-Derived Dental Pulp Stem Cells Function as Ectomesenchyme to Support Salivary Gland Tissue Formation. Dentistry. 2012;•••:S13. [Google Scholar]
- 53.Subramaniam SV, Sejpal K, Fatima A, Gaddipati S, Vemuganti GK, Sangwan VS. Coculture of autologous limbal and conjunctival epithelial cells to treat severe ocular surface disorders: long-term survival analysis. Indian J Ophthalmol. 2013;61:202–7. doi: 10.4103/0301-4738.99840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sejpal K, Ali MH, Maddileti S, Basu S, Ramappa M, Kekunnaya R, Vemuganti GK, Sangwan VS. Cultivated limbal epithelial transplantation in children with ocular surface burns. JAMA Ophthalmol. 2013;131:731–6. doi: 10.1001/jamaophthalmol.2013.2308. [DOI] [PubMed] [Google Scholar]
- 55.Ricardo JR, Cristovam PC, Filho PA, Farias CC, de Araujo AL, Loureiro RR, Covre JL, de Barros JN, Barreiro TP, dos Santos MS, Gomes JA. Transplantation of conjunctival epithelial cells cultivated ex vivo in patients with total limbal stem cell deficiency. Cornea. 2013;32:221–8. doi: 10.1097/ICO.0b013e31825034be. [DOI] [PubMed] [Google Scholar]
- 56.Kuriakose R, Saha U, Castillo G, Udupa K, Ni Z, Gunraj C, Mazzella F, Hamani C, Lang AE, Moro E, Lozano AM, Hodaie M, Chen R. The nature and time course of cortical activation following subthalamic stimulation in Parkinson's disease. Cereb Cortex. 2010;20:1926–36. doi: 10.1093/cercor/bhp269. [DOI] [PubMed] [Google Scholar]
- 57.Liu J, Sheha H, Fu Y, Giegengack M, Tseng SC. Oral mucosal graft with amniotic membrane transplantation for total limbal stem cell deficiency. Am J Ophthalmol. 2011;152:739–47. doi: 10.1016/j.ajo.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 58.Sekiyama E, Nakamura T, Kawasaki S, Sogabe H, Kinoshita S. Different expression of angiogenesis-related factors between human cultivated corneal and oral epithelial sheets. Exp Eye Res. 2006;83:741–6. doi: 10.1016/j.exer.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 59.Chen HC, Yeh LK, Tsai YJ, Lai CH, Chen CC, Lai JY, Sun CC, Chang G, Hwang TL, Chen JK, Ma DH. Expression of angiogenesis-related factors in human corneas after cultivated oral mucosal epithelial transplantation. Invest Ophthalmol Vis Sci. 2012;53:5615–23. doi: 10.1167/iovs.11-9293. [DOI] [PubMed] [Google Scholar]
- 60.Herberts CA, Kwa MS, Hermsen HP. Risk factors in the development of stem cell therapy. J Transl Med. 2011;9:29. doi: 10.1186/1479-5876-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frank MH, Frank NY. Restoring the cornea from limbal stem cells. Regen Med. 2015;10:1–4. doi: 10.2217/rme.14.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ksander BR, Kolovou PE, Wilson BJ, Saab KR, Guo Q, Ma J, McGuire SP, Gregory MS, Vincent WJ, Perez VL, Cruz-Guilloty F, Kao WW, Call MK, Tucker BA, Zhan Q, Murphy GF, Lathrop KL, Alt C, Mortensen LJ, Lin CP, Zieske JD, Frank MH, Frank NY. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature. 2014;511:353–7. doi: 10.1038/nature13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramachandran C, Basu S, Sangwan VS, Balasubramanian D. Concise review: the coming of age of stem cell treatment for corneal surface damage. Stem Cells Transl Med. 2014;3:1160–8. doi: 10.5966/sctm.2014-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li DQ, Wang Z, Yoon KC, Bian F. Characterization, isolation, expansion and clinical therapy of human corneal epithelial stem/progenitor cells. J Stem Cells. 2014;9:79–91. [PubMed] [Google Scholar]
- 65.Ouyang H, Xue Y, Lin Y, Zhang X, Xi L, Patel S, Cai H, Luo J, Zhang M, Zhang M, Yang Y, Li G, Li H, Jiang W, Yeh E, Lin J, Pei M, Zhu J, Cao G, Zhang L, Yu B, Chen S, Fu XD, Liu Y, Zhang K. WNT7A and PAX6 define corneal epithelium homeostasis and pathogenesis. Nature. 2014;511:358–61. doi: 10.1038/nature13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stoll EA. Advances toward regenerative medicine in the central nervous system: challenges in making stem cell therapy a viable clinical strategy. Mol Cell Ther. 2014;2:12. doi: 10.1186/2052-8426-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Durruthy Durruthy J, Ramathal C, Sukhwani M, Fang F, Cui J, Orwig KE, Reijo Pera RA. Fate of induced pluripotent stem cells following transplantation to murine seminiferous tubules. Hum Mol Genet. 2014;23:3071–84. doi: 10.1093/hmg/ddu012. [DOI] [PMC free article] [PubMed] [Google Scholar]